Abstract
The aim of the present study was to assess the correlation between therapeutic response and carcinoembryonic antigen (CEA) and carbohydrate antigen 15–3 (CA15-3) levels in advanced breast cancer patients with non-assessable lesions or stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors. A total of 232 female patients with recurrent tumors following radical mastectomy were recruited, including 76 patients with non-assessable lesions and 60 patients with SD. The correlation between CEA and CA15-3 changes, progression-free survival (PFS) and therapeutic response were analyzed in the non-assessable and SD patient groups. For all subjects, the association between the patients' serum tumor markers levels and the clinical presentation of the tumor, as well as the correlation between initial tumor marker levels and PFS, were analyzed. An increase in CEA (an increment of >2 ng/ml) or CA15-3 levels (an increase of >15 U/ml) following the second cycle of treatment correlated with shorter PFS in both non-assessable and SD patients, and with poor clinical outcome in SD patients. High CA15-3 levels correlated with hormone receptor-positive tumors, multiple metastases and liver metastases. Bone metastases correlated with high levels of both CEA and CA15-3. Relatively low CEA and CA15-3 concentrations were associated with triple-negative and locally invasive tumors. High CEA and CA15-3 levels at the beginning of relapse correlated with shorter PFS. The present study illustrates that CEA and CA15-3 levels correlate with several factors in recurrent breast cancer patients. Elevated levels of CEA and CA15-3 at the beginning of relapse may predict shorter PFS. Furthermore, elevation of CEA and CA15-3 levels following the second therapeutic cycle predict poor therapeutic response in patients with non-assessable lesions and SD. Our findings suggest that alterations in CEA and CA15-3 levels can predict therapeutic response in advanced breast cancer patients.
Keywords: advanced breast cancer, CEA, CA15-3, monitoring therapy, survival
Introduction
Breast cancer is the most frequent form of malignant cancer in women worldwide (1). While the primary tumor is often treatable, tumor recurrence remains the most frequent cause of breast cancer mortality. Thus, the treatment of metastatic disease is crucial for improving breast cancer survival (2). Furthermore, designing subsequent treatment strategies using accurate initial response data could improve the outcome of advanced breast cancer and reduce the use of ineffective chemotherapeutic agents (3).
One of the most popular criteria used to evaluate therapeutic strategies is referred to as Response Evaluation Criteria in Solid Tumors (RECIST) (4). According to RECIST 1.1, a patient's therapeutic response can be classified into four conditions: Complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). In SD patients receiving chemotherapy, certain patients would develop PD over time, while other patients would maintain SD or even experience remission.
Previous evidence indicates that there are a number of metastatic forms of breast cancer that are not adequately assessed by RECIST (4), including pleural/pericardial effusion, ascites, the majority of bone metastases and lesions resected by surgery. In particular, ~50% of breast cancer patients develop bone metastases (5), and a large number of these patients would be non-assessable by RECIST. Various alternative methods have been proposed to assesses the therapeutic response of bone metastases, including 18F-fluorodeoxyglucose-positron emission tomography (6) and bone-specific biochemical markers such as N-terminal telopeptide (7,8). However, these indicators are not applicable to other non-assessable lesions.
Previous studies suggest that carcinoembryonic antigen (CEA) and carbohydrate antigen 15–3 (CA15-3) are predictive markers of radiological response in metastatic breast cancer (9). Thus, these markers may be useful for monitoring the therapeutic response of metastatic breast cancer patients. Despite extensive study of CEA and CA15-3, their utility as breast cancer markers remains unclear (10). The majority of tumor markers are used for early diagnosis, determining prognosis, monitoring therapeutic efficacy and follow-up subsequent to therapy (11–16). However, CEA and CA15-3 are unsuitable for early detection due to their low expression and lack of sensitivity in breast cancer (11,17). While CEA and CA15-3 have been used to assess the follow-up of patients with breast cancer (18), their clinical value has not been assessed (11).
Although tumor markers alone are insufficient to evaluate therapeutic response (19), several studies suggest that tumor marker levels correlate with treatment response (3,20–23). For example, Robertson et al (3) reported that changes in the levels of tumor markers correlated with patients' therapeutic response, as assessed by imaging methods (3). Furthermore, reduction in CEA and CA15-3 levels predicted a positive response to systemic therapy in metastatic breast cancer patients (23). However, to date, no studies have assessed the correlation between CEA and CA15-3 levels and therapeutic response in patients with non-assessable lesions or SD.
In order to assess the predictive efficacy of CEA and CA15-3 in metastatic breast cancer, CEA and CA15-3 levels were compared with radiological response in a group of patients classified as non-assessable or SD by RECIST 1.1. In addition, it was analyzed which factors are associated with pre-treatment levels of CEA and CA15-3, including progression-free survival (PFS). The present study should clarify the prognostic value of CEA and CA15-3 as tumor markers in metastatic breast cancer.
Patients and methods
Patients
All data were retrospectively collected from 232 female breast cancer patients in the Affiliated Tumor Hospital of Harbin Medical University (Harbin, China). Patients were included in the study if they underwent radical mastectomy but experienced subsequent tumor recurrence or metastasis. Patients were excluded from the study if their CEA or CA15-3 levels were not measured at the time of the initial relapse or did not undergo therapeutic intervention. Patients' age ranged from 25 to 76 years, and all patients received first-line treatment between July 2001 and February 2013 along with systemic therapies, including chemotherapy, trastuzumab, endocrine therapy and bisphosphonate treatment for bone metastases.
From the 232 enrolled subjects, patients with ≥1 measurable lesion according to RECIST were grouped as assessable patients, while those with lesions resected by surgery or non-measurable lesions (pleural/pericardial effusion, ascites and bone metastases) were grouped as non-assessable patients. At the first-line therapeutic cycle, 60 individuals classified with SD were selected to study the predictive value of CEA and CA15-3 levels in evaluating the therapeutic response. Patients classified as non-assessable by RECIST (76 patients) who had available CEA and CA15-3 data were selected to study the predictive value of the levels of these markers in assessing the therapeutic response in patients with non-assessable lesions.
Determination of tumor markers
Serum CEA concentrations were determined using an Enzyme Immunoassay kit (Dinabot, Tokyo, Japan), while serum CA15-3 levels were determined using a radioimmunoassay kit (Roche Diagnostics, Indianapolis, IN, USA). A threshold of 0–5 ng/ml CEA and 0–25 U/ml CA15-3 was used to determine the ‘normal’ levels of the respective markers. Levels >5 ng/ml CEA or >25 U/ml CA15-3 were considered elevated in patients.
Assessment
CEA and CA15-3 levels were determined within 1 week prior to the initiation of systemic therapy, and were then evaluated every 3 weeks during the course of therapy. Non-assessable patients and patients receiving chemotherapy or trastuzumab therapy underwent radiological examination, which was performed every 6 weeks during treatment.
To study the association between tumor markers levels and PFS, all 232 patients were divided into two groups based on their CEA and CA15-3 levels at the time of relapse (normal and elevated). Patients were subsequently divided into two groups based on the relative changes in CEA and CA15-3 levels at the end of the second therapy cycle: An increased group (an increment of >2 ng/ml CEA or 15 U/ml CA15-3 relative to pre-treatment levels) and a non-increase group (an increment of <2 ng/ml CEA or 15 U/ml CA15-3, or any decrease relative to pre-treatment levels).
The therapeutic response in patients with assessable lesions was classified using RECIST into four categories: CR, PR, SD and PD. For the 60 individuals classified as SD, the clinical therapeutic response following the second cycle of therapy was classified as the final clinical response. Final clinical response was divided into two categories: PD and disease controlled (DC). DC was defined as the lack of PD following all chemotherapy cycles. For patients with non-assessable lesions, PD was defined as the appearance at ≥1 new lesion and/or progression of the existing lesions. All the patients participated in follow-up treatment and testing until May 2013. The median follow-up time of the patients was 11.78 months. Recurrent disease was confirmed by biopsy or, in cases of multiple metastases, by radiological examination.
Statistical analysis
All statistical analysis were carried out using SPSS 19.0 (IBM SPSS, Armonk, NY, USA). Statistical analysis of the differences among patient groups was performed using the t-test and Mann-Whitney U-test for quantitative results, while the Kruskal-Wallis test for qualitative results. The clinical response between PD and DC was analyzed using a cross-tabulation table and the Fisher's exact test. PFS was calculated using the Kaplan-Meier method and the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
The correlation between the clinical characteristics of all 232 enrolled breast cancer patients and the CEA and CA15-3 levels at the time of relapse was initially analyzed (Table I). In particular, it was observed that CA15-3 levels were highly correlated with hormone receptor (HR) status. Significantly higher levels of CA15-3 were detected in estrogen receptor (ER)-positive, progesterone receptor-positive and HR-positive groups (P<0.001, P=0.001 and P<0.001, respectively), whereas CEA did not appear to be correlated with any HR. While there was no correlation between human epidermal growth factor receptor-2 (HER-2) status and either CEA or CA15-3 levels, both markers were negatively correlated with triple-negative breast cancer (CEA, P=0.021; and CA15-3, P<0.001).
Table I.
Correlation between patients' characteristics and initial tumor marker levels at the first relapse (n=232).
| CEA levels (ng/ml) | CA15-3 levels (U/ml) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | n | Mean/median | SE | P-value | Mean/median | SE | P-value |
| Age, years | NSb | NSa | |||||
| ≤35 | 37 | 9.77 | 4.04 | 20.96 | 7.45 | ||
| >35 | 195 | 18.48 | 58.96 | 21.42 | 5.85 | ||
| BMI | NSb | NSb | |||||
| <23.9 | 108 | 13.61 | 3.83 | 49.14 | 6.43 | ||
| ≥23.9 | 124 | 20.12 | 5.88 | 59.12 | 7.66 | ||
| Pathological type | NSa | NSa | |||||
| IDC | 188 | 2.77 | 12.43 | 51.84 | 5.37 | ||
| Others | 44 | 2.87 | 15.90 | 65.74 | 13.74 | ||
| Histological grade | NSb | NSb | |||||
| Well/moderate | 84 | 16.50 | 4.24 | 49.69 | 7.47 | ||
| Poor | 20 | 12.97 | 9.56 | 50.52 | 23.31 | ||
| Unknown | 128 | ||||||
| Tumor size | NSb | NSb | |||||
| T1 | 58 | 15.87 | 4.41 | 45.12 | 9.88 | ||
| T2-T4 | 122 | 19.98 | 6.38 | 63.47 | 7.56 | ||
| Unknown | 52 | ||||||
| Nodal status | NSa | NSb | |||||
| N0-N1 | 127 | 2.71 | 2.36 | 52.46 | 6.50 | ||
| N2-N3 | 91 | 3.00 | 8.52 | 61.31 | 9.10 | ||
| Unknown | 14 | ||||||
| Ki-67, % | NSb | NSb | |||||
| ≤14 | 50 | 21.72 | 5.48 | 60.83 | 11.31 | ||
| >14 | 58 | 13.80 | 5.40 | 54.15 | 10.82 | ||
| Unknown | 124 | ||||||
| ER expression | NSb | <0.001a | |||||
| Negative | 66 | 10.35 | 3.23 | 15.89 | 6.89 | ||
| Positive | 133 | 20.78 | 6.00 | 27.05 | 7.24 | ||
| Unknown | 33 | ||||||
| PR expression | NSb | 0.001a | |||||
| Negative | 75 | 14.50 | 5.00 | 16.45 | 6.66 | ||
| Positive | 124 | 19.03 | 5.95 | 27.41 | 7.59 | ||
| Unknown | 33 | ||||||
| HER-2 expression | NSb | NSb | |||||
| Negative | 151 | 16.16 | 4.95 | 49.55 | 5.59 | ||
| Positive | 27 | 24.13 | 11.67 | 45.56 | 12.02 | ||
| Unknown | 54 | ||||||
| HR expression | NSa | <0.001a | |||||
| Negativec | 56 | 2.29 | 2.51 | 15.89 | 7.14 | ||
| Positived | 143 | 3.00 | 5.67 | 27.05 | 6.90 | ||
| Unknown | 33 | ||||||
| Triple negative | 0.021a | <0.001a | |||||
| Yes | 36 | 2.00 | 1.10 | 11.93 | 5.43 | ||
| No | 158 | 2.92 | 5.19 | 25.79 | 6.32 | ||
| Unknown | 38 | ||||||
| Metastatic sites, n | NSb | <0.001a | |||||
| 1 | 134 | 2.77 | 2.55 | 18.18 | 5.24 | ||
| ≥2 | 98 | 2.82 | 7.80 | 33.08 | 9.36 | ||
| Sites of metastases | |||||||
| Bone | <0.001a | <0.001a | |||||
| Yes | 112 | 4.35 | 7.22 | 39.14 | 9.07 | ||
| No | 120 | 2.21 | 1.26 | 17.17 | 3.48 | ||
| Lung | NSa | NSb | |||||
| Yes | 82 | 9.24 | 9.26 | 65.58 | 9.16 | ||
| No | 150 | 2.73 | 2.34 | 48.40 | 6.00 | ||
| Liver | NSb | 0.009a | |||||
| Yes | 33 | 17.94 | 5.01 | 37.23 | 16.85 | ||
| No | 199 | 16.95 | 4.13 | 20.15 | 5.17 | ||
| Brain | NSb | NSb | |||||
| Yes | 7 | 8.48 | 5.06 | 36.27 | 7.47 | ||
| No | 225 | 17.36 | 3.72 | 55.04 | 5.22 | ||
| Local site | NSa | 0.039a | |||||
| Yes | 54 | 2.29 | 2.38 | 16.58 | 7.58 | ||
| No | 178 | 3.04 | 4.64 | 23.87 | 6.16 | ||
| Regional lymph node | 0.002a | 0.039a | |||||
| Yes | 62 | 1.99 | 1.42 | 18.54 | 8.56 | ||
| No | 170 | 3.11 | 4.86 | 25.07 | 6.13 | ||
| Non-regional lymph node | NSb | NSb | |||||
| Yes | 35 | 7.70 | 2.63 | 50.01 | 13.11 | ||
| No | 197 | 18.76 | 4.22 | 55.27 | 5.51 | ||
Mann-Whitney U-test was used for statistical calculations.
Samples were analyzed using a Student's t-test.
Either ER- or PR-positive.
Both ER- and PR-negative. NS, not significant; BMI, body mass index; IDC, invasive ductal carcinoma; HR, hormone receptor; ER, estrogen receptor; PR, progesterone receptor; SE, standard error; HER-2, human epidermal growth factor receptor-2; CEA, carcinoembryonic antigen; CA15-3, carbohydrate antigen 15-3.
In addition, it was noticed that the serum levels of CEA and CA15-3 were highly correlated with the location and number of metastatic sites in breast cancer patients. In particular, patients with multiple metastases had significantly higher CA15-3 levels than patients with a single metastatic site (P<0.001). However, CEA concentration did not appear to be correlated with the number of metastases (P>0.05). Similarly, increased levels of CA15-3, but not of CEA, were observed in patients with liver metastases (P=0.009).
The serum levels of CEA and CA15-3 were both elevated in patients with bone metastases relative to those without bone metastases (CEA, P<0.001; and CA15-3, P<0.001). By contrast, patients with localized invasion had reduced CA15-3 levels (P=0.039), while patients with regional lymph node metastases had reduced levels of both CEA (P=0.002) and CA15-3 (P=0.039) compared with patients without lymph node metastases. Neither CEA nor CA15-3 was correlated with lung or brain metastasis in breast cancer patients (all P>0.05).
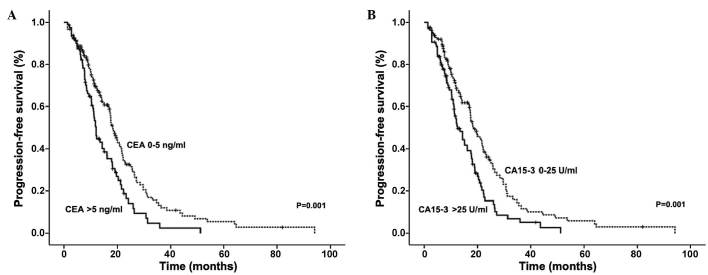
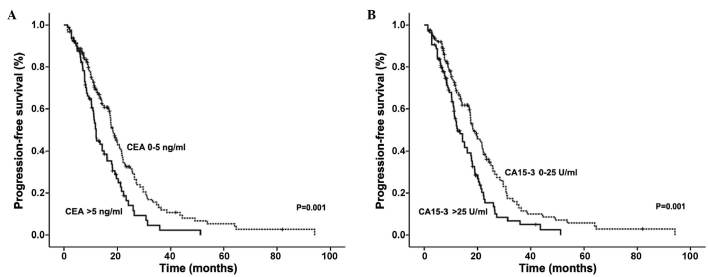
It was observed that the serum levels of CEA and CA15-3 correlated with shorter PFS in advanced breast cancer patients (Fig. 1). Patients with elevated CEA had a median PFS time of 12.10 months, compared with a PFS time of 18.33 months in patients with normal levels (P=0.001). Similarly, patients with elevated CA15-3 levels had a median PFS time of 12.50 months compared with 18.53 months in patients with normal CA15-3 levels (P=0.001).
Figure 1.
PFS according to CEA and CA15-3 levels at the beginning of relapse in 232 advanced breast cancer patients. Kaplan-Meier graphs indicating the percentage of patients with PFS based on the initial (A) CEA and (B) CA15-3 levels. PFS, progression-free survival; CEA, carcinoembryonic antigen; CA15-3, carbohydrate antigen 15-3.
CEA and CA15-3 levels following the second therapy cycle were also predictors of PFS in breast cancer patients with non-assessable lesions. Patients with increased CEA levels (an increment of >2 ng/ml relative to pre-treatment levels) had significantly shorter PFS than patients with no increased CEA levels subsequent to therapy (6.72 vs. 17.74 months, respectively; P<0.001). Similarly, patients with increased CA15-3 levels (an increment of >15 U/ml relative to pre-treatment levels) following therapy had shorter PFS than those with no increased CA15-3 levels (7.71 vs. 17.26 months, respectively; P<0.0001; Fig. 2).
Figure 2.
PFS according to the changes in CEA and CA15-3 levels at the end of the second therapeutic cycle in the 76 patients with non-assessable lesions. Kaplan-Meier graphs indicating the percentage of patients with PFS based on the changes in (A) CEA and (B) CA15-3 levels following the second cycle of chemotherapy. PFS, progression-free survival; CEA, carcinoembryonic antigen; CA15-3, carbohydrate antigen 15-3.
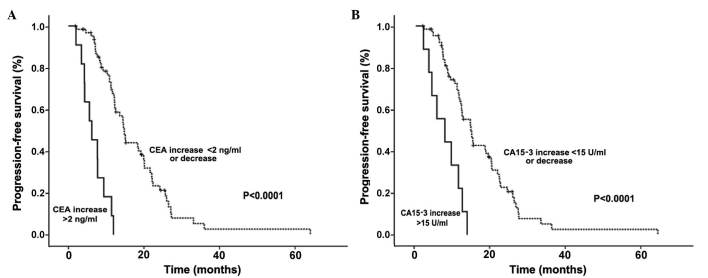
To assess the predictive value of CEA and CA15-3 in patients with SD, the correlations between the serum levels of these markers, PFS and final clinical outcome were analyzed in 60 patients classified as SD by RECIST subsequent to the second treatment cycle. An increase in CEA or CA15-3 levels correlated with a significantly shorter PFS (Fig. 3). Furthermore, this increase in CEA or CA15-3 levels was also negatively correlated with achievement of a CD state (Table II). These data indicate that, even in patients with SD, elevated CEA and CA15-3 levels correlate with a poor prognosis. Taken together, these data suggest that CEA and CA15-3 are predictive of PFS at both the early stages of relapse and throughout the treatment, particularly in non-assessable patients and in those with SD.
Figure 3.
PFS according to the variations in CEA and CA15-3 levels following the second therapeutic cycle in the 60 patients with stable disease. Kaplan-Meier graphs indicating the percentage of patients with PFS based on the changes in (A) CEA and (B) CA15-3 levels following the second cycle of chemotherapy. PFS, progression-free survival; CEA, carcinoembryonic antigen; CA15-3, carbohydrate antigen 15-3.
Table II.
Correlation analysis of CEA and CA15-3 levels and final clinical response in patients classified with stable disease following the second chemotherapy cycle.
| Changes in markers | PDa, n | DCb, n | P-valuec |
|---|---|---|---|
| CEA (ng/ml) | 0.011 | ||
| Increase of ≤2 or decrease | 6 | 47 | |
| Increase of >2 | 4 | 3 | |
| CA15-3 (U/ml) | 0.034 | ||
| Increase of ≤15 or decrease | 6 | 45 | |
| Increase of >15 | 4 | 5 |
Final clinical response refers to the comprehensive clinical assessment of the therapeutic response of the patient to the chemotherapy cycles subsequent to the second chemotherapy cycle.
If a PD response was assessed following any chemotherapy cycle, the final response was considered to be PD.
Only if no PD was assessed following any chemotherapy cycle, the patient was classified as DC.
Fisher's exact test was used for statistical calculations. PD, progressive disease; DC, disease controlled; CEA, carcinoembryonic antigen; CA15-3, carbohydrate antigen 15-3.
Discussion
Predicting a patient's therapeutic response is critical to avoid side effects from unnecessary and ineffective drugs. Few studies have analyzed predictive factors for therapeutic response in advanced breast cancer patients classified as SD by RECIST or in patients with lesions that are not-assessable by RECIST. A major reason for this is that the therapeutic response of such non-assessable lesions (e.g. ascites) cannot be adequately measured by radiological methods (4). In such cases, PFS is the only criteria to assess the therapeutic response in patients, making difficult to predict the patient's response during treatment.
Previously established models used to predict cancer patients' response to chemotherapy are complex and not applicable to patients with surgically resected lesions (24). Furthermore, due to the low sensitivity of the currently available imaging techniques, it is difficult to detect small changes in the tumor burden (25), particularly in patients with SD. Therefore, it is necessary to develop alternative methods to predict therapeutic results in patients with SD or non-assessable lesions.
CA15-3 (also known as mucin 1) is overexpressed in >90% of human breast cancers and in their subsequent metastases (26). CA15-3 promotes tumor invasion and metastasis through activation of the mitogen-activated protein kinase signaling pathway (26) and downregulation of E-cadherin (27). Thus, elevated levels of CA15-3 would predict a poor prognosis with an increased risk of metastasis (28). Consistent with this, CA15-3 levels were observed to negatively correlate with PFS (29). Similarly, CEA has also been observed to correlate with treatment response (21,23,25,30–32). These reports support the results of our study, suggesting that CEA and CA15-3 may be useful markers for predicting patient prognosis and therapeutic response.
Our data suggest that CEA and CA15-3 can predict PFS and final clinical outcome in patients with either SD or non-assessable lesions. An increase of >2 ng/ml CEA or >15 U/ml CA15-3 following the second therapeutic cycle predicted a shorter PFS. Furthermore, elevation of CEA and CA15-3 following the second cycle of chemotherapy correlated with a poor final clinical response in SD patients.
To date, few studies have analyzed the predictive potential of CEA and CA15-3 in advanced breast cancer patients who are not assessable by RECIST and in those with SD. These patient populations require an alternative determination of therapeutic response, as current imaging-based methods are not capable of accurately evaluating the therapeutic response of metastatic lesions (5). Monitoring the serum concentrations of CEA and CA15-3 provides a simple and cost-effective method to predict the therapeutic response of these patients, thus improving the design of therapeutic strategies and minimizing unnecessary side effects due to ineffective treatments.
In our study, serum CEA and CA15-3 concentrations were predictive of HR status, number of metastases and location of metastatic lesions. Specifically, CA15-3 was strongly associated with liver metastasis and the presence of multiple metastatic lesions, while both CEA and CA15-3 were associated with bone metastasis. By contrast, lower levels of CEA and CA15-3 were identified in patients with triple-negative tumors and with regional lymph node recurrence. In addition, lower CA15-3 levels were identified in patients with localized invasion. These results are consistent with previous studies that link CEA and CA15-3 levels with breast cancer prognosis (33,34). Taken together, these data indicate that elevated levels of CEA and CA15-3 may be predictive of increased tumor burden in breast cancer patients.
Elevated serum levels of CEA and CA15-3 prior to therapy predicted shorter PFS in our patient groups. While this finding is consistent with several reports (33–35), one study suggested that elevated CA15-3 concentrations correlated with longer overall breast cancer patient survival (36). This positive correlation between CA15-3 and survival could be explained by the association between CA15-3 levels and ER status observed in our study, as ER is commonly used to predict a better prognosis (37,38). One potential explanation for this discrepancy may be the relatively low ratio of HR-positive patients in the previous study compared with our study. It is possible that the predictive value of CEA and CA15-3 may be dependent on the HR status of breast cancer patients. Further research is required to better understand the role of CEA and CA15-3 in distinct breast cancer subtypes.
To conclude, our study demonstrates the utility of CEA and CA15-3 as markers predicting the therapeutic response of advanced breast cancer patients. These markers may be particularly useful in patients with non-assessable lesions or in those with SD, as defined by RECIST. Additionally, our data indicate that determining the serum concentrations of CEA and CA15-3 provides a simple yet robust method to predict a patient's therapeutic response. However, since our results are based on a retrospective analysis, other tumor markers such as HER-2, epidermal growth factor receptor or tissue polypeptide antigen (25,39–41) were not included in our analysis. Analyzing these markers in addition to CEA and CA15-3 could potentially provide even more accurate predictions of therapeutic response than those reported in the present study. In conclusion, the determination of CEA and CA15-3 levels can provide a powerful tool to complement RECIST in assessing and predicting the therapeutic response of advanced breast cancer patients.
Acknowledgements
Financial support for the present study was provided by the Bureau of Technology and Science of Harbin (Harbin, China; grant number 2009RFXXS017 awarded to L.C.) and the Natural Science Foundation of Heilongjiang Province (Harbin, China; grant number LC2012C08 awarded to Q.M.).
Glossary
Abbreviations
- RECIST
Response Evaluation Criteria In Solid Tumors
- CR
complete response
- PR
partial response
- SD
stable disease
- PD
progressive disease
- CEA
carcinoembryonic antigen
- CA15-3
carbohydrate antigen 15-3
- PFS
progression free-survival
- RIA
radioimmunoassay
- DC
disease controlled
- ER
estrogen receptor
- HR
hormone receptor
- HER-2
human epidermal growth factor receptor-2
- EGFR
epidermal growth factor receptor
References
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Esteva FJ, Valero V, Pusztai L, Boehnke-Michaud L, Buzdar AU, Hortobagyi GN. Chemotherapy of metastatic breast cancer: What to expect in 2001 and beyond. Oncologist. 2001;6:133–146. doi: 10.1634/theoncologist.6-2-133. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JF, Jaeger W, Syzmendera JJ, Selby C, Coleman R, Howell A, Winstanley J, Jonssen PE, Bombardieri E, Sainsbury JR, et al. European Group for Serum Tumour Markers in Breast Cancer: The objective measurement of remission and progression in metastatic breast cancer by use of serum tumour markers. Eur J Cancer. 1999;35:47–53. doi: 10.1016/S0959-8049(98)00297-4. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, Onaya H, Endo M, Sone M, Arai Y. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1) Gan To Kagaku Ryoho. 2009;36:2495–2501. (In Japanese) [PubMed] [Google Scholar]
- 5.Clamp A, Danson S, Nguyen H, Cole D, Clemons M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004;5:607–616. doi: 10.1016/S1470-2045(04)01596-7. [DOI] [PubMed] [Google Scholar]
- 6.Stafford SE, Gralow JR, Schubert EK, Rinn KJ, Dunnwald LK, Livingston RB, Mankoff DA. Use of serial FDG PET to measure the response of bone-dominant breast cancer to therapy. Acad Radiol. 2002;9:913–921. doi: 10.1016/S1076-6332(03)80461-0. [DOI] [PubMed] [Google Scholar]
- 7.Costa L, Demers LM, Gouveia-Oliveira A, Schaller J, Costa EB, de Moura MC, Lipton A. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol. 2002;20:850–856. doi: 10.1200/JCO.20.3.850. [DOI] [PubMed] [Google Scholar]
- 8.Lipton A, Demers L, Curley E, Chinchilli V, Gaydos L, Hortobagyi G, Theriault R, Clemens D, Costa L, Seaman J, et al. Markers of bone resorption in patients treated with pamidronate. Eur J Cancer. 1998;34:2021–2026. doi: 10.1016/S0959-8049(98)00277-9. [DOI] [PubMed] [Google Scholar]
- 9.Massacesi C, Rocchi MB, Marcucci F, Pilone A, Galeazzi M, Bonsignori M. Serum tumor markers may precede instrumental response to chemotherapy in patients with metastatic cancer. Int J Biol Markers. 2003;18:295–300. doi: 10.5301/JBM.2008.3624. [DOI] [PubMed] [Google Scholar]
- 10.Molina R, Auge JM, Farrus B, Zanón G, Pahisa J, Muñoz M, Torne A, Filella X, Escudero JM, Fernandez P, et al. Prospective evaluation of carcinoembryonic antigen (CEA) and carbohydrate antigen 15.3 (CA 15.3) in patients with primary locoregional breast cancer. Clin Chem. 2010;56:1148–1157. doi: 10.1373/clinchem.2009.135566. [DOI] [PubMed] [Google Scholar]
- 11.Duffy MJ. Serum tumor markers in breast cancer: Are they of clinical value? Clin Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Luo RC. Diagnostic value of combined detection of TPS, CA153 and CEA in breast cancer. Di Yi Jun Yi Da Xue Xue Bao. 2005;25(1293–1294):1298. (In Chinese) [PubMed] [Google Scholar]
- 13.Ebeling FG, Stieber P, Untch M, Nagel D, Konecny GE, Schmitt UM, Fateh-Moghadam A, Seidel D. Serum CEA and CA 15-3 as prognostic factors in primary breast cancer. Br J Cancer. 2002;86:1217–1222. doi: 10.1038/sj.bjc.6600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marić P, Ozretić P, Levanat S, Oresković S, Antunac K, Beketić-Oreskovic L. Tumor markers in breast cancer - evaluation of their clinical usefulness. Coll Antropol. 2011;35:241–247. [PubMed] [Google Scholar]
- 15.Agrawal AK, Jelen M, Rudnicki J, Grzebieniak Z, Zyśko D, Kielan W, Słonina J, Marek G. The importance of preoperative elevated serum levels of CEA and CA15-3 in patients with breast cancer in predicting its histological type. Folia Histochem Cytobiol. 2010;48:26–29. doi: 10.2478/v10042-010-0030-2. [DOI] [PubMed] [Google Scholar]
- 16.Parker C. Active surveillance: Towards a new paradigm in the management of early prostate cancer. Lancet Oncol. 2004;5:101–106. doi: 10.1016/S1470-2045(04)01384-1. [DOI] [PubMed] [Google Scholar]
- 17.Di Gioia D, Heinemann V, Nagel D, Untch M, Kahlert S, Bauerfeind I, Koehnke T, Stieber P. Kinetics of CEA and CA15-3 correlate with treatment response in patients undergoing chemotherapy for metastatic breast cancer (MBC) Tumour Biol. 2011;32:777–785. doi: 10.1007/s13277-011-0180-7. [DOI] [PubMed] [Google Scholar]
- 18.Mariani L, Miceli R, Michilin S, Gion M. Serial determination of CEA and CA 15.3 in breast cancer follow-up: An assessment of their diagnostic accuracy for the detection of tumour recurrences. Biomarkers. 2009;14:130–136. doi: 10.1080/13547500902770090. [DOI] [PubMed] [Google Scholar]
- 19.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC., Jr American Society of Clinical Oncology: American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 20.Williams MR, Turkes A, Pearson D, Griffiths K, Blamey RW. An objective biochemical assessment of therapeutic response in metastatic breast cancer: A study with external review of clinical data. Br J Cancer. 1990;61:126–132. doi: 10.1038/bjc.1990.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson JF, Pearson D, Price MR, Selby C, Blamey RW, Howell A. Objective measurement of therapeutic response in breast cancer using tumour markers. Br J Cancer. 1991;64:757–763. doi: 10.1038/bjc.1991.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon AR, Jackson L, Chan SY, Badley RA, Blamey RW. Continuous chemotherapy in responsive metastatic breast cancer: A role for tumour markers? Br J Cancer. 1993;68:181–185. doi: 10.1038/bjc.1993.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurebayashi J, Nishimura R, Tanaka K, Kohno N, Kurosumi M, Moriya T, Ogawa Y, Taguchi T. Significance of serum tumor markers in monitoring advanced breast cancer patients treated with systemic therapy: A prospective study. Breast Cancer. 2004;11:389–395. doi: 10.1007/BF02968047. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Yonemori K, Katsumata N, Shimizu C, Hirakawa A, Hirata T, Kouno T, Tamura K, Ando M, Fujiwara Y. Prediction of progressive disease using tumor markers in metastatic breast cancer patients without target lesions in first-line chemotherapy. Ann Oncol. 2010;21:2195–2200. doi: 10.1093/annonc/mdq213. [DOI] [PubMed] [Google Scholar]
- 25.Sölétormos G, Nielsen D, Schiøler V, Mouridsen H, Dombernowsky P. Monitoring different stages of breast cancer using tumour markers CA 15-3, CEA and TPA. Eur J Cancer. 2004;40:481–486. doi: 10.1016/j.ejca.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder JA, Thompson MC, Gardner MM, Gendler SJ. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;276:13057–13064. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Kitajima Y, Sato S, Miyazaki K. Combined evaluation of mucin antigen and E-cadherin expression may help select patients with gastric cancer suitable for minimally invasive therapy. Br J Surg. 2003;90:95–101. doi: 10.1002/bjs.4014. [DOI] [PubMed] [Google Scholar]
- 28.Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: An immunohistologic study of 71 patients and review of the literature. Cancer. 2001;91:1973–1982. doi: 10.1002/1097-0142(20010601)91:11<1973::AID-CNCR1222>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Cheng JP, Yan Y, Wang XY, Lu YL, Yuan YH, Jia J, Ren J. MUC1-positive circulating tumor cells and MUC1 protein predict chemotherapeutic efficacy in the treatment of metastatic breast cancer. Chin J Cancer. 2011;30:54–61. doi: 10.5732/cjc.010.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sölétormos G, Nielsen D, Schiøler V, Skovsgaard T, Dombernowsky P. Tumor markers cancer antigen 15.3, carcinoembryonic antigen, and tissue polypeptide antigen for monitoring metastatic breast cancer during first-line chemotherapy and follow-up. Clin Chem. 1996;42:564–575. [PubMed] [Google Scholar]
- 31.Cheung KL, Graves CR, Robertson JF. Tumour marker measurements in the diagnosis and monitoring of breast cancer. Cancer Treat Rev. 2000;26:91–102. doi: 10.1053/ctrv.1999.0151. [DOI] [PubMed] [Google Scholar]
- 32.Tondini C, Hayes DF, Gelman R, Henderson IC, Kufe DW. Comparison of CA15-3 and carcinoembryonic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res. 1988;48:4107–4112. [PubMed] [Google Scholar]
- 33.Lee JS, Park S, Park JM, Cho JH, Kim SI, Park BW. Elevated levels of serum tumor markers CA 15-3 and CEA are prognostic factors for diagnosis of metastatic breast cancers. Breast Cancer Res Treat. 2013;141:477–484. doi: 10.1007/s10549-013-2695-7. [DOI] [PubMed] [Google Scholar]
- 34.Yerushalmi R, Tyldesley S, Kennecke H, Speers C, Woods R, Knight B, Gelmon KA. Tumor markers in metastatic breast cancer subtypes: Frequency of elevation and correlation with outcome. Ann Oncol. 2012;23:338–345. doi: 10.1093/annonc/mdr154. [DOI] [PubMed] [Google Scholar]
- 35.Bidard FC, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, Cottu P, Beuzeboc P, Rolland E, Mathiot C, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: A prospective observational study. Breast Cancer Res. 2012;14:R29. doi: 10.1186/bcr3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura R, Nagao K, Miyayama H, Matsuda M, Baba K, Matsuoka Y, Yamashita H. Elevated serum CA15-3 levels correlate with positive estrogen receptor and initial favorable outcome in patients who died from recurrent breast cancer. Breast Cancer. 2003;10:220–227. doi: 10.1007/BF02966721. [DOI] [PubMed] [Google Scholar]
- 37.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakshatri H, Srour EF, Badve S. Breast cancer stem cells and intrinsic subtypes: Controversies rage on. Curr Stem Cell Res Ther. 2009;4:50–60. doi: 10.2174/157488809787169110. [DOI] [PubMed] [Google Scholar]
- 39.Molina R, Augé JM, Escudero JM, Filella X, Zanon G, Pahisa J, Farrus B, Muñoz M, Velasco M. Evaluation of tumor markers (HER-2/neu oncoprotein, CEA, and CA 15.3) in patients with locoregional breast cancer: Prognostic value. Tumour Biol. 2010;31:171–180. doi: 10.1007/s13277-010-0025-9. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen AC, Sorensen PD, Jacobsen EH, Madsen JS, Brandslund I. Sensitivity of CA 15-3, CEA and serum HER2 in the early detection of recurrence of breast cancer. Clin Chem Lab Med. 2013;51:1511–1519. doi: 10.1515/cclm-2012-0488. [DOI] [PubMed] [Google Scholar]
- 41.Bao H, Yu D, Wang J, Qiu T, Yang J, Wang L. Predictive value of serum anti-p53 antibodies, carcino-embryonic antigen, carbohydrate antigen 15-3, estrogen receptor, progesterone receptor and human epidermal growth factor receptor-2 in taxane-based and anthracycline-based neoadjuvant chemotherapy in locally advanced breast cancer patients. Anticancer Drugs. 2008;19:317–323. doi: 10.1097/CAD.0b013e3282f3d018. [DOI] [PubMed] [Google Scholar]