Abstract
Fatty acid synthase (FASN) is a key enzyme involved in fatty acid biosynthesis and serves an important role in breast cancer development. The aim of the present study was to investigate the effects of patuletin on the gene expression and activity of FASN in the human breast cancer SK-BR-3 cell line, and the apoptotic effects of patuletin to breast cancer cells. Quantitative reverse transcription polymerase chain reaction, western blotting and intracellular FASN activity assays were used to evaluate FASN gene expression, protein expression and activity in patuletin-treated SK-BR-3 cells. MTT assays and flow cytometry were used to measure cell growth and cell apoptosis, respectively, following patuletin treatment. As a result, it was demonstrated that patuletin dose-dependently reduces FASN expression and intracellular activity in human breast cancer cells, and induces apoptosis in FASN over-expressing SK-BR-3 cells. Notably, apoptosis is associated with the reduction of intracellular FASN activity. The present study demonstrates that patuletin may be considered as a novel natural inhibitor of FASN, may induce anti-proliferative and pro-apoptotic effects in certain human breast cancer cells and may be useful for preventing and/or treating human breast cancer.
Keywords: patuletin, fatty acid synthase, cancer, breast cancer cells, cell apoptosis
Introduction
Fatty acid synthase (FASN) is a multi-enzyme that catalyzes the de novo synthesis of palmitate (C16:0, a long-chain saturated fatty acid) from acetyl-CoA and malonyl-CoA, in the presence of NADPH (1). FASN is not only a key factor in the role of fatty acid biosynthesis for energy storage (2,3), but also its expression level increases significantly in adipose tissues and a variety of human carcinomas, including liver, breast, prostate, lung, endometrium, ovary, colon and pancreatic cancer (4–13). This prominent difference of FASN expression between normal and neoplastic tissues makes FASN a potential diagnostic tumor marker (14).
Breast cancer is the most common type of cancer and a leading cause of cancer-associated mortalities among females, with a common feature of abnormal cell apoptosis in its development (15). In addition, high levels of FASN expression has been demonstrated to be associated with poor clinical outcome in breast carcinomas, suggesting that FASN expression and tumor aggressiveness are closely associated (4,16). It was identified that obesity may serve a crucial role in the incidence and progression of breast cancer (17). According to the close association between FASN, obesity and breast cancer, the studies of FASN inhibitors have indicated their role as targets for chemotherapy in breast cancer and a novel strategy for antineoplastic intervention (18). In fact, previous studies demonstrated that certain synthetic and natural FASN inhibitors, including C75, desoxyrhaponticin, rhaponticin and α-mangostin may lead to selective cytotoxicity in FASN over-expressing cancer cell lines (18–20). This result suggested again that the pharmacological inhibition of FASN may represent a potential target for drug development.
In previous studies, a number of dietary polyphenols, including α-mangostin, resveratrol, curcumin and quercetin exhibited high inhibitory activity against FASN (21–30). Although the detailed mechanism of the inhibitory effect of polyphenols on FASN was not fully understood, the structure activity association analysis demonstrated that the flavonoids containing two hydroxyl groups in the B ring and 5, 7-hydroxyl groups in the A ring with C-2, 3 double bond were the most potent inhibitors on FASN (31). Patuletin (3,5,7,3′,4′-pentahydroxy-6-methoxy-flavone) (Fig. 1A), a natural flavonoid primarily present in the genus Eriocaulon (32), exhibits anti-inflammatory, anti-oxidant and anti-bacterial properties (33–36). However, no anti-neoplastic effects of patuletin have been identified at present. Increased expression of FASN has previously been demonstrated in different breast cancer cells (37). Among them, human breast cancer SK-BR-3 cells exhibited higher expression levels of FASN compared with other breast cancer cells, including MCF-7 and MDA-MB-231 cells. The present study aimed to identify for the first time that patuletin induces apoptosis in FASN over-expressing human breast cancer SK-BR-3 cells.
Figure 1.
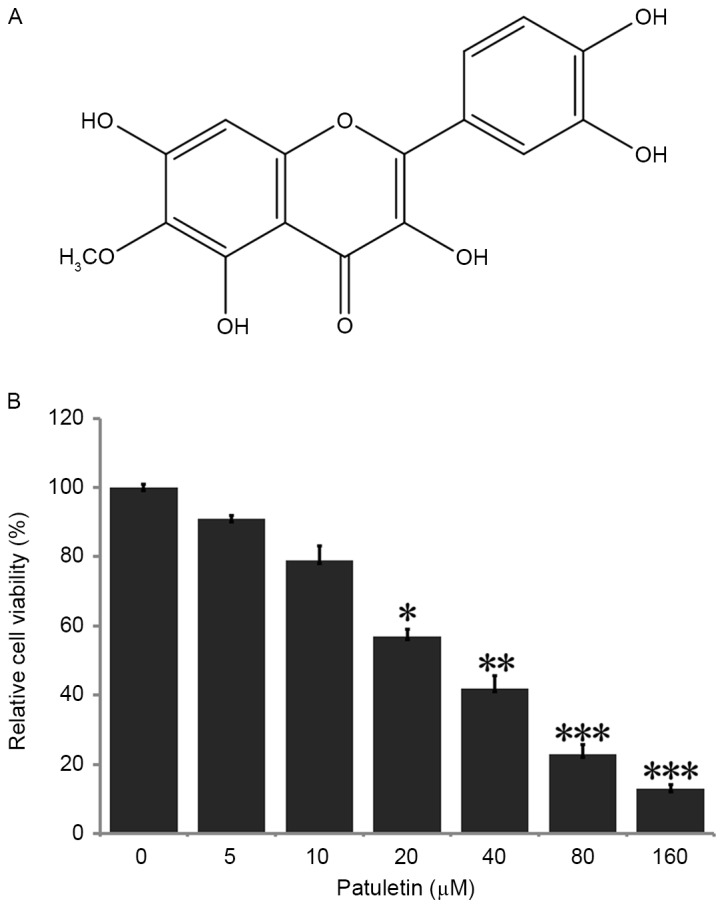
Patuletin dose-dependently inhibits the survival of SK-BR-3 cells. (A) Chemical structure of patuletin. (B) Cell survival rate was determined by MTT assay. SK-BR-3 cells were cultured with patuletin for 24 h at different concentrations of 5, 10, 20, 40, 80 and 160 µM. Bars represent means ± standard deviation. P-values were measured by one-way analysis with Dunnett's post-hoc test. *P<0.05 vs. 0 µM patuletin-treated group; **P<0.01 vs. 0 µM patuletin-treated group; ***P<0.001 vs. 0 µM patuletin-treated group.
Materials and methods
Reagents
Acetyl-Coenzyme A (CoA), Malonyl-CoA, dexamethasone, NADPH, ethyl acetate (EtOAc), chloroform, methanol, MTT, 3-isobutyl-1-methylxanthine (IBMX), EDTA and DTT were all purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit was purchased from BD Biosciences (San Jose, CA, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, PBS, penicillin-streptomycin, trypsin-EDTA, dimethyl sulfoxide (DMSO), TRIzol, SuperScript III First-Strand Synthesis system were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Rabbit antibodies against FASN (cat no. 3180) and β-actin (cat no. 4967) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Extraction and isolation of patuletin
The whole plants of Eriocaulon buergerianum were collected in Zhejiang (China) by the research group. Patuletin from air-dried whole plants of E. buergerianum (3.0 kg) was extracted with 95% ethanol at room temperature. The concentrated crude extract was dissolved in H2O and partitioned with EtOAc. The EtOAc portion (225 g) was chromatographed on a silica gel column eluting with a chloroform-methanol gradient system to yield patuletin (300 mg). Isolated patuletin was ≥98% pure as determined by HPLC-UV (Agilent Technologies, Inc., Santa Clara, CA, USA).
Cell culture
The human breast cancer SK-BR-3 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were incubated at 5% CO2 and 37°C in a medium containing 89% DMEM (high glucose), 10% bovine fetal serum and 100 U/ml penicillin-streptomycin. For passage, the cells were digested by 0.25% trypsin-EDTA every 4 days.
Cell viability assay
SK-BR-3 cells were seeded in 96-well plate firstly, at a density about 5×103 cells/well and then treated with purified patuletin in different concentrations (5, 10, 20, 40, 80 or 160 µM) for 24 h. Thereafter, 5 mg/ml MTT solution was added into each well and incubated for 4 h at 37°C. Then, the medium with MTT was aspirated, 200 µl DMSO/well was added to the wells and the cells were incubated for 15 min. Finally, the concentration was measured at 492 nm by a microplate spectrophotometer (BioTek China, Beijing, China). PBS was used as blank control, and cells without patuletin treatment were used as negative control.
Cell lysis and immunoblotting
Cells were lysed as previously described (38). Protein concentration of cell lysates was measured by the Pierce BCA protein assay kit using bovine serum albumin as a standard control. 50 µg protein was loaded per lane, separated by SDS-PAGE (12% gel), and then electrophoretically transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Then the protein samples were blocked with 5% skimmed milk for 1–2 h at room temperature to prevent nonspecific antibody binding, and probed with primary antibodies against FASN and β-actin at a dilution of 1:1,000 overnight at 4°C. Subsequently, membranes were washed twice with TBST (10 mM Tris, 10 mM NaCl, 0.1% Tween-20), and incubated 1 h at room temperature with corresponding peroxidase conjugated secondary antibody (cat no. 7074) and developed with a commercial enhanced chemiluminescence kit (West Pico chemiluminescent substrate; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) according to the manufacturer's protocol. Blots were probed with an antibody against β-actin as the control.
Cell apoptosis assay
Cell apoptosis detection was performed using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences) according to the manufacturer's protocol. First, cells were collected after 24 h treatment with patuletin at different concentrations (20, 40 and 80 µM). Then, cells (1×106 cells/tube) were washed twice with cold PBS and resuspended in 100 µl 1X binding buffer (Biomiga Inc., San Diego, CA, USA). Cell suspension was incubated with 5 µl Annexin V-FITC and 10 µl propidium iodide (PI) for 15 min at room temperature and kept in a dark place. Immediately following that, 400 µl 1X binding buffer was added and the cells were analyzed by a CellQuest Pro software (FACSstation 6.0; BD Biosciences) in a BD FACSCalibur™ flow cytometer (BD Biosciences) within 1 h. Those cells stained with Annexin V+/PI− were early apoptotic cells and those stained with Annexin V+/PI+ were late apoptotic cells.
FASN gene expression analysis
FASN gene expression analysis was performed in SK-BR-3 cells treated with patuletin at different concentrations (5, 10, 20, 40, 80 and 160 µM) for 24 h. Cells were washed with PBS twice for RNA extraction. Total RNA was isolated from SK-BR-3 cells using TRIzol reagent (Thermo Fisher Scientific, Inc.), following the manufacturer's protocol. A total of ~2 µg RNA was reverse transcribed into complementary DNA (cDNA) using SuperScript III First-Strand Synthesis system (Thermo Fisher Scientific, Inc.), from the control and treated cells. Polymerase chain reaction (PCR) was performed in 20 µl of the final volume, using primers for analyses of the FASN and β-actin genes. The conditions for PCR were as follows: Initial denaturation at 95°C for 5 min and followed by 45 cycles (95°C for 15 sec, 55°C for 15 sec, 72°C for 20 sec). (FASN sense, 5′-TATGCTTCTTCGTGCAGCAGTT-3′ and antisense, 5′-GCTGCCACACGCTCCTCTAG-3′; β-actin sense, 5′-AAAGACCTGTACGCCAACACAGTGCTGTCTGG-3′ and antisense, 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′) The β-actin gene, which is a housekeeping gene, was used as an internal control, and samples without reverse transcription were used as negative control. Quantitative PCR was performed in 25 µl final volume containing 2 µl cDNA, SYBR Green Master Mix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) on a 7500 Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). FASN gene and β-actin gene expression levels were determined with the comparative Cq method in triplicate experiments (39).
Intracellular fatty acids assay
SK-BR-3 cells were collected after 24 h treatment with patuletin at different concentrations (20, 40, 80 µM, respectively). Then, cells were washed twice with cold PBS and extracted by homogenization with pure chloroform containing 1% Triton X-100 (Sigma-Aldrich; Merck KGaA). The extract was centrifuged at 10,800 × g for 5–10 min at 4°C, to collect the organic phase. Next, the organic phase was air and vacuum dried to remove chloroform. The dissolved dried lipids were applied to detect the amount of intracellular fatty acid by Fatty Acid Assay kit (BioVision, Inc., Milpitas, CA, USA), following the manufacturer's protocol. The fatty acids concentration was measured at 570 nm by a microplate spectrophotometer.
Cell FASN activity assay
Intracellular FASN activity was assessed as described previously (40). SK-BR-3 cells were harvested and collected in cold assay buffer containing 100 mM potassium phosphate buffer, 1 mM EDTA, 0.6 mM PMSF and 1 mM dithiolthreitol (pH 7.0). Then, the cell suspension was centrifuged at 10,800 × g for 30 min at 4°C, and the supernatant was collected for the overall reaction assay. A total of 25 ml supernatant was added into the reaction mix containing 25 mM KH2PO4-K2HPO4 buffer, 0.25 mM EDTA, 0.25 mM dithiothreitol, 30 mM acetyl-CoA, 100 mM malonyl-CoA, 350 mM NADPH (pH 7.0) to a total volume of 200 ml. The protein content in the supernatant was determined using a bicinchoninic acid (BCA) assay (Pierce; Thermo Fisher Scientific, Inc.) and results were expressed as the specific activity of FASN at the same protein concentration.
Quantification of fatty acid
Following treatment with patuletin at the corresponding concentrations (0, 20, 40 and 80 µM), cells were harvested using trypsin-EDTA, washed twice with PBS, and stored at −80°C. The amount of intracellular fatty acid was determined with a Free Fatty Acid Assay kit (Sigma-Aldrich; Merck KGaA), according to the manufacturer's protocol.
Statistical analysis
All values are presented as mean ± standard deviation. To determine if differences between experimental and control groups existed in cancer cell viability, apoptosis, FASN gene expression and activity and in intracellular fatty acids concentration, the results were evaluated and analyzed by one-way analysis of variance and the Dunnett's post-hoc test using GraphPad software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibitory effects of patuletin on the viability of SK-BR-3 cells
To identify whether patuletin affected the viability of the breast cancer SK-BR-3 cell line, cells were treated with 0–160 µM patuletin for 24 h, and following this, the ability of cell survival was examined by MTT assay. As demonstrated in Fig. 1B, SK-BR-3 cell viability was reduced significantly subsequent to treatment with 20, 40, 80 and 160 µM patuletin. When compared with the negative control (0 µM patuletin), cell survival rate was markedly reduced to 13% following treatment with 160 µM patuletin. Patuletin demonstrated high inhibition of cell population in a dose-dependent manner, with a half-maximal inhibitory concentration (IC50) value of 24 µM.
Patuletin reduces gene expression of FASN in SK-BR-3 cells
The effect of patuletin on FASN gene expression in SK-BR-3 cells was measured by PCR and reverse transcription-PCR. As demonstrated in Fig. 2, treatment of SK-BR-3 cells for 24 h with increasing concentrations of patuletin (from 5–160 µM) resulted in a significant reduction in FASN mRNA expression.
Figure 2.
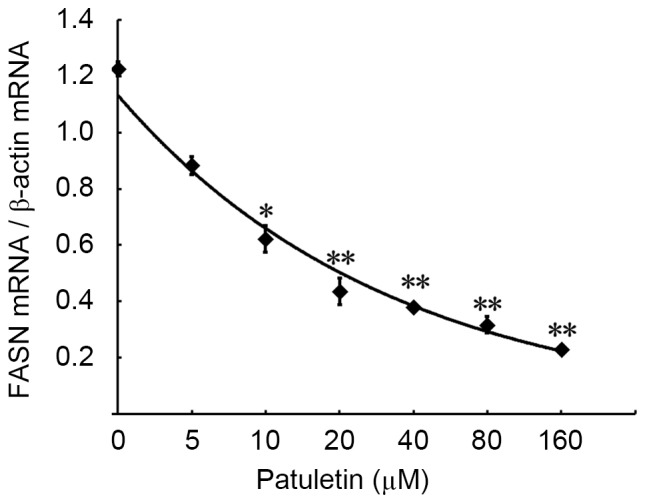
Inhibitory effects of patuletin on FASN mRNA levels in SK-BR-3 cells. The effect of patuletin on FASN gene expression in SK-BR-3 cells was measured by quantitative reverse transcription PCR. Increasing concentrations of patuletin, incubated with SK-BR-3 cells for 24 h, resulted in a concentration-associated reduction on FASN gene expression. P-values were measured by one-way analysis with Dunnett's post-hoc test. *P<0.05 vs. 0 µM patuletin-treated group; **P<0.01 vs. 0 µM patuletin-treated group. FASN, fatty acid synthase; PCR, polymerase chain reaction.
Patuletin inhibits intracellular FASN activity in SK-BR-3 cells
The effect of patuletin on the FASN activity in SK-BR-3 cells was measured by western blotting analysis. As demonstrated in Fig. 3A, SK-BR-3 cells treated with patuletin for 24 h exhibited much lower levels of FASN compared with the control. When SK-BR-3 cells were treated with patuletin at different concentrations for 24 h, intracellular FASN activity was significantly reduced to 65.0, 34.3 and 20.6%, respectively (P<0.05, P<0.01 and P<0.001, respectively; Fig. 3B). This suggests that intracellular FASN activity was significantly suppressed by patuletin, and that the inhibition was dose-dependent.
Figure 3.
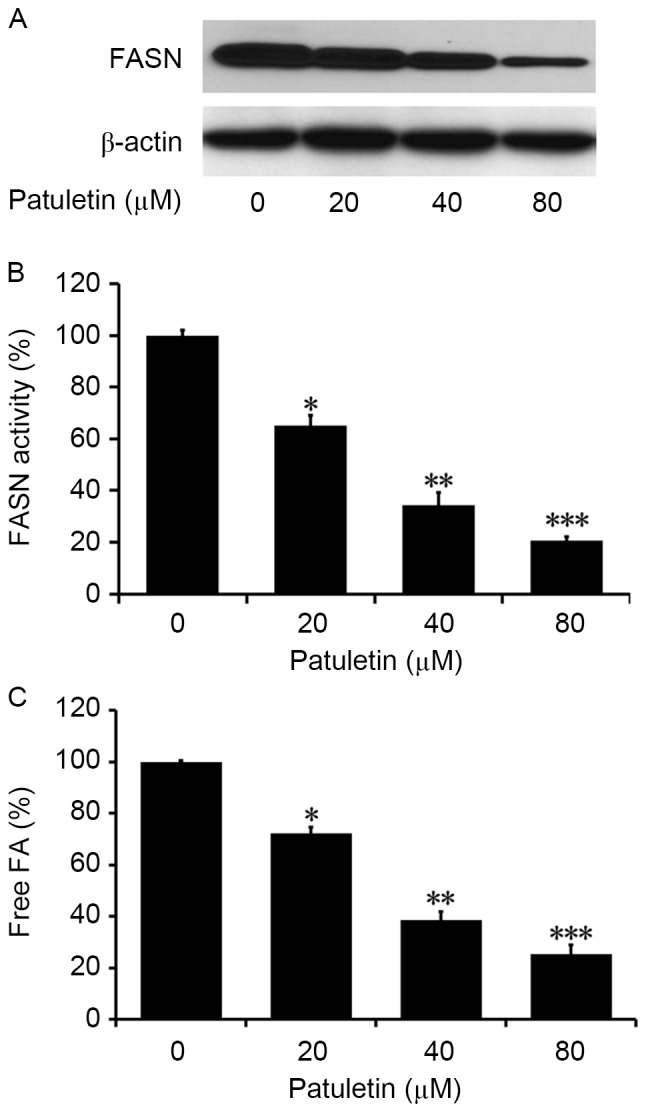
Inhibitory effects of patuletin on intracellular FASN expression and activity. SK-BR-3 cells were treated with 20, 40 and 80 µM patuletin for 24 h, and then analyzed by different methods. Data were normalized to control cells without patuletin (0 µM). (A) Effect of patuletin on FASN protein expression analyzed by western blotting. (B) Intracellular FASN activity assay. Relative FASN activity was presented as means ± SD. *P<0.05 vs. with 0 µM patuletin-treated group; **P<0.01 vs.0 µM patuletin-treated group; ***P<0.001 vs. 0 µM patuletin-treated group. (C) The amount of intracellular FA was measured by Fatty Acid Assay kit. Data was presented as means ± SD. (n=3). *P<0.05 vs. 0 µM patuletin-treated group; **P<0.01 vs. 0 µM patuletin-treated group; ***P<0.001 vs. 0 µM patuletin-treated group. FASN, fatty acid synthase; SD, standard deviation; FA, fatty acid.
Patuletin reduced intracellular fatty acids in SK-BR-3 cells
The levels of intracellular fatty acids in SK-BR-3 cells treated with 20, 40 and 80 µM patuletin was measured by Fatty Acids Assay kit. As demonstrated in Fig. 3C, compared with the control (0 µM patuletin), the levels of intracellular fatty acids in treated cells significantly decreased to 63.8, 52.4 and 31.2% (P<0.05, P<0.01 and P<0.001, respectively).
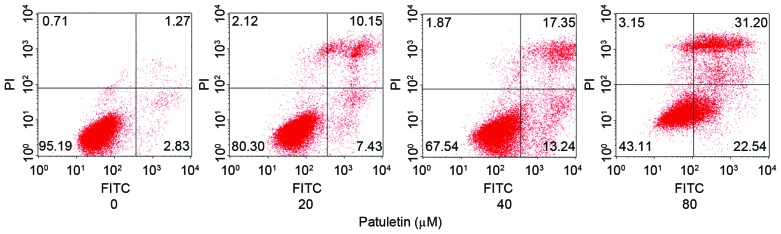
Patuletin induced SK-BR-3 cells apoptosis
The apoptotic rate of SK-BR-3 cells following 20, 40 and 80 µM patuletin treatment for 24 h was measured using a Annexin V-FITC Apoptosis Detection kit and analyzed by flow cytometry. As demonstrated in Fig. 4, patuletin markedly induced SK-BR-3 cell apoptosis in a dose-dependent manner.
Figure 4.
Apoptotic effect of patuletin on SK-BR-3 cells. SK-BR-3 cells were treated with 0, 20, 40 and 80 µM patuletin for 24 h, and then the apoptotic fraction of the SK-BR-3 cells was detected by Annexin V-FITC and PI double staining. The concentrations of patuletin in each part of the figure (left-right) were 0, 20, 40 and 80 µM, respectively. FITC, fluorescein isothiocyanate; PI propidium iodide.
Discussion
The present study focuses on the effects of patuletin on FASN gene expression and activity in human breast cancer SK-BR-3 cells. To the best of our knowledge, it was demonstrated for the first time that patuletin dose-dependently decreases the gene and protein expression levels of FASN and its activity in the human breast cancer SK-BR-3 cell line. In addition, this natural flavone markedly inhibits cell proliferation and induces apoptosis in SK-BR-3 cells.
Natural polyphenolic compounds include of a wide variety of biologically active compounds, a number of which have been suggested to exhibit antineoplastic properties (40–42). However, the anti-cancer activity of patuletin has not yet been examined, to the best of our knowledge. In the present study, the inhibition of FASN activity was associated with the apoptosis of cancer cells, which suggested that efficient FASN inhibitors may be potential target drugs for the treatment of cancer. It was also demonstrated that the natural polyphenolic compound patuletin may inhibit intracellular FASN activity, and therefore induce breast cancer cell apoptosis.
FASN is a key multi-enzyme that catalyzes fatty acid synthesis. The expression level of FASN is relatively low in the majority of normal tissues, however; increased expression of FASN has been identified in human breast cancer cells, particularly in SK-BR-3 cells (37). According to previous studies on tumor proliferation, FASN may contribute to the generation of tumor cell membranes (43). Therefore, FASN inhibitors such as C75 and orlistat are promising potential anti-cancer drugs for the prevention and/or treatment of a variety of cancers such as cervical, prostate, leukemia and colon cancer (44–46). It is essential to identify more effective FASN inhibitors that may be applied practically as chemotherapeutic drugs.
The present study identified that patuletin not only downregulated mRNA and protein expression of FASN, but also demonstrated a high inhibitory activity on intracellular FASN. The decrease of intracellular FASN activity and fatty acids levels in SK-BR-3 cells revealed that patuletin acted on FASN as an inhibitory target. The intracellular activity of FASN directly affected the amount of intracellular fatty acids as FASN serves a key role in de novo fatty acid biosynthesis.
Like certain FASN inhibitors such as C75 and cerulenin (18), patuletin has been suggested to induce apoptosis of breast cancer cells. The results demonstrated that the apoptotic ratio of patuletin treated SK-BR-3 cells increased from 4.10% (control) to 53.74% (80 µM patuletin). The mechanism of cancer cell apoptosis through the inhibition of intracellular FASN expression may be explained by accumulating malonyl-CoA, which was considered as a trigger of cancer cell death and apoptosis (47,48). The present study also concluded that the signal pathways in cancer cell apoptosis exhibit close associations with the inhibition of FASN, therefore FASN inhibitors may be ideal drugs for the treatment of cancer.
In conclusion, patuletin induced apoptosis in breast cancer SK-BR-3 cells via inhibiting intracellular FASN activity and downregulating the mRNA and protein expression levels of FASN. As patuletin demonstrated a significant promotion of apoptosis in SK-BR-3 cells, it exhibits potential for application as an anti-cancer drug candidate for the treatment of human breast cancers.
Acknowledgements
The present study was sponsored by Natural Science Foundation of Heilongjiang Province (grant no. C201439), Heilongjiang Postdoctoral Fund (grant no. LBH-Z13142) and China Postdoctoral Science Foundation (grant no. 2014M551267).
References
- 1.Wakil SJ. Fatty-acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 2.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–2120. [PubMed] [Google Scholar]
- 3.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Alo' PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474–482. doi: 10.1002/(SICI)1097-0142(19960201)77:3<474::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W, Verhoeven G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 6.Pizer ES, Lax SF, Kuhajda FP, Pasternack GR, Kurman RJ. Fatty acid synthase expression in endometrial carcinoma: Correlation with cell proliferation and hormone receptors. Cancer. 1998;83:528–537. doi: 10.1002/(SICI)1097-0142(19980801)83:3<528::AID-CNCR22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 7.Gansler TS, Hardman W, III, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28:686–692. doi: 10.1016/S0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 8.Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150:201–208. [PMC free article] [PubMed] [Google Scholar]
- 9.Orita H, Coulter J, Tully E, Kuhajda FP, Gabrielson E. Inhibiting fatty acid synthase for chemoprevention of chemically induced lung tumors. Clin Cancer Res. 2008;14:2458–2464. doi: 10.1158/1078-0432.CCR-07-4177. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Nie F, Ouyang J, Wang X, Ma X. Inhibitory effects of sea buckthorn procyanidins on fatty acid synthase and MDA-MB-231 cells. Tumor Biol. 2014;35:9563–9569. doi: 10.1007/s13277-014-2233-1. [DOI] [PubMed] [Google Scholar]
- 11.Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, Murari R, Zotti G, Di Tondo U. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27:2523–2527. [PubMed] [Google Scholar]
- 12.Fan H, Tian W, Ma X. Curcumin induces apoptosis of HepG2 cells via inhibiting fatty acid synthase. Target Oncol. 2014;9:279–286. doi: 10.1007/s11523-013-0286-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Tian WX, Ma XF. Inhibitory effects of onion (Allium cepa L.) extract on proliferation of cancer cells and adipocytes via inhibiting fatty acid synthase. Asian Pac J Cancer Prev. 2012;13:5573–5579. doi: 10.7314/APJCP.2012.13.11.5573. [DOI] [PubMed] [Google Scholar]
- 14.Walter K, Hong SM, Nyhan S, Canto M, Fedarko N, Klein A, Griffith M, Omura N, Medghalchi S, Kuhajda F, Goggins M. Serum fatty acid synthase as a marker of pancreatic neoplasia. Cancer Epidemiol Biomarkers Prev. 2009;18:2380–2385. doi: 10.1158/1055-9965.EPI-09-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsburg OM, Love RR. Breast cancer: A neglected disease for the majority of affected women worldwide. Breast J. 2011;17:289–295. doi: 10.1111/j.1524-4741.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swinnen JV, Heemers H, Deboel L, Foufelle F, Heyns W, Verhoeven G. Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Oncogene. 2000;19:5173–5181. doi: 10.1038/sj.onc.1203889. [DOI] [PubMed] [Google Scholar]
- 17.Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Bustos M, Martínez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: A molecular nutrition approach. Biochim Biophys Acta. 2011;1807:664–678. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Kuhajda FP. Fatty acid synthase and cancer: New application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 19.Li P, Tian W, Wang X, Ma X. Inhibitory effect of desoxyrhaponticin and rhaponticin, two natural stilbene glycosides from the Tibetan nutritional food Rheum tanguticum Maxim. ex Balf., on fatty acid synthase and human breast cancer cells. Food Funct. 2014;5:251–256. doi: 10.1039/C3FO60484E. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Tian W, Ma X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol Cancer. 2014;13:138. doi: 10.1186/1476-4598-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan H, Wu D, Tian W, Ma X. Inhibitory effects of tannic acid on fatty acid synthase and 3T3-L1 preadipocyte. Biochim Biophys Acta. 2013;1831:1260–1266. doi: 10.1016/j.bbalip.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Ma X, Tian W. Pomegranate husk extract, punicalagin and ellagic acid inhibit fatty acid synthase and adipogenesis of 3T3-L1 adipocyte. J Funct Food. 2013;5:633–641. doi: 10.1016/j.jff.2013.01.005. [DOI] [Google Scholar]
- 23.Quan X, Wang Y, Ma X, Liang Y, Tian W, Ma Q, Jiang H, Zhao Y. α-Mangostin induces apoptosis and suppresses differentiation of 3T3-L1 cells via inhibiting fatty acid synthase. PLoS One. 2012;7:e33376. doi: 10.1371/journal.pone.0033376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang HZ, Ma QY, Fan HJ, Liang WJ, Huang SZ, Dai HF, Wang PC, Ma XF, Zhao YX. Fatty acid synthase inhibitors isolated from Punica granatum L. J Braz Chem Soc. 2012;23:889–893. doi: 10.1590/S0103-50532012000500014. [DOI] [Google Scholar]
- 25.Jiang HZ, Quan XF, Tian WX, Hu JM, Wang PC, Huang SZ, Cheng ZQ, Liang WJ, Zhou J, Ma XF, Zhao YX. Fatty acid synthase inhibitors of phenolic constituents isolated from Garcinia mangostana. Bioorg Med Chem Lett. 2010;20:6045–6047. doi: 10.1016/j.bmcl.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 26.Liang Y, Tian W, Ma X. Inhibitory effects of grape skin extract and resveratrol on fatty acid synthase. BMC Complement Altern Med. 2013;13:361. doi: 10.1186/1472-6882-13-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie F, Liang Y, Xun H, Sun J, He F, Ma X. Inhibitory effects of tannic acid in the early stage of 3T3-L1 preadipocytes differentiation by down-regulating PPARγ expression. Food Funct. 2015;6:894–901. doi: 10.1039/C4FO00871E. [DOI] [PubMed] [Google Scholar]
- 28.Jiang B, Liang Y, Sun X, Liu X, Tian W, Ma X. Potent inhibitory effect of chinese dietary spices on fatty acid synthase. Plant Foods Hum Nutr. 2015;70:257–262. doi: 10.1007/s11130-015-0486-5. [DOI] [PubMed] [Google Scholar]
- 29.Zeng XF, Li WW, Fan HJ, Wang XY, Pan J, Wang ZR, Ma S, Li LL, Ma XF, Yang SY. Discovery of novel fatty acid synthase (FAS) inhibitors based on the structure of ketoaceyl synthase (KS) domain. Bioorg Med Chem Lett. 2011;21:4742–4744. doi: 10.1016/j.bmcl.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 30.Zhao YX, Liang WJ, Fan HJ, Ma QY, Tian WX, Dai HF, Jiang HZ, Li N, Ma XF. Fatty acid synthase inhibitors from the hulls of Nephelium lappaceum L. Carbohydrate Res. 2011;346:1302–1306. doi: 10.1016/j.carres.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Tian WX. Inhibition of fatty acid synthase by polyphenols. Curr Med Chem. 2006;13:967–977. doi: 10.2174/092986706776361012. [DOI] [PubMed] [Google Scholar]
- 32.Yasukawa K, Kasahara Y. Effects of flavonoids from French marigold (Florets of Tagetes patula L.) on acute inflammation model. Int J Inflam. 2013;2013:309493. doi: 10.1155/2013/309493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koleckar V, Brojerova E, Rehakova Z, Kubikova K, Cervenka F, Kuca K, Jun D, Hronek M, Opletalova V, Opletal L. In vitro antiplatelet activity of flavonoids from Leuzea carthamoides. Drug Chem Toxicol. 2008;31:27–35. doi: 10.1080/01480540701688444. [DOI] [PubMed] [Google Scholar]
- 34.Fang JJ, Ye G, Chen WL, Zhao WM. Antibacterial phenolic components from Eriocaulon buergerianum. Phytochemistry. 2008;69:1279–1286. doi: 10.1016/j.phytochem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Könczöl A, Engel R, Szabó K, Hornok K, Tóth S, Béni Z, Prechl A, Máthé I, Balogh Tibor G. Topical analgesic, anti-inflammatory and antioxidant properties of Oxybaphus nyctagineus: Phytochemical characterization of active fractions. J Ethnopharmacol. 2014;155:776–784. doi: 10.1016/j.jep.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Mao W, Cao X, Liang S, Ding Z, Li N. Inhibition of rat lens aldose reductase by quercetagetin and patuletin. Yan Ke Xue Bao. 1991;7(29–30):33. [PubMed] [Google Scholar]
- 37.Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, Park BW, Kim KS. Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem. 2007;282:26122–26131. doi: 10.1074/jbc.M702854200. [DOI] [PubMed] [Google Scholar]
- 38.Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. P Natl Acad Sci USA. 2004;101:147–152. doi: 10.1073/pnas.0307075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Fan H, Liang Y, Jiang B, Li X, Xun H, Sun J, He W, Lau HT, Ma X. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol Rep. 2016;35:2651–2656. doi: 10.3892/or.2016.4682. [DOI] [PubMed] [Google Scholar]
- 41.Adaramoye O, Erguen B, Nitzsche B, Höpfner M, Jung K, Rabien A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem Biol Interact. 2017;274:100–106. doi: 10.1016/j.cbi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Szeja W, Grynkiewicz G, Rusin A. Isoflavones, their glycosides and glycoconjugates. synthesis and biological activity. Curr Org Chem. 2017;21:218–235. doi: 10.2174/1385272820666160928120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menendez JA, Mehmi I, Atlas E, Colomer R, Lupu R. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-κB. Int J Oncol. 2004;24:591–608. [PubMed] [Google Scholar]
- 44.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 45.Cioccoloni G, Bonmassar L, Pagani E, Caporali S, Fuggetta MP, Bonmassar E, D'Atri S, Aquino A. Influence of fatty acid synthase inhibitor orlistat on the DNA repair enzyme O6-methylguanine-DNA methyltransferase in human normal or malignant cells in vitro. Int J Oncol. 2015;47:764–772. doi: 10.3892/ijo.2015.3025. [DOI] [PubMed] [Google Scholar]
- 46.Rae C, Haberkorn U, Babich JW, Mairs RJ. Inhibition of fatty acid synthase sensitizes prostate cancer cells to radiotherapy. Radiat Res. 2015;184:482–493. doi: 10.1667/RR14173.1. [DOI] [PubMed] [Google Scholar]
- 47.Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- 48.Zhou W, Simpson PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, Pinn ML, Ronnett GV, Kuhajda FP. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003;63:7330–7337. [PubMed] [Google Scholar]