Abstract
Sarcoidosis, a chronic, inflammatory disease that affects various different organs, is characterized by noncaseating epitheloid granulomas. This systemic inflammatory process is associated with an increased risk of cancer. Several cases of sarcoidosis that mimic metastatic tumor progression in radiological findings have been reported so far. However, there are also cases that have presented a coexistence of sarcoidosis and metastasis, which have caused a diagnostic and therapeutic dilemma. Due to inadequate current therapies, a reliable differentiation between benign and malignant lesions is crucial. This review focuses on the residual risk of the coexistence of metastases within radiological suspicious lesions in patients with a history of solid tumors and sarcoidosis, as well as immunological findings, in order to explain the potential associations. Sarcoidosis has the potential to promote metastasis as it includes tumor-promoting and immune-regulating cell subsets. Notably, myeloid derived suppressor cells may serve a pivotal role in metastatic progression in patients with sarcoidosis. In addition, the present review also evaluates the potential novel diagnostic approaches, which may be able to differentiate between metastatic lesions and sarcoidosis. The risk of coexistent metastasis in sarcoidosis lesions must be considered by clinical practitioners, and a multidisciplinary approach may be required to avoid misdiagnosis and the subsequent unnecessary surgery or insufficient treatments.
Keywords: review, diagnosis, sarcoidosis, cancer, neoplasm metastasis, tumor microenvironment
1. Introduction
Sarcoidosis is a chronic, inflammatory, systemic disease affecting primarily the lungs, the mediastinum and the lymphatic system but also salivary glands, heart, nervous system, joints and various other organs (1). Diagnosis depends on the existence of typical clinicoradiological findings in association with noncaseating epitheloid cell granulomas in biopsy and the absence of known, alternative or local causes provoking granulomas (1). Granulomas are nonspecific inflammatory lesions and can occur during mycobacterial, fungal or parasitic infections as well as other diseases like Wegener's granulomatosis (2). Due to the differentiation of granulomas the pathologists play a pivotal role in finding the correct diagnosis (2). In sarcoidosis, granuloma formation is characterized by infiltrating Th1 helper cells and macrophages. The latter show a transformation process into epitheloid cells and can fuse into multinucleate giant cells. Although small amounts of necrosis can be observed, the sarcoid granuloma is referred to the group of nonnecrotizing or noncaseating granulomas (2). Caseating granulomas are typically found in infectious diseases like syphilis or tularemia or infection with tuberculous and nontuberculous mycobacterium (2). In some, especially oncologic patients treated with immunotherapy, noncaseating granulomas can be found although they do not fulfill the criteria for systemic sarcoidosis and are thus referred to as sarcoid-like reactions (3). Due to the toxicity profile of immunotherapies immune-related adverse events can provoke those sarcoid-like reactions which may occur in the organ of tumor origin or in the tumor-draining lymph nodes (3). Sarcoidosis is associated with an increased risk for cancer development in several organs like lung, liver, stomach or for melanoma and lymphoma. Sarcoid-like reactions can be found in 13.8% of patients with Hodgkin-disease, 7.3% with non Hodgkin lymphoma and 4.4% of cases with carcinomas (4,5). Furthermore, simultaneous occurrence of sarcoidosis and cancer is associated with a diminished survival rate (6). Although no increased risk for malignancy in the head and neck has been described so far, there are a few cases that report the simultaneous occurrence of sarcoidosis and malignoma of the head and neck (7–9). Notably, in follow-up computed tomography (CT) scans or those done for the detection of primary tumors or metastatic lesions (e.g., 18-Fluorodeoxyglucose positron emission tomography-(PET)-CT, 18FDG-PET-CT) sarcoidosis can mimic cancer recurrence or metastatic progress (10,11). However, it is possible that metastatic lesions coexist next to lymph nodes with sarcoid-like lesions and it is unclear whether sarcoidosis has an influence on metastasis of malignoma. Therefore, a review of the current literature was performed to analyze the residual risk of metastasis within radiological suspicious lesions in patients with a history of solid tumors and sarcoidosis.
2. Methods
In this review we analyzed reported cases of patients with solid tumors whose staging or follow-up analysis revealed an unclear lymphadenopathy owing to metastasis or sarcoidosis. A systematic literature search was done in Pub Med data base (from inception to April 2017) without any limitation using the terms: Sarcoidosis [title] AND metastasis. All cases with a solid tumor and sarcoidosis were included and the provided information concerning age, gender, tumor region, tumor entity, tumor classification, therapy, as well as information about diagnosis of sarcoidosis were collected. Analyzing the data, the risk of simultaneous occurrence of metastasis and sarcoidosis in concordance to positive radiological and histological findings was elucidated.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors. For this retrospective study and review of anonymized clinical records an ethical permission was not required.
3. Results
Review of the literature
Review of the literature revealed 115 cases in Pub Med Data Base based on the search criteria mentioned above. Cases without malignancy were excluded and 59 cases with cancer and sarcoidosis were identified and are listed in Table I. The median age at cancer diagnosis was 49.5 +/- 13.4 years and cases of 31 female and 23 male patients were reported. In 5 cases, the sex of the patient was not documented. Systemic sarcoidosis treatment consisted of oral steroids (n=12) or chloroquine (n=1). Local steroids were applied in one case with uveitis. No therapy was initiated in 15 cases and 30 cases did not provide any information about the sarcoidosis therapy approach. However, independent from the treatment strategy the patients' outcome was described to be good with a stable status or complete remission. The most frequent cancer origin was breast (n=12) followed by malignoma of the thyroid gland (n=8). Sarcoidosis occurred in 20 cases after an average of 34.4 months and in 7 cases 10.25 years before diagnosis of malignoma. In most reported cases (n=24) sarcoidosis was revealed simultaneously with diagnosis of malignoma. In 25 cases, surgery was performed to remove the tumor. 24 patients had a combination of surgery and radio-, chemo- or radiochemotherapy. Two cases were only treated by radiochemotherapy and in seven cases, no information about therapeutic aspects concerning the tumor was provided. Occurrence of sarcoidosis after surgery was reported in 3/25 cases and in 17/24 cases after surgery and radio-/chemotherapy. In 17% of the cases, simultaneous detection of sarcoidosis or sarcoid-like lesions and metastasis was reported (n=10). These reports are now described in more detail.
Table I.
Characteristics of patients from literature review.
| Tumor characteristics | Patient | Sarcoidosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Tumor region | Tumor entity | TNM classification | Therapy | Age | Sex | Diagnoses tool | Biopsy | Sarcoidosis manifestations | Time between Diagnostic (months) | (Refs.) |
| Altinkaya et al, 2015 | Breast | Ductal invasive carcinoma | T1N0M0 | OP | 70 | F | PET | EBUS | LN mediastinal | 0 | (31) |
| Conte et al, 2015 | Breast | Ductal invasive carcinoma | pT3pN1 | OP | 50 | F | PET | OP+Biopsy | LN pelvic, supradiaphragmatic | 0 | (12) |
| Zivin et al, 2014 | Breast | Ductal invasive carcinoma | nk | OP | 32 | F | PET | Biopsy+EBUS | LN hilar + mediastinal | nk | (32) |
| El Hammoumi et al, 2015 | Breast | Lobular carcinoma | T1N2Mx | OP + aRCTx (Tamoxifen) | 48 | F | CT | Biopsy | LN mediastinal | 36 | (11) |
| Kim et al, 2014 | Breast | Ductal invasive carcinoma | T1N2M0 | OP + aRCTx | 44 | F | PET | Mediastinoscopy | LN paraesophageal, hilar | 24 | (33) |
| Akhtari et al, 2014 | Breast | Ductal invasive carcinoma | T2N0Mx | OP + aCTx + aRT | 47 | F | PET | FNA | LN supraclav., mediastinal, periportal | 0 | (34) |
| Braza and Nelson, 2014 | Breast | Ductal invasive carcinoma | T1N0M0 | nk | 68 | F | MRI | Bone biopsy | Lesions lumbosacral spine | nk | (35) |
| DeFilippis et al,2013 | Breast | Lobular carcinoma | nk | OP + aRTx | 63 | F | MRI | Biopsy | LN axillary | 0 | (36) |
| Bush et al, 2011 | Breast | Ductal invasive carcinoma | N0 | OP | 42 | F | PET | Biopsy | LN cervical, abdominal, bone, spleen | 0 | (37) |
| Viswanath et al, 2009 | Breast | Ductal invasive carcinoma | T4aN1M0 | OP + aRCTx | 50 | F | CI | Biopsy | Dermal lesions | 24 | (38) |
| Whittington et al, 1986 | Breast | Carcinoma | N0 | nk | nk | F | nk | Mediastinoscopy | LN mediastinal | 0 | (39) |
| Whittington et al, 1986 | Breast | Carcinoma | N0 | nk | nk | F | nk | EBUS | LN hilar | 0 | (39) |
| El Hammoumi et al, 2015 | Cervix | Epidermoid Carcinoma | nk | naCTx + OP | 47 | F | PET | Mediastinoscopy | LN mediastinal | 36 | (11) |
| Tamauchi et al, 2015 | Endometrium | Adenocarcinoma | T1bN0Mx | OP + aCTx | 67 | F | CT | OP | LN hilar, paraaortic, pelvine | 0 | (40) |
| Powell et al, 2005 | Endometrium | Adenocarcinoma | N0 | OP | 67 | F | CT | FNA | LN mediastinal, liver lesions | 48 | (41) |
| Takanami et al, 2008 | Esophageal | Squamous cell carcinoma | N0 | OP | 72 | M | PET | Biopsy | LN mediastinal + hilar | −168 | (42) |
| Takanami et al, 2008 | Esophageal | Squamous cell carcinoma | pT1b | OP | 59 | M | PET | Biopsy | LN mediastinal + hilar | 0 | (42) |
| Arana et al, 2013 | Ethmoid sinus | Adenocarcinoma | T3N0M0 | OP + aRCTx | 42 | F | PET | Mediastinoscopy | LN mediastinal + hilar | 0 | (7) |
| Kachalia et al, 2014 | Lung | Adenocarcinoma | TxNxM2 | OP | 80 | F | X-ray | Mediastinoscopy | LN | 0 | (13) |
| Kim et al, 2011 | Lung | Adenocarcinoma | T2N0M0 | OP | 65 | F | PET | Biopsy | LN mediastinal | 0 | (43) |
| Mimura et al, 2011 | Lung | Squamous cell carcinoma | pT1N0M0 | OP | 69 | M | CT | Biopsy | LN mediastinal | −120 | (44) |
| Urushiyama et al, 2015 | Lung | Squamous cell carcinoma | N0 | OP | 60 | M | CT | Biopsy | LN mediastinal + hilar | −24 | (45) |
| Bouaziz et al, 2006 | Lung | Squamous cell carcinoma | nk | nk | 49 | M | MRI | EBUS | LN mediastinal, hepatic nodules | 0 | (46) |
| Shields et al, 2005 | Lung | Papillary carcinoma | M1 | OP | 57 | F | PET | Radiology | Salivary and lacrimal glands, LN hilar | 0 | (14) |
| Sato et al, 2003 | Lung | Adenocarcinoma | N1 | nk | nk | nk | nk | Thoracoscopy | LN mediastinal, interlobar | 0 | (15) |
| Muramatsu et al, 2000 | Lung | Squamous cell carcinoma | N0 M0 | OP | 64 | M | CT | Biopsy | LN mediastinal | 0 | (47) |
| Abdel-Galiil et al, 2008 | Maxilla | Squamous cell carcinoma | nk | OP | 51 | M | PET | Mediastinoscopy | LN mediastinal+hilar + peribronchial | 24 | (8) |
| Yao et al, 2005 | Oropharynx | Squamous cell carcinoma | T3N2cM0 | RCTx | 49 | M | PET | Mediastinoscopy | LN mediastinal, pretracheal, subcarinal | 2 | (9) |
| Yonenaga et al, 2006 | Ovar | Mucinous Cystadenocarcinoma | nk | OP + aCTx | 21 | F | PET | nk | Spleen, Liver | 36 | (48) |
| Kim et al, 2013 | Ovar | Papillary cystadenocarcinoma | nk | OP + aCTx | 52 | F | PET | EBUS | LN paratracheal, supraclavicular, diaphragmal | 12 | (49) |
| Pollock and Catalano, 1979 | Parotid gland | Ductal carcinoma | N2 | OP + aRCTx | 38 | M | CI | Biopsy | LN hilar | −60 | (50) |
| Montini and Tulchinsky, 2012 | Rectum | Cancer | nk | nk | 45 | M | PET | Biopsy skeletal | LN mediastinal, | 0 | (51) |
| Abdi et al, 1987 | Renal | Renal cell carcinoma | N2 | OP + aRTx (IFNα) | 57 | F | CT | EBUS | LN mediastinal | 24 | (52) |
| Fukutani et al, 1987 | Renal | Renal cell carcinoma | NO | OP | 75 | F | nk | Biopsy | LN pelvic | 0 | (53) |
| Khan and Khan, 1974 | Renal | Hypernephroma | M1 | OP | 52 | M | X-ray | Biopsy | LN hilar | 0 | (19) |
| Gharavi et al, 2013 | Sacrum | Chordoma | nk | OP | 48 | M | PET | Biopsy | LN iliacal + femoral | 12 | (54) |
| Wilgenhof et al, 2012 | Skin | Melanoma | M1c | OP + aCTx (Dacarbazine, Cisplatin) | 48 | F | PET | EBUS | LN hilar | 84 | (55) |
| Vogel et al, 2012 | Skin | Melanoma | N1 | OP + aCTx (αCTLA-4) | 49 | M | PET | EBUS | LN mediastinal, hilar | 168 | (56) |
| Heinzerling et al, 2010 | Skin | Melanoma | pT4N0M0 | OP + aCTx (INFα) | 50 | M | nk | Biopsy | LN mediastinal | 7 | (57) |
| Chiagne et al, 2011 | Skin | Melanoma | nk | OP | 35 | M | PET | Biopsy | LN inguinal | 0 | (18) |
| Heinzerling et al, 2010 | Skin | Melanoma | pT3bpN1acM0 | OP + aCTx (INFα) | 47 | M | PET | Biopsy | LN mediastinal+ hilar + peribronchial | nk | (57) |
| Heinzerling et al, 2010 | Skin | Melanoma | N1 | OP + aCTx (INFα) | 47 | M | PET | Mediastinoscopy | LN mediastinal+ hilar + peribronchial | 2 | (57) |
| Suarez-Garcia et al, 2009 | Skin | Melanoma | N1 | OP + aCTx (INFα) | 42 | M | CI | Biopsy | Dermal lesions | 3 | (58) |
| Massaguer et al, 2004 | Skin | Melanoma | nk | CTx (IFNα) | nk | F | CT | Mediastinoscopy | LN mediastinal | nk | (59) |
| Matsubara et al, 2015 | Stomach | Adenocarcinoma | N0 | OP | 64 | F | nk | Endoscopy | Gastric sarcoidosis | −120 | (60) |
| El Hammoumi et al, 2015 | Stomach | Adenocarcinoma | nk | OP + aCTx | 59 | F | CT | Mediastinoscopy | LN paratracheal | 36 | (11) |
| Tissot et al, 1985 | Stomach | Adenocarcinoma | nk | nk | 63 | F | OP | Biopsy | Combined gastric lesions | −336 | (61) |
| Claus et al, 2012 | Testis | Seminoma | T2N0M1 | OP + aCTx (Carboplatin) | 34 | M | CT | EBUS | LN mediastinal | 24 | (62) |
| Claus et al, 2012 | Testis | Seminoma | T1N0M0 | OP + aCTx (Carboplatin) | 36 | M | CT | Biopsy | LN abdominal | 0 | (62) |
| Teo et al, 2013 | Testis | Seminoma | T1N2M1a | OP + aCTx (Cisplatin, Etoposid) | 20 | M | CT | EBUS | LN mediastinal | 60 | (63) |
| Tjan-Heijnen et al, 1998 | Testis | Seminoma | N2 | OP + aRTx | 41 | M | CT | Mediastinoscopy | LN mediastinal | 24 | (64) |
| Salih et al, 2015 | Thyroid | Papillary thyroid carcinoma | T2N1Mx | OP | 48 | F | X-ray | Neck dissection | LN cervical and hilar | 0 | (10) |
| Lebo et al, 2015 | Thyroid | Papillary thyroid carcinoma | T1bN1aMx | OP | 41 | F | PET | Mediastinoscopy | Cervical + mediastinal | 0 | (16) |
| Myint et al, 2015 | Thyroid | Papillary thyroid carcinoma | nk | OP + I-131 | 68 | F | PET | Bone biopsy | LN hilar + mediast. Bone | nk | (65) |
| Ergin and Nasr, 2014 | Thyroid | Papillary thyroid carcinoma | N0 | OP | nk | nk | nk | OP | Cervical | pre | (17) |
| Ergin and Nasr, 2014 | Thyroid | Papillary thyroid carcinoma | N0 | OP | nk | nk | nk | FNA | Cervical | post | (17) |
| Ergin and Nasr, 2014 | Thyroid | Papillary thyroid carcinoma | N1 | OP | nk | nk | PET | OP | Cervical | 0 | (17) |
| Ergin and Nasr, 2014 | Thyroid | Papillary thyroid carcinoma | N0 | OP | nk | nk | nk | OP | Cervical | 0 | (17) |
| Zimmermann-Belsing et al, 2000 | Thyroid | Papillary adenocarcinoma | N2 | OP | 27 | M | Scintigraphy | Biospy | LN hilar | −36 | (66) |
nk, not known; OP, operation; aCTx, adjuvant chemotherapy; aRTx, adjuvant radiotherapy; aRCTx, adjuvant combined radiochemotherapy; naCTx, neoadjuvant chemotherapy; INFα, Interferon alpha; PET, positron emission tomography; CT, computed tomography; F, female; M, male; MRI, magnetic resonance imaging; CI, clinical investigation; EBUS, endobronchial ultrasound based biopsy; FNA, fine needle aspiration; LN, lymph node.
The first case describes a 50-year-old female patient suffering from ductal invasive breast carcinoma with local lymph node metastasis (pT3pN1). Chest X-ray and 18FDG-PET-CT were performed for staging of the tumor and showed a bilateral mediastinal lymphadenopathy and an increased FDG-uptake in supra-diaphragmatic and pelvic lymph nodes. Biopsy of an example lesion obtained sarcoidosis (12). Secondly, an 80-year-old female's CT scan revealed a tumor suspicious mass in the upper lobe of the right lung, multiple smaller nodules and hilar lymphadenopathy. Subsequent biopsy of the mass and a mediastinal lymph node showed just noncaseating granulomas but no malignant cells and led to insufficient treatment. Six months later, symptoms like cough and chest pain were exacerbated and a thoracocentesis revealed adenocarcinoma cells. Further staging examination showed pleural, pericardial and diaphragmatic metastasis. Due to tumor progress, palliative care was initiated (13). The third case describes a 57-year-old female patient who was found to have a choroidal mass in the left eye. Total body gallium 67 scan showed an increased uptake in salivary and lacrimal glands and was misinterpreted as typical for sarcoidosis. Progress of symptoms resulted in enucleation and revealed a choroideal metastasis of a papillary lung carcinoma (14). Sato et al (15) report on a patient with concomitant sarcoidosis and lung adenocarcinoma. Thoracoscopic biopsy of altered lymph nodes did not detect metastasis but sarcoidosis. Surgery was performed and a permanent pathological slide showed that several nodes contained both sarcoidosis and lung cancer metastasis (15). Three other reports describe patients suffering from papillary thyroid carcinoma that underwent thyreoidectomy and modified neck dissection. Pathology revealed concurrent existence of sarcoidosis and regional lymph node metastasis (10,16,17). One patient (27-year-old, male) shows a papillary thyroid carcinoma upon a previously diagnosed sarcoidosis. Local lymph nodes contained sarcoidosis mixed with metastasis. A 35-year-old male with a previous history of melanoma developing metastatic involvement and sarcoidosis in regional lymph nodes was described by Chaigne et al (18). Khan and Khan (19) described a 52-year-old patient with cough and chest pain. Radiologic examination showed bilateral hilar lymphadenopathy. Furthermore, within a biopsy of an enlarged lymph node, a metastasis of a left kidney hypernephroma was detected (19).
Taken together, these ten patients had a median age of 49 years ranging from 27 to 80 years at the time point of simultaneous detection of metastasis and sarcoidosis. The gender ratio was 0.6:1 (male to female) although no information concerning the sex was provided in two cases. In 8 cases (80%), the metastases were localized in regional lymph nodes whereas just 2 cases showed distant metastases. Furthermore, the region and entity of the associated tumor differed greatly (breast: n=1, lung: n=3, thyroid: n=4, skin: n=1, kidney: n=1).
4. Discussion
Sarcoidosis and metastasis
Although there are several published cases concerning coexistence of malignoma and sarcoidosis, the causal relationship between these entities is still unclear. On the one hand, it is possible that patients with sarcoidosis develop malignancies and, on the other hand, there are oncologic patients developing sarcoidosis and sarcoid-like reactions, especially after chemotherapy (3). Several cases describe a mimicking of metastatic disease by sarcoidosis but just a few cases actually report a simultaneous occurrence of sarcoidosis and metastases.
Active sarcoidosis is characterized by an enhanced local expression of T helper 1 (Th1) and T helper 17 (Th17) chemokines and cytokines like IFN-γ, TNF-α, IL-17A and IL-22. In various chronic, autoimmune, inflammatory diseases, such as sarcoidosis, the percentage of IL-17A+/IFN-γ+ double-producing Th-cells is increased in peripheral blood and is related to high disease activity. Furthermore, in these pathological conditions, a dysfunctional response of regulatory T-cells (Tregs) has been described that is characterized by an insufficient immunosuppressive function (20). Interestingly, cytotoxic T-lymphocyte antigen 4 (CTLA-4) expression is decreased while PD-1 (programmed death-1) expression is increased in Th17-cells in the mediastinal lymph nodes during sarcoidosis (20). CTLA-4 is present on Th-cells and mediates an inhibitory effect on further T cells responses. Hence, a diminished CTLA-4 level maintains inflammatory reactions. Similarly, PD-1 is expressed on the surface of T-cells upon activation and is involved in limiting inflammatory reactions (21). Its ligand, PD-1L, can be found on tumor cells and provokes upregulation of PD-1 in T cells. Consequently, activation of tumor antigen-specific T-cells in pancreatic adenocarcinoma is inhibited (22). Hence, the bivalent adaptive immune response in patients with sarcoidosis and metastasis promotes both pathological conditions by maintaining sarcoidosis-related inflammation due to the decreased anti-inflammatory CTLA-4 expression while limiting tumor-specific T cell activation, marked by an increased PD-1 expression, that enables tumor escape from the immune system and metastasis. Increased PD-1 expression on T-cells in sarcoidosis lymph nodes could thus be a possible predictor of metastasis on the basis of sarcoidosis. Furthermore, myeloid-derived suppressor cells (MDSC) might play a pivotal role in the pathogenic association between sarcoidosis and metastasis. MDSC pursue immunoregulatory and T-cell suppressive functions (23). Although it has not been described yet, an influence of MDSC on sarcoidosis is assumable because of their important role in other inflammatory diseases (24). Th-17 cells are the main source of IFN-γ production in sarcoidosis and IFN-γ induces MDSC differentiation and promotes their immunosuppressive function (20,25). In cancer models, MDSC accumulation was promoted by several cytokines and growth factors, such as IL-6, IL-1ß and S100A8/A9, resulting in an anti-inflammatory tumor microenvironment (23). MDSC themselves express cytokines and chemokines like IL-6, TNF, IL-1β, IL-23 and S100A9 and have the potential to attract both further myeloid cells and tumor cells (26). Furthermore, in a melanoma and lung carcinoma mouse model, S100A9 expressing MDSC were identified as important players in enabling tumor metastasis (27). Consequently, they are generally recognized as dominant tumor-promoting forces (28). Patients suffering from sarcoidosis have increased serum levels of S100A8/A9 and an enhanced cytoplasmatic S100A8/A9 expression in monocytes and multinuclear giant cells in granulomas (29). Because of the sarcoidosis-related inflammation, marked by the expression of IFN-γ, IL-6, IL-23 and S100A8/A9, we can suspect a similar microenvironment to tumors that are characterized by an increased accumulation and immunosuppressive function of MDSC. Accordingly, we can assume that sarcoidosis has the potential to promote metastasis by inducing tumor-promoting and immune-regulating cell subsets. Further analysis is necessary to verify the influence of MDSC on sarcoidosis as well as the cellular immune response concerning the association between sarcoidosis and metastasis.
Differentiation between benign and malignant lesions
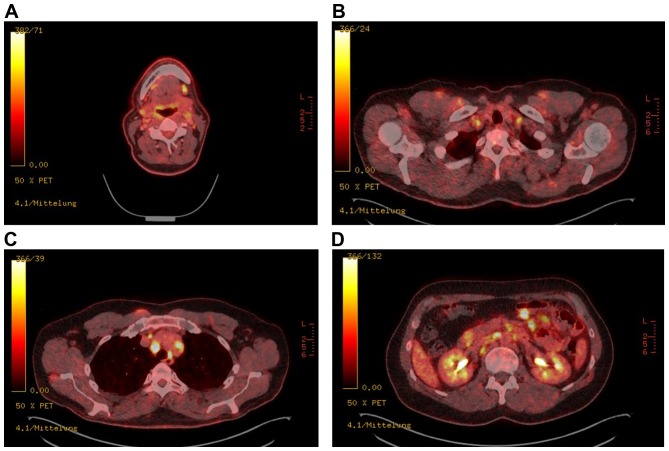
PET-CT scan is a very useful diagnostic tool to identify malignant lesions with a sensitivity between 47 and 100% and a specificity of 86–100% (30). Unfortunately, elevated FDG uptake can also be detected in inflammatory diseases such as sarcoidosis causing a diagnostic dilemma. The case of a 61-year-old carpenter with a history of adenocarcinoma of the paranasal sinus and simultaneous occurrence of multiple cervical metastases and sarcoidosis detected during follow-up investigation impressively demonstrates the risk to overlook metastatic lesions within sarcoidosis (Fig. 1). Especially PET scans for staging or restaging of oncologic diseases supply important information about tumor progression. Decisions on curative or palliative therapy are based on this information, emphasizing the importance to avoid misdiagnosis. Inclusion of an additional tracer would be helpful to differentiate between inflammation and tumor. F18-labeled 3′deoxy-3′fluorothymidine (FLT) is such a promising tracer to minimize diagnostic and subsequent therapeutic mistakes. By measuring DNA synthesis instead of metabolic activity that seems to be more specific to detect tumor diseases, FLT might be a useful candidate to discriminate between tumor and sarcoidosis lesions (7).
Figure 1.
PET-CT showing multiple high uptake lesions: 18-Fluorodeoxyglucose positron emission tomography and computed tomography (18FDG-PET-CT) of a patient with a history of paranasal sinus adenocarcinoma shows increased uptake of (A) submandibular, (B) cervical, (C) mediastinal and (D) mesenterial lymph nodes suspicious of multiple metastatic lesions. Bioptical examination revealed cervical metastases of the known adenocarcinoma and mediastinal sarcoid-like lesions without malignancy. Courtesy of Professor W. Heindel, Institute of Clinical Radiology, University Hospital Münster. 18FDG-PET-CT, 18-fluorodeoxyglucose-positron emission tomography-computed tomography.
An additional helpful tool to discriminate inflammatory bone marrow involvement, like skeletal sarcoidosis, from metastatic disease might be the diffusion whole-body magnetic resonance imaging (b-values 50–900s/mm2). In contrast to malignant lesions (cut-off value of 774 µm2/s), sarcoidosis or other inflammatory skeletal reactions show high signal intensity on diffusion-weighted images and a lower apparent diffuse coefficient (ADC) (12).
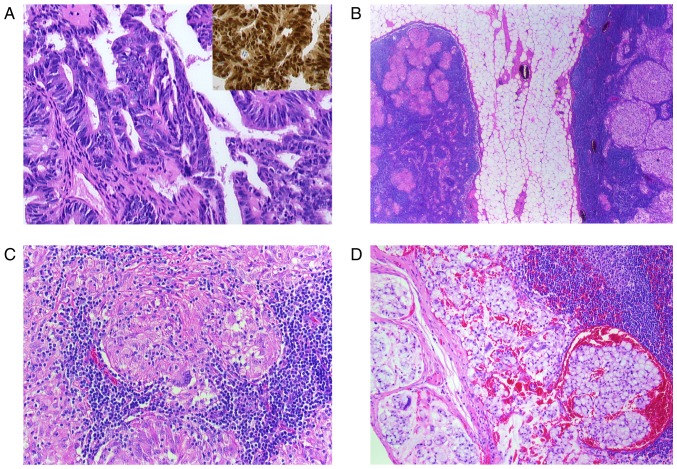
Bioptical evaluation of radiologically altered lymph nodes is necessary for selection of appropriate oncological treatment strategy. However, examination of each PET-CT positive lesion is not feasible and the chance to detect metastatic lesions next to sarcoidosis is rather rare (Fig. 2). Thus, even if pathological findings were suspicious of sarcoidosis, concomitant metastasis cannot be certainly excluded. Hence, correlation between the radiological and histological findings with the probability of distant or local metastasis corresponding to the tumor entity is important for the careful assessment of the residual metastasis risk.
Figure 2.
(A-D) Histological findings of a resected paranasal sinus adenocarcinoma and lymph node. (A) Microphotograph shows the adenocarcinoma with atypical tumor cells (H&E; magnification, ×400) and CDX2 nuclear positive cells, characteristic for intestinal-type adenocarcinoma (inset, H&E, magnification, ×400). (B) Two lymph nodes surrounded by perinodal fat tissue. The right node with noncaseating epitheloid granulomas and the left one with metastatic lesions (H&E, magnification, ×5). (C) Lymph node with noncaseating epitheloid granuloma in higher magnification (H&E, magnification, ×200). Multinuclear giant cells are identifiable. (D) Metastatic carcinoma infiltration in a lymph node (H&E, magnification, ×200). H&E, hematoxylin and eosin; CDX2, caudal type homeobox 2.
5. Conclusion
Correlation between PET-Scan, histological findings and knowledge about typical tumor behavior is necessary to avoid misdiagnosis. Nevertheless, a residual risk of overlooking metastases in systemic inflammatory diseases still remains existent. Therefore, it is important for clinical practice to be aware of the simultaneous occurrence of sarcoidosis and metastatic malignancy. Further cell subset analysis in these pathologies might additionally reveal immunological distinct cell populations as useful markers to distinguish between sarcoidosis, cancer and the coexistence of these two and help in overcoming the current diagnostic dilemma.
Acknowledgements
This study was supported by a fellowship from the medical faculty of the University of Münster, Germany to C. Spiekermann.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Rosen Y. Pathology of sarcoidosis. Semin Respir Crit Care Med. 2007;28:36–52. doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 3.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326–333. doi: 10.1016/j.clindermatol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13:147–156. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 5.Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160:1668–1672. doi: 10.1164/ajrccm.160.5.9904045. [DOI] [PubMed] [Google Scholar]
- 6.Boffetta P, Rabkin CS, Gridley G. A cohort study of cancer among sarcoidosis patients. Int J Cancer. 2009;124:2697–2700. doi: 10.1002/ijc.24261. [DOI] [PubMed] [Google Scholar]
- 7.Yi Arana C, McCue P, Rosen M, Machtay M, Axelrod R, Morris GJ. Sarcoidosis mimicking metastatic bone disease in head and neck cancer. Semin Oncol. 2013;40:529–534. doi: 10.1053/j.seminoncol.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Galiil K, Anand R, Sharma S, Brennan PA, Ramchandani PL, Ilankovan V. Incidence of sarcoidosis in head and neck cancer. Br J Oral Maxillofac Surg. 2008;46:59–60. doi: 10.1016/j.bjoms.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Yao M, Funk GF, Goldstein DP, DeYoung BR, Graham MM. Benign lesions in cancer patients: Case 1. Sarcoidosis after chemoradiation for head and neck cancer. J Clin Oncol. 2005;23:640–641. doi: 10.1200/JCO.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 10.Salih AM, Fatih SM, Kakamad FH. Sarcoidosis mimicking metastatic papillary thyroid cancer. Int J Surg Case Rep. 2015;16:71–72. doi: 10.1016/j.ijscr.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Hammoumi M, El Marjany M, Moussaoui D, Doudouh A, Mansouri H, Kabiri el H. Mediastinal sarcoidosis mimicking lymph malignancy recurrence after anti-neoplastic therapy. Arch Bronconeumol. 2015;51:e33–e35. doi: 10.1016/j.arbres.2014.07.014. (In English, Spanish) [DOI] [PubMed] [Google Scholar]
- 12.Conte G, Zugni F, Colleoni M, Renne G, Bellomi M, Petralia G. Sarcoidosis with bone involvement mimicking metastatic disease at (18)F-FDG PET/CT: Problem solving by diffusion whole-body MRI. Ecancermedicalscience. 2015;9:537. doi: 10.3332/ecancer.2015.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kachalia AG, Ochieng P, Kachalia K, Rahman H. Rare coexistence of sarcoidosis and lung adenocarcinoma. Respir Med Case Rep. 2014;12:4–6. doi: 10.1016/j.rmcr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields JA, Shields CL, Eagle RC., Jr Choroidal metastasis from lung cancer masquerading as sarcoidosis. Retina. 2005;25:367–370. doi: 10.1097/00006982-200504000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Sasano S, Oyama K, Sakuraba M, Onuki T, Nitta S. Lung cancer associated with sarcoidosis. Jpn J Thorac Cardiovasc Surg. 2003;51:21–24. doi: 10.1007/s11748-003-0061-0. [DOI] [PubMed] [Google Scholar]
- 16.Lebo NL, Raymond F, Odell MJ. Selectively false-positive radionuclide scan in a patient with sarcoidosis and papillary thyroid cancer: A case report and review of the literature. J Otolaryngol Head Neck Surg. 2015;44:18. doi: 10.1186/s40463-015-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ergin AB, Nasr CE. Thyroid cancer & sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:239–243. [PubMed] [Google Scholar]
- 18.Chaigne B, Perrinaud A, Penaud A, Machet MC, Venel Y, Marchand-Adam S, Machet L. Melanoma lymph node metastasis occurring simultaneously with multifocal sarcoidosis affecting lymph nodes and the lung: A diagnostic pitfall. Eur J Dermatol. 2011;21:798–799. doi: 10.1684/ejd.2011.1457. [DOI] [PubMed] [Google Scholar]
- 19.Khan A, Khan FA. Hypernephroma: A rare cause of bilateral adenopathy, and an example of the importance of tissue diagnosis in suspected cases of sarcoidosis. Chest. 1974;66:722–723. doi: 10.1378/chest.66.6.722. [DOI] [PubMed] [Google Scholar]
- 20.Broos CE, Hendriks RW, Kool M. T-cell immunology in sarcoidosis: Disruption of a delicate balance between helper and regulatory T-cells. Curr Opin Pulm Med. 2016;22:476–483. doi: 10.1097/MCP.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 21.Hashemi-Sadraei N, Sikora AG, Brizel DM. Immunotherapy and checkpoint inhibitors in recurrent and metastatic head and neck cancer. Am Soc Clin Oncol Educ Book. 2016;35:e277–e282. doi: 10.14694/EDBK_157801. [DOI] [PubMed] [Google Scholar]
- 22.Seo YD, Pillarisetty VG. T-cell programming in pancreatic adenocarcinoma: A review. Cancer Gene Ther. 2017;24:106–113. doi: 10.1038/cgt.2016.66. [DOI] [PubMed] [Google Scholar]
- 23.Crook KR, Liu P. Role of myeloid-derived suppressor cells in autoimmune disease. World J Immunol. 2014;4:26–33. doi: 10.5411/wji.v4.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI. Regulatory myeloid suppressor cells in health and disease. Cancer Res. 2009;69:7503–7506. doi: 10.1158/0008-5472.CAN-09-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Wu T, Shao S, Shi B, Zhao Y. Phenotype, development, and biological function of myeloid-derived suppressor cells. Oncoimmunology. 2015;5:e1004983. doi: 10.1080/2162402X.2015.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Kosaka A, Ikeura M, Kohanbash G, Fellows-Mayle W, Snyder LA, Okada H. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro Oncol. 2013;15:891–903. doi: 10.1093/neuonc/not031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Q, Li X, Chen H, Cao Y, Xiao Q, He Y, Wei J, Zhou J. IRF7 regulates the development of granulocytic myeloid-derived suppressor cells through S100A9 transrepression in cancer. Oncogene. 2017;36:2969–2980. doi: 10.1038/onc.2016.448. [DOI] [PubMed] [Google Scholar]
- 28.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terasaki F, Fujita M, Shimomura H, Tsukada B, Otsuka K, Otsuka K, Katashima T, Ikemoto M, Kitaura Y. Enhanced expression of myeloid-related protein complex (MRP8/14) in macrophages and multinucleated giant cells in granulomas of patients with active cardiac sarcoidosis. Circ J. 2007;71:1545–1550. doi: 10.1253/circj.71.1545. [DOI] [PubMed] [Google Scholar]
- 30.Zanation AM, Sutton DK, Couch ME, Weissler MC, Shockley WW, Shores CG. Use, accuracy, and implications for patient management of [18F]-2-fluorodeoxyglucose-positron emission/computerized tomography for head and neck tumors. Laryngoscope. 2005;115:1186–1190. doi: 10.1097/01.MLG.0000163763.89647.9F. [DOI] [PubMed] [Google Scholar]
- 31.Altinkaya M, Altinkaya N, Hazar B. Sarcoidosis mimicking metastatic breast cancer in a patient with early-stage breast cancer. Ulus Cerrahi Derg. 2015;32:71–74. doi: 10.5152/UCD.2015.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zivin S, David O, Lu Y. Sarcoidosis mimicking metastatic breast cancer on FDG PET/CT. Intern Med. 2014;53:2555–2556. doi: 10.2169/internalmedicine.53.3333. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Lee SY, Oh SC, Choi CW, Kim JS, Seo JH. Case report of pulmonary sarcoidosis suspected to be pulmonary metastasis in a patient with breast cancer. Cancer Res Treat. 2014;46:317–321. doi: 10.4143/crt.2014.46.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhtari M, Quesada JR, Schwartz MR, Chiang SB, Teh BS. Sarcoidosis presenting as metastatic lymphadenopathy in breast cancer. Clin Breast Cancer. 2014;14:e107–e110. doi: 10.1016/j.clbc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Braza DW, Nelson PA. Vertebral sarcoidosis masquerading as breast metastasis. Am J Phys Med Rehabil. 2014;93:274. doi: 10.1097/PHM.0b013e318269ec2a. [DOI] [PubMed] [Google Scholar]
- 36.DeFilippis EM, Arleo EK. New diagnosis of sarcoidosis during treatment for breast cancer, with radiologic-pathologic correlation. Clin Imaging. 2013;37:762–766. doi: 10.1016/j.clinimag.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Bush E, Lamonica D, O'Connor T. Sarcoidosis mimicking metastatic breast cancer. Breast J. 2011;17:533–535. doi: 10.1111/j.1524-4741.2011.01136.x. [DOI] [PubMed] [Google Scholar]
- 38.Viswanath L, Pallade S, Krishnamurthy B, Naveen T, Preethi BL, Pramod KP, Reddy O, Padma G. Darier-roussy sarcoidosis mimicking metastatic breast cancer. Case Rep Oncol. 2009;2:251–254. doi: 10.1159/000262412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittington R, Lazarus A, Nerenstone S, Martin A. Sarcoidosis developing during therapy for breast cancer. Chest. 1986;89:762–763. doi: 10.1378/chest.89.5.762. [DOI] [PubMed] [Google Scholar]
- 40.Tamauchi S, Shimomura Y, Hayakawa H. Endometrial cancer with sarcoidosis in regional lymph nodes: A case report. Case Rep Oncol. 2015;8:409–415. doi: 10.1159/000440800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powell JL, Cunill ES, Gajewski WH, Novotny DB. Sarcoidosis mimicking recurrent endometrial cancer. Gynecol Oncol. 2005;99:770–773. doi: 10.1016/j.ygyno.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Takanami K, Kaneta T, Yamada T, Kinomura S, Yamada S, Fukuda H, Takahashi S. FDG PET for esophageal cancer complicated by sarcoidosis mimicking mediastinal and hilar lymph node metastases: Two case reports. Clin Nucl Med. 2008;33:258–261. doi: 10.1097/RLU.0b013e3181662fda. [DOI] [PubMed] [Google Scholar]
- 43.Kim JJ, Park JK, Wang YP, Choi SH, Jo KH. Lung cancer associated with sarcoidosis - A case report. Korean J Thorac Cardiovasc Surg. 2011;44:301–303. doi: 10.5090/kjtcs.2011.44.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mimura K, Mochizuki Y, Nakahara Y, Kawamura T, Sasaki S, Katsuda R. A case of primary lung cancer with swollen mediastinal lymph nodes due to pre-existing sarcoidosis. Nihon Kokyuki Gakkai Zasshi. 2011;49:208–213. (In Japanese) [PubMed] [Google Scholar]
- 45.Urushiyama H, Yamauchi Y, Suzuki S, Sunohara M, Kouyama T, Ohishi N, Fukami T, Nakajima J, Ushiku T, Oota S, et al. Case of sarcoidosis with squamous cell carcinoma which originated from solitary bronchial papilloma. Nihon Kokyuki Gakkai Zasshi. 2010;48:815–820. (In Japanese) [PubMed] [Google Scholar]
- 46.Bouaziz H, Kaffel N, Charfi N, Fourati M, Abid H, Abid M. Panhypopituitarism revealing metastasis of small-cell lung carcinoma associated with sarcoidosis. Ann Endocrinol (Paris) 2006;67:259–264. doi: 10.1016/S0003-4266(06)72596-3. (In French) [DOI] [PubMed] [Google Scholar]
- 47.Muramatsu M, Kuriyama M, Takahashi K, Miyamoto H, Uekusa T, Danbara T, Fukuchi Y. A case of resected squamous cell carcinoma of the lung complicated with sarcoidosis. Nihon Kokyuki Gakkai Zasshi. 2000;38:720–725. (In Japanese) [PubMed] [Google Scholar]
- 48.Yonenaga Y, Kushihata F, Inoue H, Watanabe J, Tohyama T, Sugita A, Takada Y. Sarcoidosis manifesting as hepatic and splenic nodules mimicking ovarian cancer metastases: A case report. Oncol Lett. 2015;10:2166–2170. doi: 10.3892/ol.2015.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MH, Lee K, Kim KU, Park HK, Lee MK, Suh DS. Sarcoidosis mimicking cancer metastasis following chemotherapy for ovarian cancer. Cancer Res Treat. 2013;45:354–358. doi: 10.4143/crt.2013.45.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollock JL, Catalano E. Metastatic ductal carcinoma of the parotid gland in a patient with sarcoidosis. Arch Dermatol. 1979;115:1098–1099. doi: 10.1001/archderm.115.9.1098. [DOI] [PubMed] [Google Scholar]
- 51.Montini KM, Tulchinsky M. False-positive bone metastases on PET/CT secondary to sarcoidosis in a patient with rectal cancer. Clin Nucl Med. 2012;37:307–310. doi: 10.1097/RLU.0b013e31823eaaf0. [DOI] [PubMed] [Google Scholar]
- 52.Abdi EA, Nguyen GK, Ludwig RN, Dickout WJ. Pulmonary sarcoidosis following interferon therapy for advanced renal cell carcinoma. Cancer. 1987;59:896–900. doi: 10.1002/1097-0142(19870301)59:5<896::AID-CNCR2820590507>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 53.Fukutani K, Kawabe K, Moriyama N, Kitamura T, Murakami T. Carcinoma of the renal pelvis and bladder associated with sarcoidosis: A case report. Urol Int. 1987;42:224–226. doi: 10.1159/000281907. [DOI] [PubMed] [Google Scholar]
- 54.Gharavi MH, Wu HH, Toms SA. High fluorodeoxyglucose ((18)F)PET-uptake lymph nodes in a patient with chordoma: Tumor metastasis or sarcoidosis? Am J Case Rep. 2013;14:373–375. doi: 10.12659/AJCR.889329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilgenhof S, Morlion V, Seghers AC, Four Du S, Vanderlinden E, Hanon S, Vandenbroucke F, Everaert H, Neyns B. Sarcoidosis in a patient with metastatic melanoma sequentially treated with anti-CTLA-4 monoclonal antibody and selective BRAF inhibitor. Anticancer Res. 2012;32:1355–1359. [PubMed] [Google Scholar]
- 56.Vogel WV, Guislain A, Kvistborg P, Schumacher TN, Haanen JB, Blank CU. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol. 2012;30:e7–e10. doi: 10.1200/JCO.2011.37.9693. [DOI] [PubMed] [Google Scholar]
- 57.Heinzerling LM, Anliker MD, Muller J, Schlaeppi M, von Moos R. Sarcoidosis induced by interferon-α in melanoma patients: Incidence, clinical manifestations, and management strategies. J Immunother. 2010;33:834–839. doi: 10.1097/CJI.0b013e3181eef779. [DOI] [PubMed] [Google Scholar]
- 58.Suarez-Garcia C, Pérez-Gil A, Pereira-Gallardo S, Codes-Villena M, García-Escudero A, Camacho Miguel F. Interferon-induced cutaneous sarcoidosis in melanoma. Melanoma Res. 2009;19:391–394. doi: 10.1097/CMR.0b013e32832f51f2. [DOI] [PubMed] [Google Scholar]
- 59.Massaguer S, Sánchez M, Castel T. Mediastinal sarcoidosis induced by high-dose alpha-2-interferon therapy in a patient with malignant melanoma. Eur Radiol. 2004;14:1716–1717. doi: 10.1007/s00330-003-2207-y. [DOI] [PubMed] [Google Scholar]
- 60.Matsubara T, Hirahara N, Hyakudomi R, Fujii Y, Kaji S, Taniura T, Tajima Y. Early gastric cancer associated with gastric sarcoidosis. Int Surg. 2015;100:949–953. doi: 10.9738/INTSURG-D-15-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tissot E, Bringeon G, Berger F, Kalb JC. Cancer and gastric sarcoidosis. J Chir (Paris) 1985;122:479–481. (In French) [PubMed] [Google Scholar]
- 62.Claus F, De Wever L, Moerman P. Coincidence of seminoma and sarcoidosis in two patients presenting with peritoneal surface disease. Int J Urol. 2012;19:1126. doi: 10.1111/j.1442-2042.2012.03129.x. [DOI] [PubMed] [Google Scholar]
- 63.Teo M, McCarthy JE, Brady AP, Curran DR, Power DG. A case of sarcoidosis in a patient with testicular cancer post stem cell transplant. Acta Oncol. 2013;52:869–871. doi: 10.3109/0284186X.2012.689854. [DOI] [PubMed] [Google Scholar]
- 64.Tjan-Heijnen VC, Vlasveld LT, Pernet FP, Pauwels P, De Mulder PH. Coincidence of seminoma and sarcoidosis: A myth or fact? Ann Oncol. 1998;9:321–325. doi: 10.1023/A:1008220002148. [DOI] [PubMed] [Google Scholar]
- 65.Myint ZW, Chow RD. Sarcoidosis mimicking metastatic thyroid cancer following radioactive iodine therapy. J Community Hosp Intern Med Perspect. 2015;5:26360. doi: 10.3402/jchimp.v5.26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmermann-Belsing T, Christensen L, Hansen HS, Kirkegaard J, Blichert-Toft M, Feldt-Rasmussen U. A case of sarcoidosis and sarcoid granuloma, papillary carcinoma, and Graves' disease in the thyroid gland. Thyroid. 2000;10:275–278. doi: 10.1089/thy.2000.10.275. [DOI] [PubMed] [Google Scholar]