Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
The incidence of infections after CD19 CAR–T-cell immunotherapy was similar to the incidence after other salvage chemoimmunotherapies.
Infections were more frequent in patients who had ALL, more prior antitumor treatment, a higher CAR–T-cell dose, or greater CRS severity.
Abstract
Lymphodepletion chemotherapy with CD19-targeted chimeric antigen receptor–modified T (CAR-T)-cell immunotherapy is a novel treatment for refractory or relapsed B-cell malignancies. Infectious complications of this approach have not been systematically studied. We evaluated infections occurring between days 0 to 90 in 133 patients treated with CD19 CAR-T cells in a phase 1/2 study. We used Poisson and Cox regression to evaluate pre- and posttreatment risk factors for infection, respectively. The cohort included patients with acute lymphoblastic leukemia (ALL; n = 47), chronic lymphocytic leukemia (n = 24), and non-Hodgkin lymphoma (n = 62). There were 43 infections in 30 of 133 patients (23%) within 28 days after CAR–T-cell infusion with an infection density of 1.19 infections for every 100 days at risk. There was a lower infection density of 0.67 between days 29 and 90 (P = .02). The first infection occurred a median of 6 days after CAR–T-cell infusion. Six patients (5%) developed invasive fungal infections and 5 patients (4%) had life-threatening or fatal infections. Patients with ALL, ≥4 prior antitumor regimens, and receipt of the highest CAR–T-cell dose (2 × 107 cells per kg) had a higher infection density within 28 days in an adjusted model of baseline characteristics. Cytokine release syndrome (CRS) severity was the only factor after CAR–T-cell infusion associated with infection in a multivariable analysis. The incidence of infections was comparable to observations from clinical trials of salvage chemoimmunotherapies in similar patients. This trial was registered at www.clinicaltrials.gov as #NCT01865617.
Introduction
Adoptive immunotherapy with CD19-targeted chimeric antigen receptor–modified T (CAR-T) cells administered after lymphodepletion chemotherapy is a novel treatment of patients with relapsed or refractory (R/R) B-cell malignancies, including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL).1-3 This approach has produced high complete response rates in ALL and high overall response rates in NHL and CLL,4-12 and is currently being investigated in multicenter clinical trials.
Most patients who present for CD19 CAR–T-cell immunotherapy have poor immune function due to both the effects of their malignancy and prior cytotoxic treatments. The lymphodepletion chemotherapy administered immediately before CAR–T-cell infusion also causes cytopenias and may impair mucosal barriers.6,7,13,14 CAR–T-cell immunotherapy can be complicated by cytokine release syndrome (CRS) and neurotoxicity, which can require management in the intensive care unit (ICU) and treatment with corticosteroids and/or tocilizumab, a humanized interleukin-6 receptor monoclonal antibody, both of which may increase infection risk.15 Finally, CD19 CAR-T cells deplete normal CD19+ B cells in most patients, which contributes to hypogammaglobulinemia.6,7,13,14
Despite the many insults to immune function in patients who receive CD19 CAR–T-cell immunotherapy, no systematic studies of the infectious complications of this treatment have been conducted. Here, we report the epidemiology of infections during the first 90 days after CD19 CAR–T-cell immunotherapy in 133 patients with R/R B-cell malignancies, and identify factors that predispose patients to a higher risk of infection.
Methods
Patients
Patients in this study were adults ≥18 years old who were HIV-negative with R/R CD19+ ALL, CLL, or NHL treated with lymphodepletion chemotherapy and CD19 CAR-T cells before September 2016 in a phase 1/2 open-label single-institution clinical trial (https://clinicaltrials.gov/ct2/show/NCT01865617). Eligibility criteria required the absence of uncontrolled infections. The trial was conducted with approval from the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board.
Lymphodepletion chemotherapy and adoptive transfer of CD19 CAR-T cells
Manufacture of CD19 CAR-T cells was performed as previously described.4 Patients received a single cycle of lymphodepletion chemotherapy, followed by CAR–T-cell infusion at, or as close as possible to, 1 of 3 CAR–T-cell dose levels: 2 × 105 cells per kg, 2 × 106 cells per kg, or 2 × 107 cells per kg.
Supportive care and monitoring
Granulocyte colony-stimulating factor 5 μg/kg per day subcutaneously was administered after lymphodepletion when the absolute neutrophil count (ANC) was <500 cells per mm3. Antimicrobial prophylaxis consisted of acyclovir 800 mg or valacyclovir 500 mg twice a day for herpes simplex or varicella zoster virus seropositive individuals starting on the day of lymphodepletion until ≥3 months after CAR–T-cell infusion, levofloxacin 750 mg daily and fluconazole 400 mg daily while the ANC was <500 cells per mm3, and trimethoprim 160 mg/sulfamethoxazole 800 mg twice a day for 2 days each week starting after neutrophil recovery until ≥3 months after CAR–T-cell infusion. The serum immunoglobulin G (IgG) concentration was evaluated prior to and approximately monthly after CAR–T-cell infusion, and immunoglobulin (400 mg/kg, IV) was recommended if the serum IgG concentration was <400 mg/dL.
The severity of CRS was graded as previously described.14 Neurotoxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03 and did not contribute to organ-toxicity grading in CRS. Treatment with tocilizumab (4-8 mg/kg per dose IV) and/or corticosteroids was considered for patients with grade ≥2 CRS and/or neurotoxicity. Additional supportive care and monitoring details are in supplemental Methods (available on the Blood Web site).
Infection categorization
Infections were recorded if there was a microbiologic or histopathologic diagnosis, and infection-onset day was defined as the day on which the diagnostic test was performed. Bacterial infections were categorized as bacteremias or site-specific infections. Multiple positive blood cultures for different organisms on the same day were considered separate events. Repeated positive cultures for a given organism were considered a second event if they occurred >21 days after the initial diagnosis and intervening cultures were negative. If the bacterial isolate was a possible skin contaminant (eg, Diphtheroid, Bacillus, or coagulase-negative Staphylococcus species) and isolated in only 1 blood culture it was excluded, unless systemic antibiotics were administered. Site-specific bacterial infections were defined as evidence of bacterial infection by culture of a normally sterile site or by culture and evidence of tissue invasion of a nonsterile site. Infections due to respiratory viruses were categorized as upper respiratory tract infections if the virus was detected in nasopharyngeal/throat washes or swabs, sinuses, or sputum with no symptoms or clinical evidence of lower respiratory tract infection. Lower respiratory tract infections were defined as detection of respiratory viruses in bronchoalveolar lavage in association with new or changing pulmonary infiltrates and lower respiratory tract symptoms. Fungal infections were recorded if there was proven or probable invasive fungal disease based on the 2008 revised criteria.16 Infections with 1 microorganism in 2 nonadjacent organs were counted as 2 infections. The lungs, respiratory tract, and paranasal sinuses were considered adjacent.
Classification of infection severity
Infection severity was classified as mild, moderate, severe, life-threatening, or fatal as previously established.17,18 Mild infections required no treatment. Moderate infections required oral treatment only. Severe infections required IV antimicrobial therapy or were associated with other clinical circumstances that were considered severe, with the exception of bacteremia due to possible skin contaminants and fever without systemic symptoms (categorized as moderate). Life-threatening infections were complicated by symptoms considered life-threatening. Fatal infections contributed significantly to death.
Data collection and statistical considerations
Patient information was extracted from medical records and databases. Infections were retrospectively identified from the day of the first CAR–T-cell infusion (day 0) up to day 90 after infusion. Patients were censored on the date of new antitumor therapy, the last clinical contact at our center, or death.
We reported summary statistics for categorical variables along with medians and ranges for continuous variables, stratified by underlying disease type. Infection densities were calculated as the mean number of infections for every 100 patient days. We compared infection densities and calculated the 95% confidence interval (CI) for the rate ratio (RR) using the mid-P method.19 We did not perform a competing risk analysis for cumulative incidence estimates in the first 28 days due to the low incidence of loss to follow-up or death.
To identify baseline clinical characteristics that modulated infection density, we used univariate and stepwise multivariable Poisson regression with an offset to account for days at risk. To evaluate post-CAR–T-cell infusion risk factors for infection, we performed univariate and stepwise multivariable Cox proportional hazards regression. Neutrophil recovery was defined as the first of 3 consecutive days with an ANC ≥500 cells per mm3. Analyses were performed for infections occurring between CAR–T-cell infusion and day 28 given standard follow-up at our center for at least 28 days. We separately reported infections from day 29 up to day 90 for patients under care at our center for ≥28 days after CAR–T-cell infusion. P values reported are 2-sided without multiplicity adjustment. Stepwise multivariable models were constructed with an entry and removal criteria of P < .1. Statistical significance was defined as 2-sided P < .05. Analyses were performed using SAS version 9.4 (SAS Institute) and Prism 7.
Results
Patient and treatment characteristics
One hundred thirty-three adult patients with R/R CD19+ B-cell malignancies were included in the study, 47 (35%) with ALL, 24 (18%) with CLL, and 62 (47%) with NHL. Patient and treatment characteristics are in Table 1. The median age was 54 years (range, 20-73 years). Prior to receiving lymphodepletion and CD19 CAR–T-cell immunotherapy, patients had received a median of 4 treatment regimens (range, 1-11). Fifty patients (38%) had undergone prior autologous and/or allogeneic hematopoietic cell transplant (HCT). Prior to lymphodepletion chemotherapy, 26% of patients had serum IgG < 400 mg/dL, 13% had an ANC < 500 cells per mm3, and 80% had an absolute lymphocyte count (ALC) < 200 cells per mm3. Neutrophil recovery to ±500 cells per mm3 occurred a median of 6 days after CAR–T-cell infusion. Twenty patients (15%), including 6 who had preexisting neutropenia before lymphodepletion chemotherapy, did not have neutrophil recovery before day 28 or censoring, and 14 patients (11%) never had an ANC < 500 cells per mm3 after CAR–T-cell infusion. Thus, this cohort had previously received multiple immunosuppressive therapies and had substantial immunodeficiency prior to CAR–T-cell infusion.
Table 1.
Clinical and treatment characteristics
| ALL, n = 47* | CLL, n = 24 | NHL, n = 62 | Total, N = 133 | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, median (range), y | 40 (20, 73) | 61 (40, 73) | 58 (28, 70) | 54 (20, 73) |
| Female | 20 (42.6) | 7 (29.2) | 13 (21.0) | 40 (30.1) |
| White | 35 (74.5) | 21 (87.5) | 54 (87.1) | 110 (82.7) |
| Prior antitumor treatment regimens, median (range) | 3 (1, 11) | 5 (3, 9) | 4 (1, 11) | 4 (1, 11) |
| Prior autologous and/or allogeneic HCT† | 17 (36.2) | 4 (16.7) | 29 (46.8) | 50 (37.6) |
| IgG <400 mg/dL prelymphodepletion‡ | 13 (28.9) | 8 (40.0) | 13 (22.8) | 34 (25.6) |
| ALC <200 prelymphodepletion | 40 (85.1) | 12 (50.0) | 54 (87.1) | 106 (79.7) |
| ANC <500 prelymphodepletion§ | 9 (20.0) | 2 (8.3) | 5 (8.8) | 16 (12.7) |
| Treatment | ||||
| Cy/Flu lymphodepletion regimen|| | 34 (72.3) | 21 (87.5) | 49 (79.0) | 104 (78.2) |
| CAR–T-cell dose | ||||
| 2 × 105 cells per kg, level 1 | 26 (55.3) | 4 (16.7) | 5 (8.1) | 35 (26.3) |
| 2 × 106 cells per kg, level 2 | 19 (40.4) | 19 (79.2) | 48 (77.4) | 86 (64.7)¶ |
| 2 × 107 cells per kg, level 3 | 2 (4.3) | 1 (4.2) | 9 (14.5) | 12 (9.0) |
| Post-CAR–T-cell characteristics | ||||
| Time-to neutrophil recovery ≥500, median (range), d# | 6 (1, 25) | 6 (3, 19) | 5 (1, 17) | 6 (1, 25) |
| CRS grade | ||||
| 0 | 12 (25.5) | 4 (16.7) | 24 (38.7) | 40 (30.1) |
| 1-3 | 31 (66.0) | 18 (75.0) | 34 (54.8) | 83 (62.4) |
| 4-5 | 4 (8.5) | 2 (8.3) | 4 (6.5) | 10 (7.5) |
| Neurotoxicity grade | ||||
| 0 | 22 (46.8) | 16 (66.7) | 42 (67.7) | 80 (60.2) |
| 1-2 | 11 (23.4) | 2 (8.3) | 12 (19.4) | 25 (18.8) |
| 3-5 | 14 (29.8) | 6 (25.0) | 8 (12.9) | 28 (21.1) |
| Corticosteroids and/or tocilizumab** | 15 (31.9) | 6 (25.0) | 7 (11.3) | 28 (21.1) |
| ICU admission by d 28 | 9 (19.1) | 2 (8.3) | 9 (14.5) | 20 (15.0) |
Data are presented as no. (%) unless otherwise specified. Lymphocyte and neutrophil counts are in units of cells per mm3.
ALC, absolute lymphocyte count; Cy, cytarabine; Flu, fludarabine; HCT, hematopoietic cell transplant; IQR, interquartile range.
One patient who received a second cycle of lymphodepletion chemotherapy and CAR–T-cell infusion following an allogeneic HCT performed after the first cycle of lymphodepletion and CAR–T-cell infusion is included as 2 separate enrollments.
Twenty-five patients (18.8%) had a prior allogeneic HCT alone, 22 (16.5%) had a prior autologous HCT alone, and 3 patients (2.3%) with NHL had both.
n = 122.
n = 126.
Regimens included: Cy 30 to 60 mg/kg IV on day 1 and Flu 25 mg/m2 per day IV on either days 2 to 4 or days 2 to 6 (Cy/Flu) (n = 100); Cy 2 to 4 g/m2 IV on day 1 (n = 17); Cy 2 to 4 g/m2 IV on day 1 and etoposide 200 mg/m2 per day IV on days 2 to 4 (n = 9); Cy 300 to 500 mg/m2 with Flu 30 mg/m2 concurrently for 3 days (n = 3); Flu 25 mg/m2 on days 1 to 3 (n = 2); Cy 1 g/m2 IV on day 1 and Flu 25 mg/m2 on days 2 to 4 (n = 1); bendamustine 90 mg/m2 for 2 days (n = 1). CAR T cells were infused in most patients between 36 and 96 hours after completion of chemotherapy.
Fifteen patients (NHL, n = 14; CLL, n = 1) received a second CAR–T-cell infusion ∼14 days after the first infusion and without additional lymphodepletion chemotherapy.
Time-to-neutrophil recovery was determined from the first day on or after CAR–T-cell infusion that a patient had an ANC < 500 cells per mm3 until the first of 3 consecutive days with an ANC ≥ 500 cells per mm3. Patients who did not reach an ANC ≥ 500 (ALL, n = 9; CLL, n = 7; NHL, n = 4) or never had an ANC < 500 (ALL, n = 7; CLL, n = 2; NHL, n = 5) were excluded from this analysis.
One patient received only tocilizumab, 7 received only corticosteroids, and 20 received both.
Incidence of bacterial, viral, and fungal infections in the first 28 days after CAR–T-cell infusion
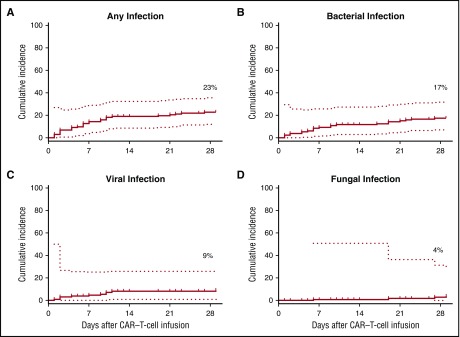
Within the first 28 days after CAR–T-cell infusion, we analyzed 3615 patient-days at risk. Cumulative-incidence curves of the time-to-first infection are shown in Figure 1 for any, bacterial, viral, and fungal infections. The first infection was identified a median of 6 days (range, 1-27 days) after CAR–T-cell infusion; 80% of first infections occurred within the first 10 days. The infection density during the first 28 days after CAR–T-cell infusion was 1.19 infections per 100 days at risk (Figure 2A; supplemental Table 1). There were 43 infections in 30 of 133 patients (23%) during this period (Table 2). Bacterial infections were the most common, with 24 events in 22 patients (17%). Twelve bacterial infections were bacteremias, and 4 of these (33%) were gram-negative organisms with acquired or intrinsic fluoroquinolone resistance. Viral infections were the next most frequent infection with 13 events in 11 patients (8%). Ten patients had respiratory virus infections, and 2 of these patients developed lower respiratory tract disease. Epstein-Barr virus (EBV) was detected by polymerase chain reaction (PCR) in plasma and cerebrospinal fluid from 1 patient with grade 4 CRS, and cytomegalovirus (CMV) was detected by PCR in plasma from 1 patient with grade 4 CRS. Neither patient had end-organ disease attributed to these viruses. Six invasive fungal infections occurred in 4 patients (3%), all of whom had severe CRS or neurotoxicity requiring tocilizumab and/or corticosteroids and 3 of whom were autologous or allogeneic HCT recipients. Invasive mold infections were diagnosed in 2 of these patients. Twenty-eight patients developed both CRS and infection. The onset of CRS occurred a median of 2 days prior to infection, and CRS was preceded by infection in only 3 patients. The median times to the onsets of CRS and the first infection were 1.9 and 6 days, respectively (P = .002, signed-rank test; supplemental Figure 1). In patients with grade ≥4 CRS, 8 of 11 infections (73%) occurred after the peak of the CRS grade.
Figure 1.
Cumulative-incidence curves of time-to-first infection for any infection and for specific infection categories. (A-D) Cumulative incidences among all patients (n = 133) of any (A), bacterial (B), viral (C), and fungal (D) infections within the first 28 days after CAR–T-cell infusion. Dotted lines represent 95% CIs.
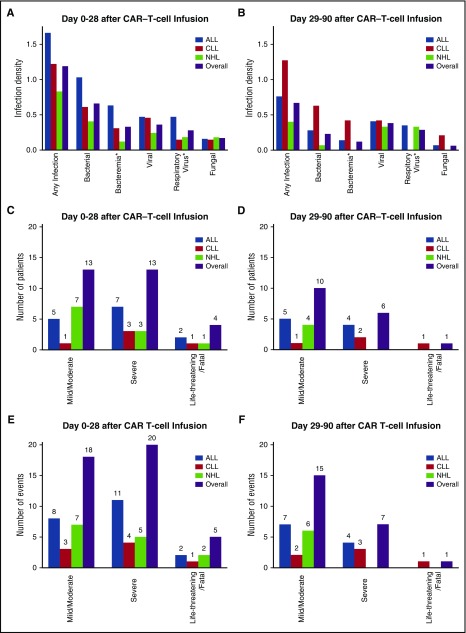
Figure 2.
Infection density and severity after CAR–T-cell infusion. (A-B) Infection densities are shown for infections occurring during the first 28 days (A) and day 29 to day 90 (B) after CAR–T-cell infusion. *Bacteremia and respiratory virus categories are subsets of the bacterial and viral categories, respectively. (C-D) Number of patients with a maximum grade of mild/moderate, severe, or life-threatening/fatal infection severity during the first 28 days (C) and between days 29 and 90 (D). (E-F) Severity of all infection events during the first 28 days (E) and days 29 to 90 (F) after CAR–T-cell infusion.
Table 2.
Incidence of specific infections through 28 days after CAR–T-cell infusion
| Type of infection | ALL, n = 47 | CLL, n = 24 | NHL, n = 62 | Total, N = 133 | ||||
|---|---|---|---|---|---|---|---|---|
| Events | No. of patients (%) | Events | No. of patients (%) | Events | No. of patients (%) | Events | No. of patients (%) | |
| Any infection | 21 | 14 (29.8) | 8 | 5 (20.8) | 14 | 11 (17.7) | 43 | 30 (22.6) |
| Bacterial infections | 13 | 12 (25.5) | 4 | 4 (16.7) | 7 | 6 (9.7) | 24 | 22 (16.5) |
| Bacteremia* | 8 | 7 (14.9) | 2 | 2 (8.3) | 2 | 1 (1.6) | 12 | 10 (7.5) |
| Bacterial site infections† | 5 | 5 (10.6) | 2 | 2 (8.3) | 5 | 5 (8.1) | 12 | 12 (9.0) |
| Viral infections | 6 | 5 (10.6) | 3 | 2 (8.3) | 4 | 4 (6.5) | 13 | 11 (8.3) |
| Respiratory virus‡ | 6 | 5 (10.6) | 1 | 1 (4.2) | 3 | 3 (4.8) | 10 | 9 (6.8) |
| Other virus§ | 0 | 0 (0.0) | 2 | 1 (4.2) | 1 | 1 (1.6) | 3 | 2 (1.6) |
| Fungal infections|| | 2 | 2 (4.3) | 1 | 1 (4.2) | 3 | 1 (1.6) | 6 | 4 (3.0) |
| Nonmold¶ | 1 | 1 (2.1) | 0 | 0 (0.0) | 3 | 2 (3.2) | 4 | 3 (2.3) |
| Mold# | 1 | 1 (2.1) | 1 | 1 (4.2) | 0 | 0 (0.0) | 2 | 2 (1.5) |
“Events” can include multiple entries per patient; the “No. of patients” columns include patients only once per category.
CMV, cytomegalovirus; EBV, Epstein-Barr virus.
Gram-positive, n = 7 (coagulase-negative Staphylococcus aureus, n = 4; streptococcal species, n = 2; Enterococcus faecium, n = 1); gram-negative, n = 4 (Escherichia coli, n = 1; Acinetobacter ursingii, n = 1; Stenotrophomonas maltophilia, n = 1; Capnocytophaga sputigena, n = 1); other, n = 1 (Mycoplasma hominis).
Gastrointestinal tract (C difficile colitis), n = 5; lower respiratory tract, n = 2; skin and soft tissue (perirectal abscesses), n = 3; urinary tract, n = 1; paranasal sinuses, n = 1.
Upper respiratory tract infection, n = 8 (rhinovirus, n = 4; parainfluenza virus 3, n = 1; influenza A, n = 1; metapneumovirus, n = 1; coronavirus, n = 1); lower respiratory tract infection, n = 2 (parainfluenza virus 3, n = 1; parainfluenza virus 4, n = 1).
CMV viremia, n = 1; EBV viremia, n = 1; EBV in cerebrospinal fluid of unclear clinical significance, n = 1.
Lower respiratory tract disease in 3 of 6 events.
Candida glabrata fungemia, n = 2; Candida glabrata pneumonia, n = 1; Candida bracarensis pneumonia, n = 1.
Aspergillus ustus proven pneumonia, n = 1; mold not otherwise specified invasive sinusitis, n = 1.
Incidence of bacterial, viral, and fungal infections between 29 and 90 days after CAR–T-cell infusion
CD19 CAR–T-cell immunotherapy results in endogenous B-cell depletion, which could predispose to infections beyond 28 days after CAR–T-cell infusion. B-cell depletion (<0.01% CD19+ normal B cells in blood leukocytes) occurred within 28 days after CAR–T-cell infusion in 116 of 118 evaluated patients (98%). By day 90, only 17 of 82 evaluated patients (21%) had evidence of endogenous B-cell recovery (≥0.01%; median day of recovery, day 69). Hypogammaglobulinemia (IgG < 400 mg/dL) was detected in 35%, 27%, and 46% of evaluated patients between days 15 to 30, 31 to 60, and 61 to 90 after CAR–T-cell infusion, respectively (supplemental Table 2). We evaluated 119 patients contributing 3431 days at risk for infections between days 29 and 90. The estimated infection density between days 29 and 90 was 0.67 infections for every 100 days at risk (Figure 2B; supplemental Table 1), which was significantly lower than the infection density in the first 28 days (RR, 0.56; 95% CI, 0.33-0.93; P = .02). Twenty-three infections occurred in 17 of 119 patients (14%) during this period (supplemental Table 3). Viral infections were the most common, with 13 infections in 11 patients (9%). Nine patients had upper respiratory tract virus infections, and lower respiratory tract disease developed in 1 of these patients (11%). Three patients without a history of HCT had nonrespiratory virus infections including CMV detection by PCR in plasma, BK polyoma virus cystitis, and CMV pneumonia. Bacterial infections were the next most frequent with 8 events in 7 patients (6%). Four of the bacterial infections were bacteremias, and 2 of these were due to gram-negative organisms with intrinsic fluoroquinolone resistance. Invasive fungal infections occurred in 2 patients who previously underwent allogeneic HCT (2%), 1 of whom had an invasive mold infection. Among patients with late infections, persistent disease and neutropenia were found in 48% and 22%, respectively (see supplemental Results).
Severity of infections after CD19 CAR–T-cell immunotherapy
To appreciate the clinical significance of infections, we evaluated infection severity. Between day 0 and 90 after CAR–T-cell immunotherapy, mild or moderate infections accounted for 33 of 66 events (50%) in 23 patients (17%) (Figure 2C-F). Severe infections accounted for 27 of 66 events (41%) in 19 patients (14%). Life-threatening infections accounted for 4 events (6%) in 3 patients (2%). Infection was the primary cause of death in a patient with CLL without neutropenia who died of acute pulmonary hemorrhage due to invasive Aspergillus ustus tracheobronchitis 90 days after CAR–T-cell infusion. Infection was a contributing factor to death in a neutropenic patient with ALL who had fatal CRS and concurrent severe Clostridium difficile pseudomembranous colitis.
Among patients with infections categorized as severe, 3 of 27 were present prior to lymphodepletion and progressed after CAR–T-cell infusion (bacterial sinusitis [n = 1] and perirectal abscess [n = 2]). One of 4 life-threatening infections was possibly present prior to lymphodepletion and progressed after CAR–T-cell infusion (invasive fungal sinusitis, species not determined). Grade ≥4 CRS was present in 2 patients who developed life-threatening infections. Both infections contributing to death developed de novo after CAR–T-cell infusion in patients with grade ≥4 CRS. These data demonstrate that most infections were mild to moderate and that life-threatening or fatal infections were infrequent after CD19 CAR–T-cell immunotherapy.
Baseline characteristics associated with increased infection density after CD19 CAR–T-cell immunotherapy
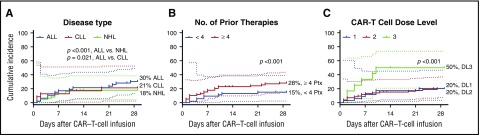
Identification of patient and treatment characteristics that increase the risk of subsequent infection would facilitate identification of those who might benefit from broader antimicrobial prophylaxis. In a univariate Poisson model, receipt of ≥4 prior antitumor treatment regimens, ANC < 500 cells per mm3 before CAR–T-cell infusion, and a CAR–T-cell dose of 2 × 107 cells per kg were associated with a significantly increased infection density (Table 3). In a stepwise multivariable model, patients with ALL, receipt of ≥4 prior antitumor treatment regimens, and receipt of 2 × 107 CAR-T cells per kg had higher infection densities (Table 3). Cumulative-incidence curves of the time to infection within 28 days after CAR–T-cell infusion stratified by informative baseline characteristics are depicted in Figure 3. These findings indicate that patients who received more intensive antitumor therapy and higher CAR–T-cell doses were at higher risk of infection after CAR–T-cell immunotherapy.
Table 3.
Pre-CAR–T-cell infusion factors and association with infection density within 28 days after CAR–T-cell infusion using Poisson regression
| Pre-CAR–T-cell infusion variables | Ratio of infection densities (95% CI) | P | Adjusted ratio of infection densities (95% CI) | P |
|---|---|---|---|---|
| Age | 1.00 (0.97-1.02) | .69 | ||
| Sex | 0.93 (0.47-1.85) | .83 | ||
| Disease type | ||||
| ALL vs CLL | 1.36 (0.60-0.37) | .46 | 2.68 (1.16-6.19) | .021 |
| ALL vs NHL | 2.01 (1.02-3.95) | .043 | 4.44 (2.06, 9.55) | <.001 |
| Prior autologous or allogeneic HCT | 0.70 (0.38-1.28) | .25 | ||
| Prior antitumor treatment regimens | ||||
| ≥4 vs <4 | 2.24 (1.10-4.54) | .017 | 3.53 (1.76-7.10) | <.001 |
| IgG <400 mg/dL prelymphodepletion | 1.01 (0.52-1.96) | .98 | ||
| ALC <200 cells per mm3 prelymphodepletion | 0.75 (0.38-1.48) | .42 | ||
| ANC <500 cells per mm3 prelymphodepletion | 1.84 (0.88-3.84) | .10 | ||
| ANC <500 cells per mm3 pre-CAR-T | 2.02 (1.08-3.78) | .024 | ||
| Lymphodepletion regimen | ||||
| Cy/Flu vs Cy/other | 0.64 (0.34-1.23) | .20 | ||
| CAR–T-cell dose, cells per kg | ||||
| 2 × 107 vs 2 × 105 | 3.06 (1.36-6.90) | <.001 | 7.86 (3.15-19.60) | <.001 |
| 2 × 107 vs 2 × 106 | 4.41 (2.13-9.17) | <.001 | 7.25 (3.51-14.99) | <.001 |
Figure 3.
Cumulative-incidence curves of time-to-first infection stratified by baseline characteristics. (A-C) Cumulative incidences of any infection within 28 days after CAR–T-cell infusion stratified by underlying disease (A), number of prior antitumor treatment regimens (B), and CAR–T-cell dose of 2 × 105 cells per kg (dose level 1), 2 × 106 cells per kg (dose level 2), or 2 × 107 cells per kg (dose level 3) (C). P values are derived from adjusted Poisson regression (Table 3). Dotted lines represent the 95% CIs. DL, dose level; Ptx, prior therapy.
Factors after CAR–T-cell infusion that were associated with increased risk for infection
Given that the incidence of infection was higher in patients who received a higher CAR–T-cell dose, and high CAR–T-cell doses are associated with an increased risk of CRS and neurotoxicity (supplemental Table 4),5,20 we used Cox proportional hazards regression to identify post-CAR–T-cell infusion factors increasing risk for first infections. In addition to baseline patient characteristics, this analysis included the following time-dependent variables: ANC < 500 cells per mm3 on the day of infection, maximum CRS and neurotoxicity grades, treatment with tocilizumab and/or corticosteroids, and ICU admission. In the univariate analysis, higher CAR–T-cell dose level, more severe CRS or neurotoxicity, treatment with tocilizumab, and ICU admission were associated with increased risk for infection (Table 4). There was an increased hazard for infection when the ANC was <500 cells per mm3, which did not achieve statistical significance (hazard ratio, 2.0; P = .1). Seventy-four percent and 91% of infections occurred in patients with an ANC <500 and <1000 cells per mm3, respectively (supplemental Figure 3). The severity of CRS was the only factor associated with infection after stepwise variable selection for a multivariable model, and there was an increased hazard for infection of 3.4 (P < .001) for each increase in CRS severity category (grade[s] 0 vs 1-3 vs 4-5). The proportions of patients with an infection in each CRS grade and category are demonstrated in supplemental Figure 2. Patients treated for CRS and/or neurotoxicity received a median of 1 dose of tocilizumab and 2 days of corticosteroids at a median daily prednisone-equivalence dose of 1.2 mg/kg. Any treatment with corticosteroids was not associated with infection, and there were insufficient events to determine whether duration or dose of tocilizumab or corticosteroids independently increased risk. Overall, the data show that most infections occurred during periods of neutropenia, and patients with more severe CRS had higher risk of infection.
Table 4.
Post-CAR-T-cell infusion factors and risk for first infection within 28 days using Cox proportional hazards regression
| Post-CAR–T-cell infusion variables | Unadjusted HR* (95% CI) | P |
|---|---|---|
| CAR–T-cell dose level, cells per kg | ||
| 2 × 107 vs 2 × 105 | 3.19 (1.07-9.51) | .038 |
| 2 × 107 vs 2 × 106 | 3.15 (1.24-8.01) | .016 |
| ANC < 500 cells per mm3 on day of infection | 2.04 (0.85-4.89) | .11 |
| CRS grade | ||
| 0 vs 1-3 vs 4-5† | 3.38 (1.99-5.73) | <.001 |
| Neurotoxicity grade | ||
| 0 vs 1-2 vs 3-5‡ | 1.76 (1.11-2.78) | .015 |
| Tocilizumab use§ | 3.45 (1.23-9.67) | .019 |
| Corticosteroid use§ | 1.50 (0.43-5.23) | .5 |
| ICU admission | 4.35 (1.78-10.65) | .001 |
HR, hazard ratio.
Data shown are from the univariate model only. CRS was the only significant variable in a stepwise multivariable model. Other baseline characteristics with P < .1 but not significant at the P < .05 level included prior antitumor treatment regimens ≥4. There was collinearity between CAR–T-cell dose level, CRS grade, neurotoxicity grade, tocilizumab use, corticosteroid use, and ICU admission.
For each increase in CRS severity category from 0 (none) to grades 1-3 to grades 4-5, there was a 3.38 increase in the hazard of infection.
For each increase in neurotoxicity severity category from 0 (none) to grades 1-2 to grades 3-5, there was a 1.76 increase in the hazard of infection.
These were analyzed as dichotomous variables (ie, yes vs no).
Infection incidence and severity among patients receiving Cy/Flu-based lymphodepletion with an optimized CD19 CAR–T-cell dose
Our data demonstrated that infection risk after CD19 CAR–T-cell immunotherapy was higher among patients who received higher doses of CAR-T cells and developed more severe CRS and/or neurotoxicity. We previously identified a preferred regimen incorporating cyclophosphamide and fludarabine (Cy/Flu)-based lymphodepletion followed by an optimized CAR–T-cell dose determined by disease type and tumor burden (see supplemental Methods), which was associated with a reduced risk of severe CRS and equivalent antitumor activity.4,5,12 In 90 patients (ALL, n = 27; CLL, n = 20; NHL, n = 43) who received the preferred regimen, within the first 28 days after CAR–T-cell infusion there were only 17 infections occurring in 14 patients (16%) at an infection density of 0.69, which was lower than in patients receiving a nonpreferred regimen (RR, 0.30; P < .001). The median time to an ANC ≥ 500 cells per mm3 was similar between patients receiving a preferred (median, 5 days; range, 1-19 days) or nonpreferred regimen (median, 6 days; range, 3-25 days). In 81 patients who received the preferred regimen and were followed at our center beyond day 28 and up to day 90, 8 infections occurred in 7 patients (9%) at an infection density of 0.44, which was lower than the infection density of patients receiving a nonpreferred regimen (RR, 0.39; P = .03). Among patients receiving the preferred regimen, 15 of 90 patients (17%) had mild or moderate infections, 5 (6%) had severe infections, and 1 (1%) had a life-threatening infection between day 0 and 90 after CAR–T-cell infusion. There were no fatal infections. These data show that patients receiving a treatment regimen optimized to reduce the severity of CRS had fewer and less severe infections after CAR–T-cell infusion compared with patients not receiving an optimized regimen.
Discussion
In this cohort of 133 patients treated with lymphodepletion chemotherapy and CD19 CAR-T cells for R/R ALL, CLL, and NHL, we demonstrated an increased risk of infections in patients who had ALL, more prior antitumor treatment, a higher CAR–T-cell dose, or more severe CRS. Patients receiving a CD19 CAR–T-cell immunotherapy regimen optimized to reduce severe CRS had fewer infections, and life-threatening or fatal infections were rare.
The incidence, distribution, and severity of infections in this cohort were similar to that observed in clinical trials of salvage or primary therapy for patients with R/R or advanced ALL, CLL, and NHL.21-23 In these studies, CTCAE grade ≥3 infections were reported in 34% to 52% of patients with ALL, 21% to 25% with CLL, and 23% to 24% with NHL. Infection densities after CD19 CAR–T-cell immunotherapy compared favorably with those observed after unrelated donor myeloablative HCT in the first 100 days (1.72-2.06 infections per 100 days at risk, depending on stem cell source) and between day 101 to day 180 (0.59-0.73 infections per 100 days at risk, depending on stem cell source).18 The distribution of specific infection categories in the early and late periods after CAR–T-cell immunotherapy aligned with those observed after unrelated donor HCT. Although comparisons to other studies are limited by differences in patient characteristics and the periods of observation, our data underscore that the risk of infection following CAR–T-cell immunotherapy is comparable to that of similar heavily pretreated patients. The incidence of bacterial infections in our study may be overestimated by inclusion of patients treated for single-positive bacterial blood cultures with possible skin contaminants in whom it was not possible to distinguish between infection and CRS as a cause of fever.
Most infections were caused by bacteria typical for patients with hematologic malignancies undergoing chemotherapy.24 Viral infections were predominantly respiratory viruses, and there was minimal detection of viremia or disease due to double-stranded DNA viruses (eg, herpesviruses, adenovirus, and BK virus) by clinically directed testing. Invasive fungal infections were the least frequent infection but an important cause of morbidity in 5% of patients. All patients who developed fungal infections had a prior HCT and/or developed severe CRS requiring immunosuppressive therapy. Despite the high net state of immunosuppression in this patient cohort before commencing lymphodepletion chemotherapy, few of the infections occurring after CAR–T-cell infusion were life-threatening or severe. Only 2 infections were primary or secondary causes of death. Among patients with severe, life-threatening, or fatal infections, 4 infections were present prior to receiving lymphodepletion and progressed after CAR–T-cell infusion. Although a delay of CAR–T-cell immunotherapy to enable resolution of infection may be preferred, this could increase risk of malignancy progression. Additional studies of the risks of immunotherapy in the setting of established infection are warranted.
Most infections occurred early after CAR–T-cell infusion. In Poisson regression models, baseline variables associated with increased infection density were a diagnosis of ALL and more preceding antitumor treatment regimens, consistent with increased risk due to greater impairment of immune function.25 A higher CAR–T-cell dose was also associated with an increased risk of infection but was collinear with CRS development and severity.20 In Cox regression models analyzing the effects of post-CAR–T-cell infusion factors, the development of CRS was the most important predictor of infection. Most infections occurred after the onset of CRS and did not appear to precipitate or exacerbate CRS. Among patients receiving CAR-T cells at a dose determined by disease and tumor burden to mitigate CRS, we observed fewer infections and no infection-related deaths, further supporting the risk conferred by CRS. It is unclear whether high cytokine levels, immunosuppressive therapies, or aggressive supportive care and ICU management contribute to the increased risk of infection in patients with severe CRS or neurotoxicity.13,14 Patients receiving chronic therapy with tocilizumab for rheumatologic diseases have an increased risk for infection,15 but the risks imparted by a few doses are unknown. Our analyses suggested that neutropenia also likely contributes to infection risk after CAR–T-cell infusion, but the study was not powered to evaluate the impact of the intensity of lymphodepletion on the risk of infection after CAR–T-cell infusion. Future studies will be required to identify lymphodepletion regimens that have limited hematopoietic toxicity while still enabling robust CAR–T-cell engraftment.
These data will guide development of improved supportive-care protocols. There are no standardized approaches to antimicrobial prophylaxis regimens in CD19 CAR–T-cell recipients, and practices at our center have been modeled on autologous HCT guidelines.26 Our approach to infection prevention through antibiotic prophylaxis and IV immunoglobulin repletion appears to be relatively effective; however, the increased risk for invasive fungal infections in patients who had undergone prior autologous or allogeneic HCT or developed life-threatening CRS and/or neurotoxicity suggests that broader prophylaxis could be considered for selected high-risk patients, similar to approaches developed for allogeneic HCT recipients.27,28 Identification of additional risk factors for infection, such as the association between infections occurring prior to lymphodepletion and those occurring after CAR–T-cell infusion, might enhance the development of infection prophylaxis regimens.
This study’s strengths were the inclusion of a large cohort of CAR–T-cell recipients with 3 disease types, who received standardized supportive-care measures, close monitoring for infections, and analyses that identified baseline and post-CAR–T-cell infusion risk factors for infection. Potential bias in the cohort of patients requiring follow-up beyond day 28 at our center limits interpretation of late-event rates. However, data regarding infections occurring between day 28 and 90 provide important insights into the epidemiology of infections after CAR–T-cell therapy.
In conclusion, the incidence and type of infections after CD19 CAR–T-cell immunotherapy were consistent with those seen in patients with R/R B-cell malignancies receiving salvage chemoimmunotherapies. Patients with greater immunosuppression and CAR–T-cell associated toxicities had the highest risk for infection, identifying a targeted group in which to study improved prophylactic strategies. Life-threatening or fatal infections were rare, especially among patients receiving optimized lymphodepletion chemotherapy and CAR–T-cell dosing regimens. Effective strategies to prevent infections in the immunocompromised host are well established in other settings and could be adapted to improve patient outcomes after CAR–T-cell immunotherapy.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the staff of the Fred Hutchinson Cancer Research Center Program in Immunology and Therapeutic Products Program, the Bezos Family Immunotherapy Clinic, and Louise Kimball, Lisa Chung, Kari Blankenship, and Toni-Ann Lupinacci for help with data collection.
This work was supported by a generous gift from the Bezos family; National Institutes of Health grants from the National Institute of Allergy and Infectious Diseases (K23 AI119133) and the National Cancer Institute (R01 CA136551); the Washington State Life Science Discovery Fund; and Juno Therapeutics.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.A.H., M.L.G., M.B., and C.J.T. designed the study and interpreted the data; J.A.H. and D.L. analyzed the data and created the figures; J.A.H., K.A.H., M.L.G., S.C., and X.C. collected data; J.A.H. and C.J.T. drafted the manuscript; and all authors contributed to the writing and revision of the manuscript and approved the final version.
Conflict-of-interest disclosure: J.A.H. has served as a consultant for Chimerix Inc and Nohla Therapeutics, Inc and has received research support from Chimerix Inc and Shire. M.B. has served as a consultant and received research support from Merck Research Laboratories, Chimerix Inc, GlaxoSmithKline, and Roche/Genentech in addition to consulting fees from Clinigen. C.J.T., D.G.M., and S.R.R. received research funding from Juno Therapeutics and hold patents. C.J.T. has served on advisory boards for Juno Therapeutics, Precision Biosciences, Bluebird Bio, Celgene, Seattle Genetics, and Adaptive Biotechnologies. S.R.R. is a cofounder of Juno Therapeutics. D.L. is an employee of and has equity in Juno Therapeutics. Fred Hutchinson Cancer Research Center receives research funding from Juno Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Joshua A. Hill, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mail Stop E-400, Seattle, WA 98109; e-mail: jahill3@fredhutch.org.
References
- 1.Turtle CJ, Riddell SR, Maloney DG. CD19-Targeted chimeric antigen receptor-modified T-cell immunotherapy for B-cell malignancies. Clin Pharmacol Ther. 2016;100(3):252-258. [DOI] [PubMed] [Google Scholar]
- 2.Frey NV, Porter DL. The promise of chimeric antigen receptor T-cell therapy. Oncology (Williston Park). 2016;30(10):880-888, 890. [PubMed] [Google Scholar]
- 3.Kenderian SS, Porter DL, Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the CART before the horse. Biol Blood Marrow Transplant. 2017;23(2):235-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turtle CJ, Hanafi LA, Berger C, et al. . Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turtle CJ, Hanafi L-A, Berger C, et al. . CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Feldman SA, et al. . B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter DL, Hwang W-T, Frey NV, et al. . Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed]
- 12.Turtle CJ, Hay KA, Hanafi L-A, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor–modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. [DOI] [PMC free article] [PubMed]
- 13.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DW, Gardner R, Porter DL, et al. . Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2015;126(8):1048]. Blood. 2014;124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro G, Taroumian S, Barroso N, Duan L, Furst D. Tocilizumab in rheumatoid arthritis: a meta-analysis of efficacy and selected clinical conundrums. Semin Arthritis Rheum. 2014;43(4):458-469. [DOI] [PubMed] [Google Scholar]
- 16.De Pauw B, Walsh TJ, Donnelly JP, et al. . National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Burik JA, Carter SL, Freifeld AG, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow : analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(12)1487-1498. [DOI] [PubMed]
- 18.Young JH, Logan BR, Wu J, et al. ; Blood and Marrow Transplant Clinical Trials Network Trial 0201. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22(2):359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin DO, Austin H. Exact estimates for a rate ratio. Epidemiology. 1996;7(1):29-33. [DOI] [PubMed] [Google Scholar]
- 20.Hay KA, Hanafi L-A, Li D, et al. . Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy [published online ahead of print 18 September 2017]. Blood. doi:10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarjian H, Stein A, Gökbuget N, et al. . Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gisselbrecht C, Glass B, Mounier N, et al. . Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallek M, Fischer K, Fingerle-Rowson G, et al. ; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164-1174. [DOI] [PubMed] [Google Scholar]
- 24.Freifeld AG, Bow EJ, Sepkowitz KA, et al. ; Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. [DOI] [PubMed] [Google Scholar]
- 25.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338(24):1741-1751. [DOI] [PubMed] [Google Scholar]
- 26.Tomblyn M, Chiller T, Einsele H, et al. ; Centers for Disease Control and Prevention. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective [published correction appears in Biol Blood Marrow Transplant 2010;16(2):294]. Biol Blood Marrow Transplant. 2009;15(10):1143-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marr KA, Seidel K, Slavin MA, et al. . Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96(6):2055-2061. [PubMed] [Google Scholar]
- 28.Ullmann AJ, Lipton JH, Vesole DH, et al. . Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335-347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.