Abstract
Acute promyelocytic leukemia (APL) is a subtype of acute myelocytic leukemia. Previous studies have reported a number of functions and therapeutic roles of microRNAs (miRs) in APL, and have suggested that miR-218 acts as a tumor suppressor in a number of types of human cancer; however, its role in APL remains unclear. In the present study, the expression of miR-218 and its effects on the viability and proliferation of HL-60 cells was investigated. Reverse transcription-quantitative polymerase chain reaction analysis demonstrated that miR-218 was frequently downregulated in APL marrow tissues compared with normal marrow tissues. Overexpression of miR-218 significantly inhibited cell proliferation, arrested the cell cycle in the G0/G1 phase and induced apoptosis. In addition, B-cell-specific Moloney murine leukemia virus integration site 1 (BMI-1) mRNA expression was negatively associated with miR-218 expression; BMI-1 mRNA and protein expression were downregulated following transfection with miR-218 mimic. These results indicate that miR-218 functions as tumor suppressor in APL, and the miR-218/BMI-1 signaling axis may be a potential novel diagnostic marker and therapeutic target for the treatment of APL.
Keywords: microRNA-218, acute promyelocytic leukemia, cell cycles, proliferation, apoptosis, B-cell-specific Moloney murine leukemia virus integration site 1
Introduction
Acute promyelocytic leukemia (APL), classified as M3 in the French-American-British classification system (1), is a subtype of acute myelocytic leukemia (AML). APL represents between 7 and 27% of all AML types worldwide; however, in China this proportion increases to between 12 and 23% (2). Compared with other AML types, APL is associated with an increased risk of hemorrhage and an increased mortality rate (3). Currently, systematical chemotherapies based on all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO) are the predominant treatment methods (4); however, single and combination drug therapies have many disadvantages, including early drug resistance, early recurrence and adverse side effects (5). Thus, the treatment outcome and prognosis of APL remain poor.
The functions and therapeutic roles for microRNAs (miRNAs/miRs) in APL have been reported (6,7). miRNAs are small (between 19 and 25 nucleotides) non-coding RNA molecules that negatively regulate gene expression by interacting with the 3′ untranslated region of targeted mRNA, eventually leading to translational suppression and/or degradation of the mRNA (8). Previous studies have demonstrated that miRNAs serve important roles in the generation and progression of a number of human solid or hematological neoplasms via regulation of cell growth, differentiation, invasion and other pathological processes (9). In patients with AML, miR-96 was identified to be downregulated and associated with leukemic burden, in addition to recurrence-free survival and overall survival (10). In addition, miR-125b was demonstrated to be highly expressed in pediatric APL compared with other subtypes of AML and was associated with treatment response and relapse in patients with pediatric APL (11). The underlying molecular mechanisms of this effect may be that miR-125b downregulates the expression of the tumor suppressor Bcl-2-antagonist/killer 1 to promote leukemic cell proliferation and inhibit cell apoptosis (11). miR-218 was demonstrated to be an important tumor suppressor in a number of types of cancer; in hepatocellular carcinoma, miR-218 has been demonstrated to be downregulated, and associated with increased tumor size (12). In vitro, overexpression of miR-218 repressed cell proliferation and induced apoptosis in HepG2 and SMMC-7721 cells (12).
B-cell-specific Moloney murine leukemia virus insertion site-1 (BMI-1) is located in human chromosome 10p11.23. Liu et al (13) demonstrated that BMI-1 was able to suppress the expression of p16INK4a and p19ARF (which are proteins from the same genetic locus as cyclin-dependent kinase inhibitor 2A) by impairing the transcription of this locus and therefore leading to a series of dysfunctions in the cell cycle and cell proliferation.
In the present study, the expression of miR-218 in APL tissues and its functions in cell growth were investigated in vitro. The results indicated that miR-218 is downregulated in APL tissues and HL-60 cells, and it may suppress cell proliferation and induce apoptosis by inhibiting BMI-1 expression.
Materials and methods
Clinical specimens and cell culture
A total of 40 marrow tissues from patients with APL (25 female; 15 male) and 20 normal marrow tissues were collected and stored in liquid nitrogen in Yuhuangding Hospital during (Yantai, China) between April 2011 to May 2014. The age range of the patients was between 24 and 65 years and the median age was 49 years. None of the patients included in the present study accepted chemotherapy prior to biopsy. All clinical values for white blood cell count (WBC), hemoglobin (HGB) and platelet count (PLT) were obtained from the medical records of patients. The present study was approved by the Ethics Committee of Yantai Yuhuangding Hospital and written informed consent was obtained from all patients. The human APL cell line HL-60 was purchased from The Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Gibco® RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and maintained at 37°C in a cell culture incubator with 5% CO2.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from marrow tissues and HL-60 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. A total of 20 µg cDNA was used for the SYBR® Green Real-Time PCR Master Mixes (Invitrogen; Thermo Fisher Scientific, Inc.) system. The relative expression of miR-218 was detected using a Bulge-Loop™ miRNA RT-qPCR Starter kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China) according to the manufacturer's protocol. The BMI-1 mRNA expression level was measured using a Quant One Step qRT-PCR kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's protocol. The Bulge-Loop™ miR-218 primer kit (cat. no. miRQ0000275-1-2) was purchased from RiboBio Co., Ltd (Guangzhou, China). BMI-1 and β-actin primers were synthesized by Beijing Augct DNA-Syn Biotechnology Co., Ltd. (Beijing, China) and the sequences were as follows: BMI-1 forward, 5′-GTGCTTTGTGGAGGGTACTTCAT-3′ and reverse, 5′-TTGGACATCACAAATAGGACAATACTT-3′; β-actin forward, 5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse, 5′-GCTGTCACCTTCACCGTTCC-3′. The 2−ΔΔCq method (14) was used to calculate differences in mRNA expression.
miRNA transfection
HL-60 cell transfection was performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Briefly, 5×105 cells were cultured in 6-well culture plates containing 1.5 ml RPMI-1640 medium per well without FBS. Amounts of 100 pmol of miR-218 mimic (cat. no., miR10000275-1-5) or scrambled miRNA mimic (cat. no., miR01201-1-5) (Guangzhou RiboBio Co., Ltd.) were mixed with 5 µl Lipofectamine® 2000 in 500 µl Opti-MEM® I Reduced Serum Medium (Gibco; Thermo Fisher Scientific, Inc.) and incubated for 15 min at room temperature. The complex was added to the cells and the full distribution over the plate surface was ensured. After 8 h of incubation, whole media were replaced and the cells were incubated for 24, 48 and 72 h.
Cell viability assay
The viability of cells was assessed using the Cell Counting Kit-8 (CCK-8, Sangon Biotech Co., Ltd., Shanghai, China). The CCK-8 assay was performed at 24, 48 and 72 h after transfection. A 10 µl volume of CCK-8 solution was added to 5×105 HL-60 cells suspended in 100 µl RPMI-1640 medium and incubated for 4 h at 37°C in the dark. Absorbance was determined using a spectrophotometer at 450 nm.
Bromodeoxyuridine (BrdU) incubation assay
HL-60 cells (7×104) were cultured on coverslips at 48 h post-transfection and their proliferative ability was detected using a BrdU Cell Proliferation Assay kit (Cell Signaling Technology, Inc., Danvers, MA, USA) according to the manufacturer's protocol. Absorbance was determined using a spectrophotometer at 450 nm.
Flow cytometric analysis
HL-60 cells were collected and diluted to 5×105 48 h after transfection. Cell cycle phase and the proportion of apoptotic cells were evaluated using Annexin V-fluorescein isothiocyanate and propidium iodine staining (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's protocol and analyzed using BD FACSCalibur flow cytometry system (BD Biosciences, Franklin Lakes, NJ, USA) with the software FCS Express v3.0 (De Novo Software, Glendale, CA, USA).
Caspase 3/7 activity assay
HL-60 cells (2×104) were collected and cultured on 96-well plates 48 h after transfection. The activity of caspase 3/7 in transfected cells was measured using the Apo-ONE® Homogeneous Caspase-3/7 assay kit (Promega Corporation, Madison, WI, USA). The data were calculated using a microplate reader at a wavelength of 499 nm.
Protein isolation and western blot analysis
Cells were washed twice with PBS and proteins were isolated using 1% Triton X-100 reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on ice. The lysate was centrifuged at 13,000 × g and 4°C for 15 min. The supernatant was obtained and the protein content was detected using a bicinchoninic acid assay kit (EMD Millipore, Billerica, MA, USA). Proteins were separated by vertical electrophoresis and transferred onto polyvinylidene fluoride membranes (EMD Millipore). Anti-BMI-1 (cat. no. sc-10745; dilution, 1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and -β-actin (cat. no. sc-4778; dilution, 1:5,000; Santa Cruz Biotechnology, Inc.) antibodies were used to detect the expression of each protein. Secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse antibodies (cat. no. ABIN361237, dilution, 1:5,000; Abgent, Inc., San Diego, CA, USA) were used and bands were developed using Immobilon Western Chemiluminescent HRP Substrate (WBKLS0500, EMD Millipore, Billerica, MA, USA).
Statistical analysis
Experiments were repeated in triplicate, and data are presented as the mean ± standard deviation. SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA) was used to calculate all statistical results. Pearson's correlation analysis was used to examine the correlation between miR-218 and BMI-1 mRNA expression levels. Differences in two groups were determined by the two-tailed Student's t-test. A one-way analysis of variance was used to analyze the difference for the data from the CCK-8 assays. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-218 is overexpressed in APL marrow tissues
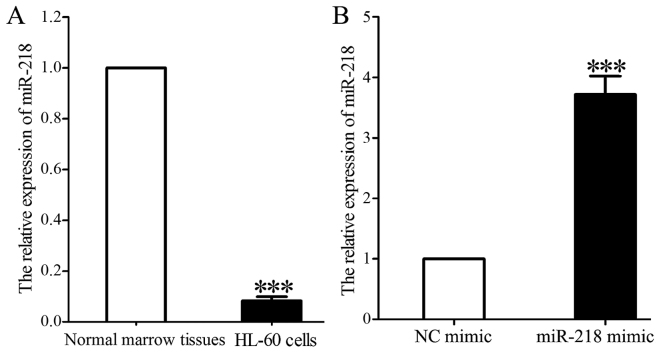
RT-qPCR analysis demonstrated that the expression of miR-218 was significantly decreased in 40 APL marrow tissues compared with 20 normal marrow tissues (2.327±0.063 vs. 5.734±0.121; P<0.001; Fig. 1). The association of miR-218 expression with clinical features is summarized in Table I. Lower levels of miR-218 were associated with a higher WBC count (P=0.004) and bone marrow (BM) promyelocyte percentage (P=0.027), and lower hemoglobin level (P=0.007) and blood platelet count (P=0.002).
Figure 1.
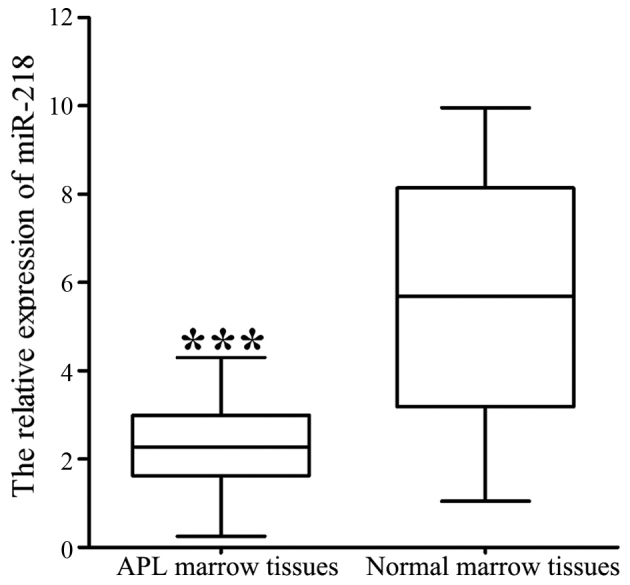
mRNA expression of miR-218 in APL marrow tissues and normal marrow tissues. ***P<0.001. APL, acute promyelocytic leukemia; miR, microRNA.
Table I.
miR-218 expression in APL marrow tissues from patients with APL (n=40), compared according to different clinicopathological characteristics.
| Clinicopathological characteristic | Classification | Relative miR-218 level | t | P-value |
|---|---|---|---|---|
| Sex | Male | 2.379±0.201 | 0.791 | 0.533 |
| Female | 2.181±0.192 | |||
| Age, years | <60 | 2.403±0.191 | 0.905 | 0.341 |
| ≥60 | 2.191±0.207 | |||
| WBC | <10×109 | 3.283±0.111 | 3.017 | 0.004a |
| ≥10×109 | 1.105±0.097 | |||
| HGB, g/l | <80 | 1.091±0.241 | 2.826 | 0.007a |
| ≥80 | 2.973±0.182 | |||
| PLT | <50×109 | 1.313±0.132 | 3.341 | 0.002a |
| ≥50×109 | 2.589±0.107 | |||
| Promyelocytes in BM, % | <50 | 2.813±0.121 | 2.382 | 0.027a |
| ≥50 | 1.321±0.237 |
P<0.05. t, student t-test; WBC, white blood cell; HGB, hemoglobin; BM, bone marrow; miR, microRNA; PLT, platelet count.
miR-218 mimic upregulates miR-218 expression in HL-60 cells
miR-218 expression in HL-60 cells was significantly decreased compared with that in normal marrow tissues (0.118±0.021 vs. 1.000; P<0.001; Fig. 2A). miR-218 expression was significantly increased in HL-60 cells 48 h following transfection with miR-218 mimic compared with that in cells transfected with control mimic (3.731±0.346 vs. 1.000; P<0.001; Fig. 2B).
Figure 2.
miR-218 expression is increased in HL-60 cells following transfection with miR-218 mimic. (A) RT-qPCR analysis of miR-218 expression in HL-60 cells compared with normal marrow tissues. (B) RT-qPCR analysis of miR-218 in HL-60 cells following transfection with control or miR-218 mimic. ***P<0.001. miR, microRNA; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; NC, control.
Overexpression of miR-218 inhibits viability and proliferation of HL-60 cells
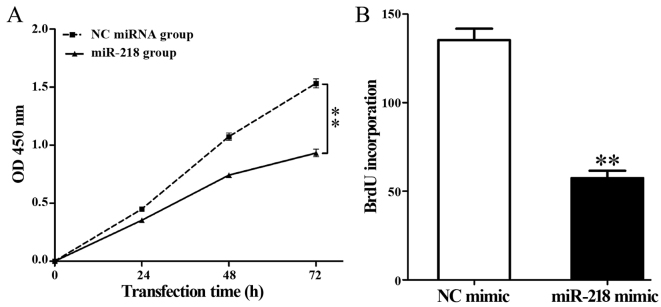
Overexpression of miR-218 significantly decreased the viability of HL-60 cells compared with the control group (P<0.01; Fig. 3A). In addition, a BrdU incubation assay demonstrated that miR-218 overexpression significantly repressed DNA synthesis in HL-60 cells compared with the control group (57.39±4.20 vs. 135.37±6.43; P=0.002; Fig. 3B).
Figure 3.
miR-218 overexpression decreases the viability and proliferation of HL-60 cells. (A) Cell Counting Kit-8 and (B) bromodeoxyuridine incorporation assay results in HL-60 cells following transfection with control or miR-218 mimic. **P<0.01. miR, microRNA; NC, control; OD, optical density.
Overexpression of miR-218 arrests cells in G0/G1 phase
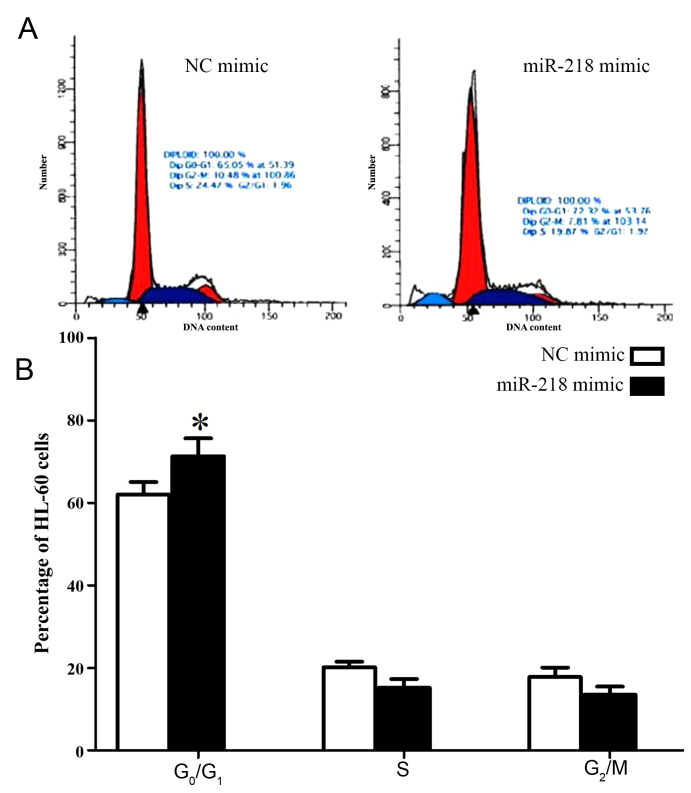
In order to investigate the molecular mechanisms underlying the effect of miR-218 on cell proliferation, the cell cycle of HL-60 cells was analyzed following transfection with miR-218 mimic. Significantly increased numbers of HL-60 cells transfected with miR-218 mimic were arrested in G0/G1 phase compared with cells transfected with control mimic (71.32±4.31 vs. 62.05±3.02, P=0.034; Fig. 4; Table II).
Figure 4.
miR-218 overexpression arrests HL-60 cells in the G0/G1 phase. (A) Representative flow cytometry histograms and (B) quantification of the percentage of HL-60 cells in each cell cycle phase following transfection with control or miR-218 mimic. *P<0.05. miR, microRNA.
Table II.
Proportion of HL-60 cells in each phase of the cell cycle following transfection with control and miR-218 mimic.
| Cell cycle | |||
|---|---|---|---|
| Group | G0/G1 phase (% of cells) | S phase (% of cells) | G2/M phase (% of cells) |
| miR-218 | 71.32±4.31a | 15.18±2.15 | 13.50±1.98 |
| NC | 62.05±3.02 | 19.17±1.33 | 18.78±2.31 |
P<0.05. miR, microRNA; NC, control.
Overexpression of miR-218 increases apoptosis and caspase 3/7 activity in HL-60 cells
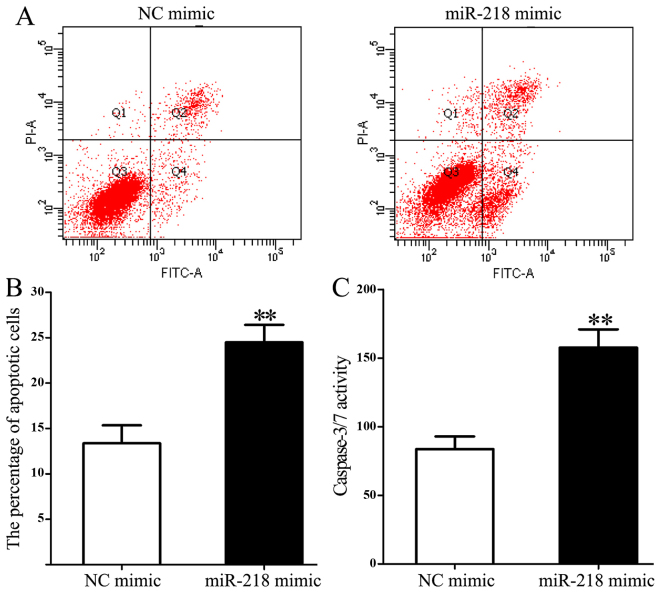
Flow cytometry was used to measure the influence of miR-218 on cell apoptosis. miR-218 overexpression significantly increased the proportion of apoptotic cells compared with the control group (24.482±1.943 vs. 13.352±1.981; P=0.003; Fig. 5A and B). In addition, miR-218 overexpression significantly increased caspase 3/7 activity compared with the control group (157.65±13.28 vs. 83.71±9.24; P=0.005; Fig. 5C).
Figure 5.
miR-218 overexpression significantly increases the percentage of apoptotic Hl-60 cells. (A) Representative flow cytometry data and (B) quantification of the percentage of apoptotic HL-60 cells based on flow cytometric analysis of annexin V-FITC/propidium iodide staining following transfection with control or miR-218 mimic. (C) Caspase 3/7 activity in HL-60 cells following transfection with control or miR-218 mimic. **P<0.01. miR, microRNA; FITC, fluorescein isothiocyanate; NC, control.
BMI-1 is a downstream target of miR-218 in APL
In order to investigate the molecular mechanisms underlying the effects of miR-218 on cell viability and apoptosis, the mRNA expression of BMI-1, a possible downstream target of miR-218 predicted by bioinformatics analysis (8), was measured in APL marrow tissues. A Pearson's correlation analysis was used to examine the correlation between miR-218 and BMI-1 mRNA expression levels. A negative correlation was revealed between miR-218 and BMI-1 expression (r=−0.546; P<0.001; Fig. 6A). In addition, mRNA and protein levels of BMI-1 were downregulated following miR-218 overexpression compared with the control group (P=0.007, Fig. 6B and C).
Figure 6.
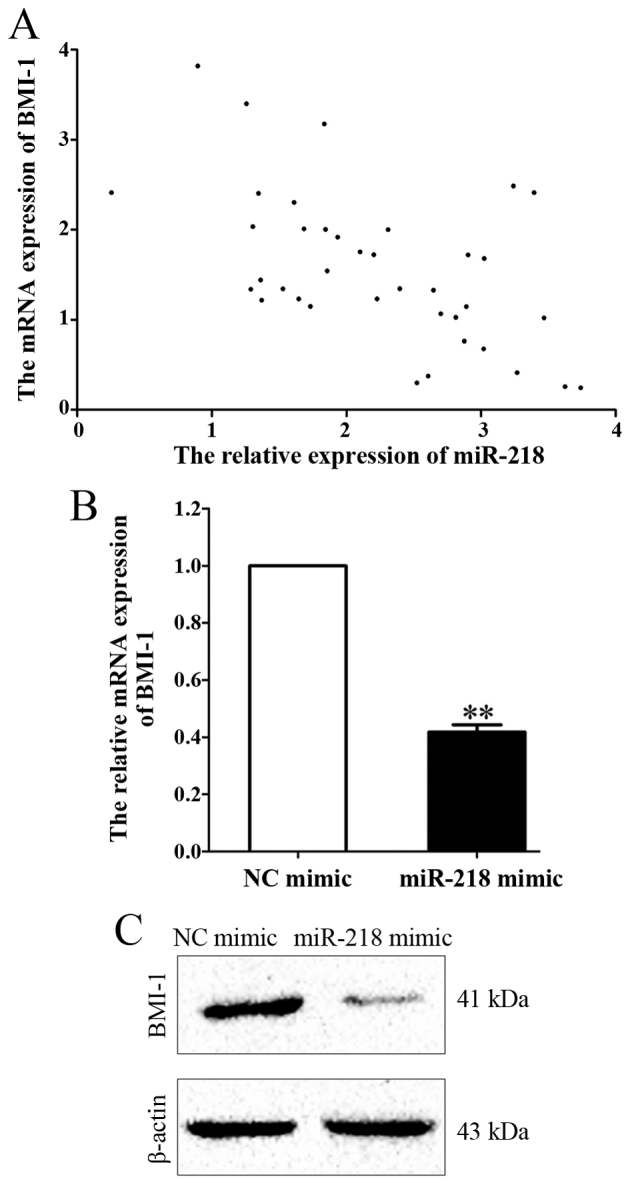
BMI-1 and miR-218 expression are negatively associated. (A) Pearson's correlation analysis of BMI-1 mRNA and miR-218 expression (r=−0.546; P<0.001). (B) Reverse transcription-quantitative polymerase chain reaction analysis and (C) western blot analysis of BMI-1 expression in HL-60 cells following transfection with control or miR-218 mimic. **P<0.01. BMI-1, B-cell-specific Moloney murine leukemia virus insertion site-1; miR, microRNA; NC, control.
Discussion
APL is a subtype of AML, and, following the identification of the promyelocytic leukemia (PML)-retinoic acid receptor (RAR)α fusion gene and the clinical applications of ATRA and ATO, APL became the first type of human cancer that was able to be treated with its tumor-specific antigen (15). However, the use of ATRA was reported to cause hyperleucocytosis and certain lethal complications, including retinoic acid syndrome (16). Therefore, further studies are required to identify novel and safe therapeutic molecules for the treatment of APL.
Previous studies have demonstrated that microRNAs serve tumor suppressor and tumor promoter roles in different types of cancer cell, and their abnormal expression is associated with tumor growth, metastasis and recurrence (17). Sharifi et al (18) reported that miR-92a suppressed proliferation and induced apoptosis of HL-60 cells by downregulating tumor protein 63 expression. In addition, miR-21 has been demonstrated as an oncogene in APL; Li et al (19) demonstrated that downregulation of miR-21 using antisense oligonucleotides enhanced HL-60 cell apoptosis induced by cytarabine (19). In the present study, the expression of miR-218 in APL marrow tissues was significantly decreased compared with normal marrow tissues and was identified to be associated with certain adverse prognostic factors, including high WBC count and marrow promyelocyte index, and low HGB and PLT counts. Therefore differential expression of miR-218 in APL marrow tissues may serve an important role in APL progression.
In a number of other types of cancer, miR-218 has been suggested as a prognostic evaluation biomarker; in glioma, low expression of miR-218 was associated with a higher World Health Organization grade and lower Karnofsky scores (20). Furthermore, overexpression of miR-218 in human osteosarcoma Saos-2 cells repressed cell migration and invasion by targeting T-lymphoma invasion and metastasis-inducing protein 1, matrix metalloproteinase (MMP) 2 and MMP9 (21). In the present study, miR-218 overexpression significantly increased apoptosis and caspase 3/7 activity. Furthermore, CCK-8 and BrdU incubation assays demonstrated that miR-218 overexpression inhibits cell viability and proliferation compared with scrambled microRNA transfection. Cell cycle analysis demonstrated that the majority of cells were arrested in the G0/G1 phase; a result that was in agreement with the result from the BrdU assay that miR-218 overexpression impairs DNA synthesis.
BMI-1 was the first functionally identified Polycomb group gene family member, and serves roles in cell cycle regulation, cell immortalization and cell senescence (22). BMI-1 is involved in the development and progression of carcinomas and is a potent target for cancer therapy (23). In addition, BMI-1 represses the cyclin-dependent kinase inhibitor 2A locus, which encodes p16INK4A and p19ARF (24). BMI-1 may promote cell cycle progression and enhance cell proliferation by downregulating p16INK4A and p19ARF expression. The results of the present study demonstrated a negative association between miR-218 and BMI-1 mRNA expression. Overexpression of miR-218 in HL-60 cells significantly repressed BMI-1 mRNA and protein expression. Therefore the growth arrest effects of miR-218 may be achieved by suppressing BMI-1 expression.
The results of the present study indicate that expression of miR-218 is downregulated in patients with APL. To the best of our knowledge, the present study is the first to demonstrate that miR-218 is associated with clinical features of APL and that miR-218 functions as a tumor suppressor by targeting BMI-1. This suggests that miR-218 is a potential marker for risk stratification in the treatment of APL.
References
- 1.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 2.Imani-Saber Z, Ghafouri-Fard S. Promyelocytic leukemia gene functions and roles in tumorigenesis. Asian Pac J Cancer Prev. 2014;15:8021–8028. doi: 10.7314/APJCP.2014.15.19.8019. [DOI] [PubMed] [Google Scholar]
- 3.Ganzel C, Douer D. Extramedullary disease in APL: A real phenomenon to contend with or not? Best Pract Res Clin Haematol. 2014;27:63–68. doi: 10.1016/j.beha.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Sandhu S, Mulligan SP. Ofatumumab and its role as immunotherapy in chronic lymphocytic leukemia. Haematologica. 2015;100:411–414. doi: 10.3324/haematol.2015.124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SJ. A potential target of Tanshinone IIA for acute promyelocytic leukemia revealed by inverse docking and drug repurposing. Asian Pac J Cancer Prev. 2014;15:4301–4305. doi: 10.7314/APJCP.2014.15.10.4301. [DOI] [PubMed] [Google Scholar]
- 6.Liang H, Li X, Wang L, Yu S, Xu Z, Gu Y, Pan Z, Li T, Hu M, Cui H, et al. MicroRNAs contribute to promyelocyte apoptosis in As2O3-treated APL cells. Cell Physiol Biochem. 2013;32:1818–1829. doi: 10.1159/000356615. [DOI] [PubMed] [Google Scholar]
- 7.Sharifi M, Salehi R, Gheisari Y, Kazemi M. Inhibition of microRNA miR-92a induces apoptosis and inhibits cell proliferation in human acute promyelocytic leukemia through modulation of p63 expression. Mol Biol Rep. 2014;41:2799–2808. doi: 10.1007/s11033-014-3134-5. [DOI] [PubMed] [Google Scholar]
- 8.Tu K, Li C, Zheng X, Yang W, Yao Y, Liu Q. Prognostic significance of miR-218 in human hepatocellular carcinoma and its role in cell growth. Oncol Rep. 2014;32:1571–1577. doi: 10.3892/or.2014.3386. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Ren X, Zhang X. Role of microRNA-150 in solid tumors. Oncol Lett. 2015;10:11–16. doi: 10.3892/ol.2015.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Lu Q, Zhu J, Fu J, Chen YX. Prognostic value of miR-96 in patients with acute myeloid leukemia. Diagn Pathol. 2014;9:76. doi: 10.1186/1746-1596-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Luo XQ, Feng DD, Zhang XJ, Wu J, Zheng YS, Chen X, Xu L, Chen YQ. Upregulation of microRNA-125b contributes to leukemogenesis and increases drug resistance in pediatric acute promyelocytic leukemia. Mol Cancer. 2011;10:108. doi: 10.1186/1476-4598-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, Zou J, Su S, Huang H, Deng Y, Wang B, Li W. MicroRNA-218 and microRNA-520a inhibit cell proliferation by downregulating E2F2 in hepatocellular carcinoma. Mol Med Rep. 2015;12:1016–1022. doi: 10.3892/mmr.2015.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YL, Jiang SX, Yang YM, Xu H, Liu JL, Wang XS. USP22 acts as an oncogene by the activation of BMI-1-mediated INK4a/ARF pathway and Akt pathway. Cell Biochem Biophys. 2012;62:229–235. doi: 10.1007/s12013-011-9287-0. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Miftakhova R, Sandberg T, Hedblom A, Nevzorova T, Persson JL, Bredberg A. DNA methylation in ATRA-treated leukemia cell lines lacking a PML-RAR chromosome translocation. Anticancer Res. 2012;32:4715–4722. [PubMed] [Google Scholar]
- 16.Liu WJ, Jiang NJ, Guo QL, Xu Q. ATRA and As2O3 regulate differentiation of human hematopoietic stem cells into granulocyte progenitor via alteration of HoxB8 expression. Eur Rev Med Pharmacol Sci. 2015;19:1055–1062. [PubMed] [Google Scholar]
- 17.Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013;10:396–404. doi: 10.1038/nrurol.2013.113. [DOI] [PubMed] [Google Scholar]
- 18.Sharifi M, Salehi R, Gheisari Y, Kazemi M. Inhibition of microRNA miR-92a induces apoptosis and inhibits cell proliferation in human acute promyelocytic leukemia through modulation of p63 expression. Mol Biol Rep. 2014;41:2799–2808. doi: 10.1007/s11033-014-3134-5. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhu X, Gu J, Hu H, Dong D, Yao J, Lin C, Fei J. Anti-miR-21 oligonucleotide enhances chemosensitivity of leukemic HL60 cells to arabinosylcytosine by inducing apoptosis. Hematology. 2010;15:215–221. doi: 10.1179/102453310X12647083620840. [DOI] [PubMed] [Google Scholar]
- 20.Cheng MW, Wang LL, Hu GY. Expression of microRNA-218 and its clinicopathological and prognostic significance in human glioma cases. Asian Pac J Cancer Prev. 2015;16:1839–1843. doi: 10.7314/APJCP.2015.16.5.1839. [DOI] [PubMed] [Google Scholar]
- 21.Jin J, Cai L, Liu ZM, Zhou XS. miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev. 2013;14:3681–3684. doi: 10.7314/APJCP.2013.14.6.3681. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Tian T, Sun W, Liu C, Fang X. Bmi-1 overexpression as an efficient prognostic marker in patients with nonsmall cell lung cancer. Medicine (Baltimore) 2017;96:e7346. doi: 10.1097/MD.0000000000007346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin X, Ojo D, Wei F, Wong N, Gu Y, Tang D. A novel aspect of tumorigenesis-BMI1 functions in regulating DNA damage response. Biomolecules. 2015;5:3396–3415. doi: 10.3390/biom5043396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng S, Luo M, Sun H, Yu X, Shen M, Zhang Q, Zhou R, Ju X, Tao W, Liu D, et al. Identification and characterization of Bmi-1-responding element within the human p16 promoter. J Biol Chem. 2010;285:33219–33229. doi: 10.1074/jbc.M110.133686. [DOI] [PMC free article] [PubMed] [Google Scholar]