Abstract
In this study, we describe an abbreviated single-step fractionation protocol for the enrichment of detergent-insoluble protein aggregates from human postmortem brain. The ionic detergent N-lauryl-sarcosine (sarkosyl) effectively solubilizes natively folded proteins in brain tissue allowing the enrichment of detergent-insoluble protein aggregates from a wide range of neurodegenerative proteinopathies, such as Alzheimer's disease (AD), Parkinson's disease and amyotrophic lateral sclerosis, and prion diseases. Human control and AD postmortem brain tissues were homogenized and sedimented by ultracentrifugation in the presence of sarkosyl to enrich detergent-insoluble protein aggregates including pathologic phosphorylated tau, the core component of neurofibrillary tangles in AD. Western blotting demonstrated the differential solubility of aggregated phosphorylated-tau and the detergent-soluble protein, Early Endosome Antigen 1 (EEA1) in control and AD brain. Proteomic analysis also revealed enrichment of β-amyloid (Aβ), tau, snRNP70 (U1-70K), and apolipoprotein E (APOE) in the sarkosyl-insoluble fractions of AD brain compared to those of control, consistent with previous tissue fractionation strategies. Thus, this simple enrichment protocol is ideal for a wide range of experimental applications ranging from Western blotting and functional protein co-aggregation assays to mass spectrometry-based proteomics.
Keywords: Biochemistry, Issue 128, Neurodegeneration, aggregation, neuropathology, proteostasis, proteinopathy, amyloid, tau, sarkosyl, fractionation
Introduction
Neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), and the closely related prion diseases are proteinopathies characterized by the gradual accumulation of detergent-insoluble protein aggregates in the brain with accompanying cognitive decline.1,2 This shared pathological feature is thought to play a central role in the etiology and pathophysiology of these neurodegenerative diseases.2 These aggregates typically consist of polymeric amyloid fibers, which are composed of repeating units of misfolded protein exhibiting cross β-structure.1,2,3,4 Biochemically, amyloid aggregates are highly resistant to chemical or thermal denaturation and solubilization,3 which presents unique challenges to their purification, analysis and study via traditional biochemical techniques.2,5,6,7,8,9,10,11 Unsurprisingly, the detergent-insoluble protein fraction has been the focus of much research into the pathophysiology of neurodegenerative diseases involving the accumulation of misfolded proteins.6,12,13,14
Biochemical fractionation techniques have often been utilized to enrich the detergent-insoluble fraction from postmortem brain homogenates.6,12,13,14 One of the most common methods involves the sequential extraction of tissue homogenates with buffers and detergents of increasing stringency, followed by ultracentrifugation to partition the soluble and insoluble fractions. A commonly used sequential fractionation protocol6,14 involves the homogenization of frozen tissue samples in a detergent-free low salt (LS) buffer and the resultant insoluble pellets are then sequentially extracted with buffers containing high salt, non-ionic detergents, high sucrose, ionic detergents and finally chaotropes like urea.6,14 An obvious drawback of such a sequential fractionation protocols is the substantial time and labor commitment required to complete them. Including homogenization and ultracentrifugation, a typical five-step fractionation protocol can take several hours or even days to complete. 4,6,7,10,15,16,17,18 Additionally, as many pathologic protein aggregates remain insoluble in all but the harshest conditions19,20 most of the generated fractions are of limited value. Thus, the less-stringent fractionation steps utilizing high salt concentrations and non-ionic detergents are largely redundant.
Previous studies have shown that the ionic detergent N-lauryl-sarcosine (sarkosyl) is an excellent candidate for a simplified single-step detergent fractionation protocol.5,6,12,13,14,21,22,23 As a denaturing detergent, sarkosyl is stringent enough to solubilize the vast majority of natively folded proteins in brain without solubilizing misfolded protein aggregates composed of beta-amyloid (Aβ),6,11 phosphorylated tau (pTau),6 TAR DNA-binding protein 43 (TDP-43),14 alpha-synuclein,12,13 scrapie,23 or U1 small nuclear ribonucleoproteins (U1 snRNPs) such as U1-70K.5,21,22As sarkosyl is less stringent than the ubiquitous anionic detergent sodium dodecyl sulfate (SDS), it preserves less robust oligomeric forms of misfolded protein aggregates that cannot withstand SDS treatment.9
Previously, we described an abbreviated detergent-fractionation protocol that achieved results comparable to the more laborious sequential fractionation methodologies.5 By omitting the less stringent fractionation steps, we were able to develop a facile single-step fractionation protocol for the enrichment of detergent-insoluble protein aggregates from postmortem human brain.5 This detailed protocol described herein is well suited for a wide range of experimental applications ranging from Western blotting and mass spectrometry-based proteomics to functional protein misfolding and aggregation seeding assays.5,6,21
Protocol
Ethics Statement: All brain tissues were obtained from the Emory Alzheimer's Disease Research Center (ADRC) Brain Bank. Human postmortem tissues were acquired under proper Institutional Review Board (IRB) protocols.
1. Homogenization and Fractionation
- Tissue selection NOTE: Frozen postmortem frontal cortex tissue from healthy control (Ctl) and pathologically confirmed AD cases were selected from the Emory ADRC brain bank (n=2). Post-mortem neuropathological evaluation of amyloid plaque distribution was performed according to the Consortium to Establish a Registry for Alzheimer's Disease semi-quantitative scoring criteria24, although neurofibrillary tangle pathology was assessed in accordance with the Braak staging system.16
- Obtain frozen postmortem brain tissue from healthy control (Ctl) and pathologically confirmed AD cases. Utilizing containers of dry ice, forceps and a razor blade, excise ~ 250 mg portions of grey matter from each tissue sample on tared disposable weigh boats, taking care to prevent the tissue from thawing. Record the weight of each piece to be homogenized.
- Excise ~ 250 mg of grey matter using forceps and razor by visually inspecting to locate the interface between the outer grey matter and inner white matter. Avoid white matter and more importantly, any large blood vessels or bloody regions, meninges, or arachnoid mater. Keep the tissue frozen during the cutting process by working quickly and frequently placing weighing boat with brain tissue into polystyrene containers with dry ice.
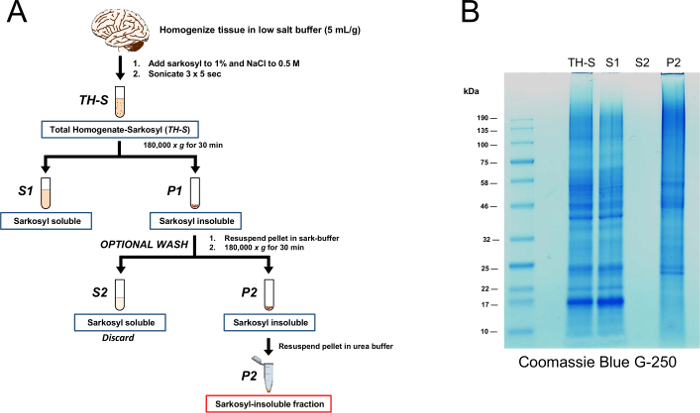
- Record the exact weight of grey matter excised from each frozen piece using an analytical balance. Calculate the volume of low salt (LS) buffer (see Table 1) needed for dounce homogenization (5 mL/g or 20% w/v).
- Prepare 10 mL LS buffer with 1x protease and phosphatase inhibitor cocktail and chill on ice.
- Remove weighed tissue and weighing boat from dry ice and dice into roughly 2 x 2 mm pieces as it thaws. Transfer to a 2 mL pre-chilled dounce homogenizer tube on ice.
- Add 5 mL/g (20% w/v) of ice-cold low salt (LS) buffer with 1X protease and phosphatase inhibitor cocktail.
- Homogenize the brain tissue on ice using approximately 10 strokes of high clearance pestle A and 15 strokes of low clearance pestle B.
- Transfer all homogenate to a 2 mL polypropylene tube using a glass Pasteur pipette. Label the tube with “TH” (Total Homogenate), the tissue case number, and homogenate volume. Aliquot 0.8 mL into a labeled 1.5 mL tube. Any excess homogenate can be similarly aliquoted and stored at -80 °C.
- To each 0.8 mL aliquot, add 100 µL each of 5 M NaCl and 10% (w/v) sarkosyl to concentrations of 0.5 M and 1% w/v, respectively. Mix tubes well by inversion and incubate on ice for 15 min. Label tubes with "TH-S" (Total Homogenate-Sarkosyl) to indicate that the homogenates are in sarkosyl-buffer (Table 1).
- Sonicate each tube for three 5 s pulses at 30% amplitude on ice (maximum intensity = 40%) using a microtip probe.
- Determine the protein concentrations of the homogenates using the bicinchoninic acid (BCA) assay25 method. NOTE: The average protein concentration of TH-S homogenates prepared at 5 mL LS per gram of tissue is 15-20 mg/mL. The homogenates can be fractionated immediately or stored at -80 °C until use.
- Dilute TH-S homogenates to 10 mg/mL using ice-cold sark buffer (Table 1) with 1x protease and phosphatase inhibitors.
- Transfer 5 mg protein (0.5 mL) of each TH-S homogenate into 500 µL polycarbonate ultracentrifuge tubes and pair-balance with sark-buffer. Load tubes into a pre-chilled rotor and ultracentrifuge at 180,000 x g for 30 min at 4 °C. Transfer the sarkosyl-soluble supernatants (S1) to 1.5 mL tubes and store at -80 °C. NOTE: (Optional wash) Add 200 µL of sark-buffer to the ultracentrifuge tubes containing the detergent-insoluble fractions (P1) and dislodge the pellets (P1) from the bottom of the ultracentrifuge tubes using a 200 µL pipette tip.
- Briefly pulse-spin the inverted tubes for 2-3 s (≤2,500 x g) on a microcentrifuge to transfer the pellets (P1) and buffer to the 1.5 mL tubes below. Resuspend the insoluble pellets (P1) in the sark-buffer by pipetting up and down to ensure the pellet is disrupted.
- Pair-balance, and centrifuge at 180,000 x g for an additional 30 min at 4 °C.
- Discard the optional wash supernatant (S2) and incubate the sarkosyl-insoluble pellets (P2) in 50-75 µL of urea buffer (Table 1) with 1x PIC (protease and phosphatase inhibitor cocktail) for 30 min at room temperature to solubilize the pellet. NOTE: Warm urea buffer to room temperature before use to avoid SDS precipitation. For detergent-sensitive applications, omit SDS from urea buffer and consider washing the insoluble pellet (P2) in low salt buffer before resuspending in urea buffer.
- Transfer the resuspended pellets (P2) to 0.5 mL tubes and use brief (1 s) microtip sonication at 20% amplitude (maximum intensity = 40%) to fully solubilize the pellets.
- Determine the protein concentrations of the sarkosyl-soluble (S1) and -insoluble (P2) fractions using the BCA assay method. Use these fractions immediately or store at -80 °C until use.
2. Immunoblotting
- Western blotting NOTE: Western blotting was performed according to previously reported procedures with slight modifications.5,10
- Prepare 40 µg samples of total homogenates (TH-S), sark-soluble (S1) and sark-insoluble (P2) fractions in 1X Laemmli SDS-page sample buffer with 5 mM TCEP pH 7. Incubate samples at 95 °C for 5 min.
- Load samples on a precast 10-well 4-12% Bis-Tris SDS-PAGE gel. Electrophorese at 80 V for the first centimeter (10 min) and 120 V for the remainder (60 min) or until the tracking dye reaches the bottom of the gel.
- Use a fresh razor blade and gel knife to split-open the pre-cast gel cassette. Gently cut away the combs and very bottom of the gel with a fresh razor blade.
- Resuspend the insoluble pellets (P1) in 200 µl sark-buffer with 1x PIC by pipetting up and down.
- Transfer to a 0.2 µm nitrocellulose membrane using a dry transfer method on a blotting machine (see the Materials Table).
- Block the membrane in blocking buffer (BB) without Tween 20 for 30 min, followed with BB with 0.05% Tween 20 (BB/T) for 30 min. After blocking, rinse the membrane in TBS/T (0.1% Tween 20) for 5 min to remove excess blocking buffer.
- Prepare 1:1,000 dilutions of the primary antibodies pT231, Tau-2 (pan tau), AT8 and EEA1 in BB/T (0.05% Tween 20).
- Incubate the membranes in primary antibody solution overnight at 4 °C with circular agitation. Rinse the membrane in TBS/T for 3 x 15 min.
- Probe the membrane with a 1:20,000 dilution of the fluorescently-labeled near-IR secondary antibody in BB/T for 60 min at room temperature in darkness on shaker. Rinse the membrane for 3 x 10 min in TBS/T and 2 x 5 min in TBS.
- Scan the membrane using an infrared imaging system at the appropriate excitation wavelength (e.g. 700 nm (red channel) for infrared dye 680 goat anti-mouse IgG (H+L) secondary and 800 nm (green channel) for infrared dye 790 donkey anti-rabbit IgG (H+L) secondary).
- Use the imaging software to quantify signal intensities and perform densitometry measurements as per the manufacturer's instructions, ensuring auto-background setting is set to average the entire background.
Representative Results
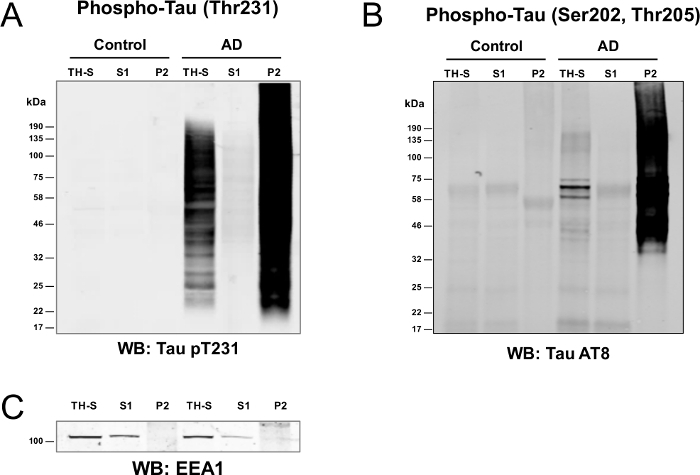
The abbreviated single-step sarkosyl-fractionation protocol was used to enrich detergent-insoluble protein aggregates from control and AD postmortem brain (Figure 1). Proteins from TH-S, S1, S2 and P2 fractions were resolved by SDS-PAGE, fixed for 15 min in Coomassie blue fixative buffer followed by gentle staining with Coomassie Brilliant Blue G-250 staining buffer. The resuspension step is optional since there were undetectable levels of protein in the S2 fraction (see 1.1.14).Western blot analysis (Figure 2Aand B) clearly demonstrates that in AD brain, the pathologic high molecular-weight phosphorylated tau aggregates were sarkosyl-insoluble, and very little pTau remained in the sarkosyl-soluble fractions.5,6,7,8,10,22 Conversely, the majority of pTau in control brain partitioned into the sarkosyl-soluble fraction.5,6,10 In contrast, EEA1 primarily partitions into the S1 fraction in both control and AD brain (Figure 2C). Here, EEA1 acts as a general marker for most native, non-pathologic proteins that are inherently sarkosyl-soluble5. Notably, there is slightly less signal for EEA1 in the soluble fraction (S1) compared to the TH-S fraction, indicating that a minor pool of EEA1 is likely sarkosyl-insoluble, yet below the limit of detection by western blot with this particular antibody. It is equally possible that the slight decrease in signal strength of EEA1 in the soluble fraction (S1) is due to non-specific binding of EEA1 to the ultracentrifuge tubes could also explain the slight deviation from expected results.
To benchmark the efficacy of the abbreviated single-step fractionation protocol (Figure 3A) against the more traditional multi-step sequential fractionation methodology (Figure 3B), the relative levels of sarkosyl-insoluble amyloid precursor protein (APP), tau (MAPT), small nuclear ribonucleoprotein U1-70K (snRNP70) and apolipoprotein E (APOE) were compared by label-free mass spectrometry (MS) based proteomics across pooled control (Ctl), mild cognitive impairment (MCI) and AD cases from previous studies7,18 (Figure 3). Measurements of detergent-insoluble APP effectively represents the Aβ peptide, as all fully tryptic APP peptides quantified mapped to the Aβ region of the full length 695 residue APP protein7. With both methods, the sarkosyl-insoluble levels of all four pathologic proteins trended upwards in MCI and AD cases relative to controls, consistent with the strong correlation of cognitive decline and pathological burden. Overall, enrichment of these proteins in the detergent-insoluble fractions (P2) were remarkably consistent using both the single-step18 and multi-step fractionation protocols7.
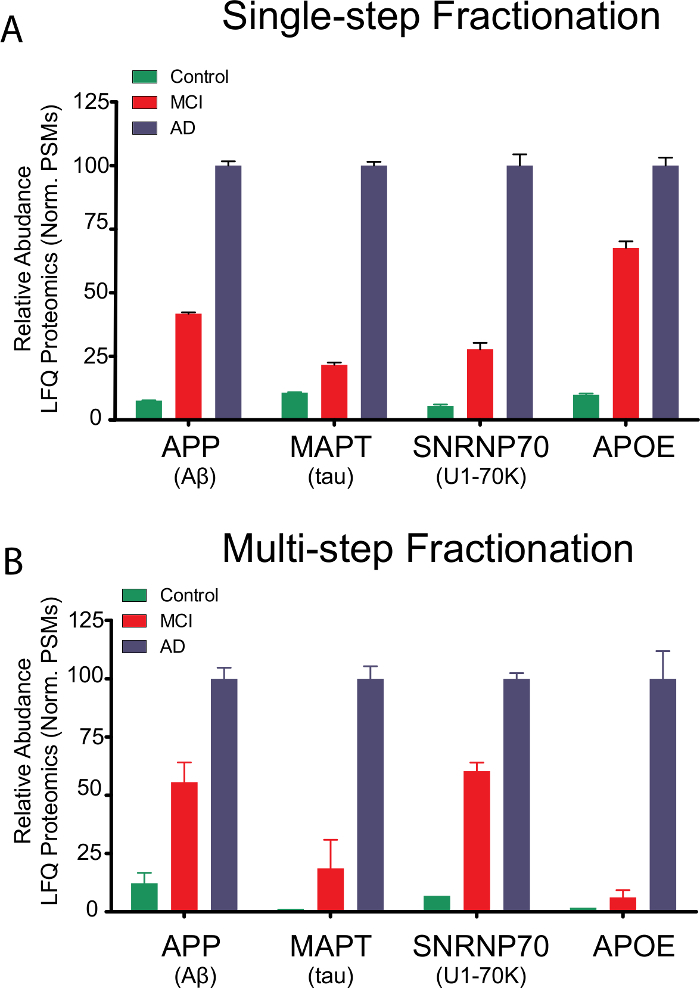
There are, however, slight differences between the two methodologies that may prove advantageous or deleterious depending on the exact nature and goals of an individual experiment. The comparative proteomic data summarized in Figure 3 may inform which protocol to use depending on specific experimental parameters and applications. As one might expect, there is a general trade-off between sample enrichment and purity, with the simplified fractionation protocol affording more enriched samples with less protein loss than the multi-step method.
For example, control insoluble fractions prepared using the simplified protocol (Figure 3A) exhibit slightly higher background levels of disease-relevant proteins than those prepared via the multi-step approach. Conversely, the single-step fractionation technique may be advantageous for low-abundance proteins as the overall sample losses are significantly reduced. For example, the relative enrichment of sarkosyl-insoluble APOE in MCI cases is significantly higher in the insoluble fractions prepared via the abbreviated protocol (Figure 3A).
Although both approaches are more than adequate to observe any significant increase in disease-relevant or pathologic misfolded protein aggregates, the more numerous and extensive fractionation and wash steps of the multi-step approach may generate a cleaner and more specific proteomic signature. Nevertheless, our data verify that the single-step fractionation method is consistent with published findings that pathologic aggregates such as tau neurofibrillary tangles (NFTs), Aβ plaques and scrapie prions (PrSc) are insoluble in sarkosyl, while their natively folded counterparts remain soluble.5,6,7,8,10,11,23,26
By calculating the relative amounts of protein that partition into the detergent-insoluble fractions of control (n=3) and AD (n=3) brain (Table 2), only ~ 10% of the total protein pool is sarkosyl-insoluble. When 5 mg total protein of brain homogenate (TH-S) is fractionated, 10.4% and 11.3% of the proteome partitions into the detergent-insoluble fraction in control and AD cases, respectively. Although the total amount of sarkosyl-insoluble protein enriched in AD brain is slightly higher than control, no significant differences were observed across the two groups (p=0.703). This indicates that protein misfolding and aggregation is not widespread in AD brain, but rather limited to a narrow and specific subset of proteins such as tau, Aβ and U1 snRNPs.7,21,22
Figure 1: Sarkosyl fractionation scheme. (A) A postmortem human brain was homogenized in low salt buffer, after which sarkosyl and NaCl were added to final concentrations of 1% w/v and 0.5 M, respectively and incubated for 15 min on ice. After sonication, the homogenates were fractionated by ultracentrifugation at 180,000 x g for 30 min affording the sarkosyl-soluble supernatant (S1) and the sarkosyl-insoluble pellet (P1). The P1 pellet was (OPTIONALLY) washed with sark-buffer and re-sedimented by ultracentrifugation to afford the final sarkosyl-insoluble pellet (P2). This P2 fraction was solubilized in urea buffer for 30 min at room temperature, followed by 3 x 5 s sonication with a clean microtip probe. (B) Proteins (20 µg) from TH-S, S1, S2 (10 µL) and P2 fractions were resolved by SDS-PAGE, fixed for 15 min in Coomassie blue fixative buffer followed by gentle staining with Coomassie Brilliant Blue G-250 staining buffer overnight at room temperature. Please click here to view a larger version of this figure.
Figure 2: Enrichment of phosphorylated-tau in the detergent insoluble fraction of AD brain. (A and B) Protein (40 μg) of the total (TH-S), soluble (S1) and insoluble (P2) fractions were blotted with an antibody against tau phosphorylated at threonine 231 (pT231) or AT8 (pSer202, pThr205) to demonstrate the enrichment of pathologic phospho-tau species in the insoluble fraction (P2) of AD brain. In AD brain, high molecular weight aggregated tau exclusively partitions into the detergent-insoluble (P2) fraction. (C) EEA1 served as a soluble protein marker and relative loading control for the total (TH-S) and soluble (S1) fractions of control and AD brain. Please click here to view a larger version of this figure.
Figure 3: Proteomic analysis of pathologic AD proteins using a single-step or multi-step fractionation protocol. Relative levels of the representative sarkosyl-insoluble proteins APP (Aβ), tau, snRNP70 (U1-70K) and APOE across different diseases among pooled control, MCI and AD cases using either the single step (A) or multi-step (B) sarkosyl fractionation approach. For both datasets, protein level was estimated by peptide spectral matches (PSMs) of these identified proteins, and normalized to set the maximum to 100. PSMs from Control, MCI, and AD cases (n=5 each) were used for the single-set dataset. For the multi-step approach, PSMs from two replicate pools of control, MCI and AD cases (n=5 each) were analyzed. Error bars indicate the values of mean ± S.E.M. Please click here to view a larger version of this figure.
| 10% w/v sarkosyl solution: 10 g N-lauryl-sarcosine sodium salt per 100 ml of ddH2O (stir at RT overnight) |
| Low Salt (LS) buffer: 50 mM HEPES pH 7.0, 250 mM sucrose, 1 mM EDTA |
| Sarkosyl (sark) buffer: LS buffer + 1% (w/v) sarkosyl + 0.5 M NaCl |
| Urea buffer (store at -20 °C): 50 mM Tris-HCl pH 8.5, 8M urea, 2% SDS |
| 4X SDS loading buffer: 200 mM Tris-HCl pH 6.8, 40% glycerol, 8% sodium dodecyl suflate (SDS), 0.4% bromophenol blue (BPB), and 20 mM TCEP pH 7.0 in ddH2O |
| Coomassie fixative buffer: 40% Methanol, 10% acetic acid in ddH2O |
| Coomassie Brilliant Blue G-250 staining buffer: 25% Methanol, 5% acetic acid, 0.1% coomassie blue G-250 in ddH2O |
| Coomassie destain buffer: 25% methanol, 5% acetic acid in ddH2O |
| 1X Tris buffered saline (TBS): 50 mM Tris-HCl pH 7.45, 150 mM NaCl in ddH2O |
| 1X Tris buffered saline with Tween 20 (TBS/T): 50 mM Tris-HCl pH 7.45, 150 mM NaCl, 0.1% Tween 20 in ddH2O |
| 1X Blocking buffer (BB): 50 mM Tris-HCl pH 7.45, 150 mM NaCl, 1% proprietary protein (see materials list), 750 mM Methylchloroisothiazolinone in ddH2O |
| 1X Blocking buffer with 0.05% Tween 20 (BB/T): 50 mM Tris-HCl pH 7.45, 150 mM NaCl, 1% proprietary protein (see materials list), 0.05% Tween 20, 750 mM Methylchloroisothiazolinone in ddH2O |
Table 1: Buffers list.
| Sample | Volume Sample (µL) | Concentration (µg/µL) | Total Protein (µg) | Average Total Protein (µg) and S.E.M. | % TH-S (5 mg) |
| Ctl 1 | 70 | 9.89 | 692.3 | ||
| Ctl 2 | 70 | 7.38 | 516.6 | 518.7 (±99.63) | 10.4% |
| Ctl 3 | 70 | 4.96 | 347.2 | ||
| AD 1 | 70 | 9.77 | 683.9 | ||
| AD 2 | 70 | 6.67 | 466.9 | 567.0 (±63.20) | 11.3% |
| AD 3 | 70 | 7.86 | 550.2 |
Table 2: Protein amount in sarkosyl-insoluble fractions (P2). On average, the percentage of protein that partitions into the sarkosyl-insoluble fraction of control (n=3) and AD (n=3) brain was 10.4% and 11.3%, respectively. There was no statistically significant difference of the amount of sarkosyl-insoluble proteins from control and AD brain, as evidenced by the by student's t-test (p = 0.703) and standard error of the mean (S.E.M.) values.
Discussion
Herein we introduce and discuss an abbreviated single-step detergent-fractionation protocol that is applicable to a wide variety of experimental applications ranging from mass spectrometry-based proteomics analysis to functional protein misfolding assays and western blotting.5,6,7,10 This methodology is perhaps most effective when used to study neurodegenerative proteinopathies such as Alzheimer's, ALS, Huntington's disease, prion diseases and the various tauopathies. Compared to original five-step sequential fractionation protocol, this single-step procedure can be completed in a single day and affords very similar results.5,6,7,9,14,22
An important aspect of this protocol is the use of sarkosyl as the fractionation detergent. In contrast with other detergents like Triton X-100 or sodium dodecyl sulfate (SDS), sarkosyl appears to be well suited to this task; in terms of stringency and solubilizing strength, it is strong enough to solubilize the majority of natively folded proteins while preserving the detergent-insolubility, structure and function of disease-relevant protein aggregates that are inherently sensitive to SDS.5,9 Additionally, unlike SDS, sarkosyl remains soluble at low temperatures and in high salt conditions.
Another key feature of this protocol is the resuspension of the first sarkosyl-insoluble (P1) pellets following the first round of ultracentrifugation. This wash step allows the initial pellets (P1) to be thoroughly extracted and washed of any residual cross-contaminating soluble or total homogenate proteins, affording a pure and well-defined detergent-insoluble protein fraction. Without the optional pellet (P1) resuspension and wash step, residual soluble proteins may be carried-over into the detergent-insoluble fraction, possibly confounding subsequent results and experiments (i.e. immunoblotting or proteomics analysis).
The insoluble and high molecular weight nature of pathologic misfolded protein aggregates (i.e. tau, phospho-tau and Aβ species) present unique challenges toward their isolation and analysis via traditional biochemical techniques.5,6,7,8,9,12,17,21,26 Unlike soluble proteins, insoluble aggregates such as amyloids (Aβ) and hyperphosphorylated tau neurofibrillary tangles (NFTs) cannot be readily purified from postmortem tissue by traditional methods such as immunoprecipitation or fast protein liquid chromatography (FPLC).3,5,6,7,11,12,23,27 Thus, the abbreviated sarkosyl-fractionation protocol is one of the only techniques that allows for the facile enrichment and isolation of detergent-insoluble protein aggregates from postmortem brain within a comparatively short time-frame.5,6,8,13,15,17,21,27
While additional steps can be employed to isolate a pure, homogenous sample of aggregated protein, the sarkosyl fractionation protocol allows for the facile enrichment of detergent-insoluble protein aggregates with comparatively little time commitment.5,6 In support of this hypothesis, proteomic studies of the sarkosyl-insoluble proteome confirm that the vast majority of known pathologic aggregation-prone proteins partition into the sarkosyl-insoluble fraction-in both traditional multi-step6,11,21 as well as our novel single-step fractionation approach.5,7,9,10
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors thank Drs. Jim Lah and Allan Levey, Emory Department of Neurology, for helpful comments and suggestions. This work was partly funded by the Accelerating Medicine Partnership grant (U01AG046161-02), the Emory Alzheimer's Disease Research Center (P50AG025688) and a National Institute on Aging grant (R01AG053960-01) to N.T.S. This research was also supported in part by the Neuropathology Core of the Emory Neuroscience NINDS Core Facilities grant, P30NS055077.
References
- Taylor JP, Hardy J, Fischbeck KH. Toxic Proteins in Neurodegenerative Disease. Science. 1991;296(5575):1991. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nature Medicine. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Ramírez-Alvarado M, Merkel JS, Regan L. A systematic exploration of the influence of the protein stability on amyloid fibril formation in vitro. Proc. Natl. Acad. Sci. 2000;97(16):8979–8984. doi: 10.1073/pnas.150091797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamley IW. The Amyloid Beta Peptide: A Chemist's Perspective. Role in Alzheimer's and Fibrillization. Chem Rev. 2012;112(10):5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- Diner I, et al. Aggregation Properties of the Small Nuclear Ribonucleoprotein U1-70K in Alzheimer Disease. J. Biol. Chem. 2014;289(51):35296–35313. doi: 10.1074/jbc.M114.562959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal YM, et al. Proteomics Analysis Reveals Novel Components in the Detergent-Insoluble Subproteome in Alzheimer's Disease. J. Proteome Res. 2009;8(11):5069–5079. doi: 10.1021/pr900474t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, et al. Changes in the detergent-insoluble brain proteome linked to amyloid and tau in Alzheimer's Disease progression. PROTEOMICS. 2016. [DOI] [PMC free article] [PubMed]
- Julien C, Bretteville A, Planel E. In: Amyloid Proteins: Methods and Protocols. Sigurdsson EM, Calero M, Gasset M, editors. Humana Press; 2012. pp. 473–491. [Google Scholar]
- Nizhnikov AA, et al. Proteomic Screening for Amyloid Proteins. PLoS ONE. 2014;9(12):e116003. doi: 10.1371/journal.pone.0116003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried NT, et al. Quantitative analysis of the detergent-insoluble brain proteome in frontotemporal lobar degeneration using SILAC internal standards. J. Proteome Res. 2012;11(5):2721–2738. doi: 10.1021/pr2010814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjer RL, et al. Proteomic determination of widespread detergent insolubility, including Aβ but not tau, early in the pathogenesis of Alzheimer's disease. FASEB J. 2005. [DOI] [PubMed]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical Characterization of the Core Structure of α-Synuclein Filaments. J. Biol. Chem. 2002;277(21):19213–19219. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, et al. Phosphorylated α-Synuclein Is Ubiquitinated in α-Synucleinopathy Lesions. J. Biol. Chem. 2002;277(50):49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- Neumann M, et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science. 2006;314(5796):130. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Bishof I, Diner I, Seyfried N. An Intrinsically Disordered Low Complexity Domain is Required for U1-70K Self-association. FASEB J. 2015;29(Suppl 1) [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Noguchi A, et al. Isolation and Characterization of Patient-derived, Toxic, High Mass Amyloid β-Protein (Aβ) Assembly from Alzheimer Disease Brains. J. Biol. Chem. 2009;284(47):32895–32905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B, et al. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease. Proc. Natl. Acad. Sci. 2013;110(41):16562–16567. doi: 10.1073/pnas.1310249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Pettegrew JW. Alzheimer's β-Amyloid Protein Is Covalently Modified when Dissolved in Formic Acid. J Neurochem. 1990;54(6):2050–2056. doi: 10.1111/j.1471-4159.1990.tb04910.x. [DOI] [PubMed] [Google Scholar]
- Yang L-S, Gordon-Krajcer W, Ksiezak-Reding H. Tau Released from Paired Helical Filaments with Formic Acid or Guanidine Is Susceptible to Calpain-Mediated Proteolysis. J Neurochem. 1997;69(4):1548–1558. doi: 10.1046/j.1471-4159.1997.69041548.x. [DOI] [PubMed] [Google Scholar]
- Bai B, et al. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease. Proc Natl Acad Sci U S A. 2013;110(41):16562–16567. doi: 10.1073/pnas.1310249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, et al. U1 small nuclear ribonucleoproteins (snRNPs) aggregate in Alzheimer's disease due to autosomal dominant genetic mutations and trisomy 21. Mol Neurodegener. 2014;9(1):15. doi: 10.1186/1750-1326-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L-W, Raymond LD, Hayes SF, Raymond GJ, Caughey B. Conformational change, aggregation and fibril formation induced by detergent treatments of cellular prion protein. J Neurochem. 2001;79(3):669–678. doi: 10.1046/j.1471-4159.2001.00606.x. [DOI] [PubMed] [Google Scholar]
- Mirra SS, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Smith PK, et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Guo JL, et al. Unique pathological tau conformers from Alzheimer's brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016;213(12):2635–2654. doi: 10.1084/jem.20160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev SV, et al. A distinct subfraction of Aβ is responsible for the high-affinity Pittsburgh compound B-binding site in Alzheimer's disease brain. J Neurochem. 2014;131(3):356–368. doi: 10.1111/jnc.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]


