Abstract
A fascinating difference between teleost and mammals is the lifelong potential of the teleost retina for retinal neurogenesis and regeneration after severe damage. Investigating the regeneration pathways in zebrafish might bring new insights to develop innovative strategies for the treatment of retinal degenerative diseases in mammals. Herein, we focused on the induction of a focal lesion to the outer retina in adult zebrafish by means of a 532 nm diode laser. A localized injury allows investigating biological processes that take place during retinal degeneration and regeneration directly at the area of damage. Using non-invasive optical coherence tomography (OCT), we were able to define the location of the damaged area and monitor subsequent regeneration in vivo. Indeed, OCT imaging produces high-resolution, cross-sectional images of the zebrafish retina, providing information which was previously only available with histological analyses. In order to confirm the data from real-time OCT, histological sections were performed and regenerative response after the induction of the retinal injury was investigated by immunohistochemistry.
Keywords: Neurobiology, Issue 128, Cellular biology, zebrafish, laser treatment, retina, degeneration, regeneration, Müller cells, optical coherence tomography
Introduction
Vision is probably the most essential sense of the human being and its impairment has a high socio-economic impact. In the industrialized world, retinal degenerative diseases account for the majority of vision loss and blindness among the adult population1. Retinitis pigmentosa (RP) is the most common inherited cause of blindness in people between the ages of 20 and 60, affecting approximately 1.5 million people worldwide2,3. It is a heterogeneous family of inherited retinal disorders characterized by progressive loss of the photoreceptors (PRs) followed by degeneration of retinal pigment epithelium and, subsequently, gliosis and remodeling of inner neurons4. The course of the disease can be explained by the incremental loss of the two PR cell types, usually starting with rods, which are responsible for achromatic vision in dim light, and cones, which are essential for color vision and visual acuity5. A single gene defect is sufficient to cause RP. So far more than 130 mutations in over 45 genes have been associated with the disease6. This leads to varying disease phenotypes and is one reason that gene therapy is non-generalizable and thus an intricate therapeutic approach. Therefore, there is an urgent need to develop new general therapeutic approaches to treat retinal degenerations in blinding diseases.
Retinal degeneration often involves PR loss; therefore, PR cell death is a hallmark of the degenerative processes in the retina7. It has already been demonstrated that PR cell death stimulates Müller glia cell (MC) activation and proliferation8. MCs, the major glial cell type in the vertebrate retina, were once considered to be nothing more than a "glue" between retinal neurons. In recent years, many studies have shown that MCs act as more than mere structural support9. Among the different functions, MCs participate also in neurogenesis and repair10. Indeed, in response to diffusible factors from the degenerating retina, MCs significantly increase glial fibrillary acidic protein (GFAP) expression. Therefore, GFAP labelling can be used as a marker for MC activation as a secondary response to retinal injury and degeneration11.
Recently, we developed a novel adaptation of focal injury using a laser to induce retinal degeneration in zebrafish (Danio rerio). Focal injury is advantageous for studying certain biological processes such as the migration of cells into the injured site and the precise timing of events that take place during retinal regeneration12. Furthermore, the zebrafish has become important in visual research because of the similarities between its visual system and that of other vertebrates. Gross morphological and histological features of human and teleost retinae display few differences. Accordingly, human and zebrafish retinae contain the same major cell classes organized in the same layered pattern, where light-sensing photoreceptors occupy the outermost layer, while the retinal projection neurons, the ganglion cells, reside in the innermost neuronal layer, proximal to the lens. The retinal interneurons, the amacrine, bipolar, and horizontal cells, localize in between the photoreceptor and ganglion cell layers13. Furthermore, the zebrafish retina is cone-dominated and therefore closer to the human retina than, for example, the intensively studied rodent retina. A fascinating difference between teleost and mammals is the persistent neurogenesis in fish retina and retinal regeneration after damage. In zebrafish, MCs can dedifferentiate and mediate regeneration in injured retina14,15. In chicken, MCs have some capacity also to re-enter the cell cycle and to dedifferentiate. Following retinal injury in adult fish, MCs adopt certain characteristics of progenitor and stem cells, migrate to the damaged retinal tissue and produce new neurons16. Gene expression profiling of mammalian MCs revealed unexpected similarities to retinal progenitors, and evidence for intrinsic neurogenic potential of MCs in the chicken, rodent, and even human retina is growing17. Nevertheless, why the regenerative response in birds and mammals is lower compared with the robust response in fish is not yet understood. Therefore, understanding the endogenous repair mechanisms in zebrafish may suggest strategies for stimulating retinal regeneration in mammals and humans. Employing the endogenous repair mechanism of MCs as a therapeutic tool for the treatment of patients with retinal degeneration would have an outstanding impact for our society.
Herein, we provide the steps necessary to employ the degeneration/regeneration model in ophthalmic research. We focused first on inducing focal damage in the neurosensory retina, then on the imaging of events developing at the injury site, and finally visualizing involvement of the adjacent MCs. The general protocol is relatively easy to perform and opens a wide variety of possibilities for evaluating the retina afterwards.
Protocol
All experiments adhered to the Statement for the Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology (ARVO) and respect the related regulations of the governmental authorities.
1. Animals
Maintain TgBAC (gfap:gfap-GFP) zebrafish 167 (AB) strain aged between 6 - 9 months under standard conditions in water with a temperature of 26.5 °C and a 14/10 h light/dark cycle18.
Follow the animal care guidelines of the involved institutions for the animal experiments after approval by the governmental authorities.
2. Reversible Systemic Anesthesia
Prepare the stock solution of ethyl 3-aminobenzoate methanesulfonate salt (tricaine) by dissolving 400 mg of tricaine powder in 97.9 mL of tank water and 2.1 mL of 1 M of Tris buffered saline (TBS). Adjust to pH 7.0 with 1 M Tris (pH 9) and store at 4 °C in the dark up to one month. NOTE: Tricaine should be prepared in water like the natural living conditions of the animal, preferably original tank water should be used.
Dilute the stock solution 1:25 in tank water and use immediately.
Place the zebrafish into a 10 cm Petri dish containing 50 mL of anesthesia solution until they become immobile and do not respond to external stimuli (approximately 2 - 5 min, depending on weight and age).
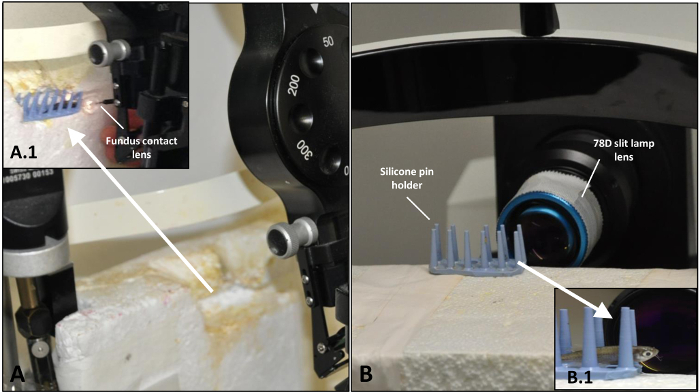
Transfer each fish by hand to a custom-made silicone pin holder for laser treatment (Figure 1A). CAUTION! The fish can remain anesthetized outside of the tank for up to 10 min only.
To reverse anesthesia after the treatment and/or imaging, place the zebrafish in container containing tank water.
To support recovery, create a flow of fresh tank water over the gills by moving the zebrafish back and forth in the water.
3. Laser Focal Injury on Retina
NOTE: A 532 nm diode laser is used to create focal light damage on the retina of the zebrafish. The experimental set-up of the laser enables the establishment of a reproducible focal retinal injury in adult zebrafish.
Set up the output power of the laser: 70 mW; aerial diameter: 50 µm; pulse duration: 100 ms. CAUTION! The use of laser light requires appropriate personal protection and labelling of the area.
Apply 1 - 2 drops of 2% hydroxypropylmethylcellulose topically in the eye before treatment and use a 2.0 mm fundus laser lens to focus the laser-aiming beam on the retina. CAUTION! hydroxypropylmethylcellulose drops are viscous and may cause problems in breathing if it goes on the gills.
Place four laser spots around the optic nerve on the left eye and use the right, untreated eye as internal control.
4. In vivo Imaging of the Retinal Morphology
On day 0, visualize the zebrafish retina directly after the laser induction without reviving them from anesthesia. At all other time points, employ general anesthesia (see Section 2: Reversible systemic anesthesia). Place the immobilized zebrafish on a custom-made silicone pin holder (Figure 1B, B.1).
To obtain optimal images, cut a commercially available hydrogel contact lens to fit the zebrafish eye (Ø = 5.2 mm, r = 2.70 mm, center thickness = 0.4 mm) by means of a hole punch. Fill the concave surface of the lens with methylcellulose and place it over the cornea.
Equip the OCT system with a 78D non-contact slit lamp lens.
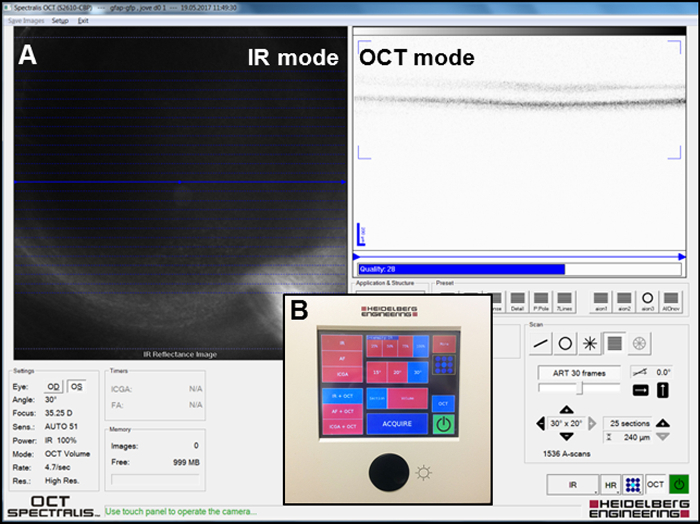
Focus the infrared (IR) image in the IR + OCT mode (Figure 2A) to visualize the fundus of the eye and take the IR pictures by clicking the "Acquire" button (Figure 2B) to localize the laser spots on the retina using the system's software.
Visualize a three-dimensional section of the retinal layers in the IR + OCT mode and take the pictures by clicking the "Acquire" button (Figure 2B). Observe the severity of injury in the outer nuclear layer (ONL) (see Section 3: Laser focal injury on retina) in these images.
To reverse anesthesia after the treatment and/or imaging place the zebrafish in a container containing tank water.
To support recovery, create a flow of fresh tank water over the gills by moving the zebrafish back and forth in the water.
Perform similar in vivo imaging of retinal morphology on day 1, 3, 7, 14 and week 6.
5. Hematoxylin & Eosin (H&E) Staining
Euthanize zebrafish by submersion into cold (4 °C) anesthesia solution on ice for at least 10 min and enucleate the eyes immediately by means of small curved forceps.
Fix the whole eyes in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) at 4 °C for 20 h and then dehydrate the samples in a graded alcohol series (xylene 100% for 5 min twice, ethanol 100% for 5 min twice, ethanol 96% for 3 min twice and ethanol 70% 3 min once). CAUTION! PFA can be irritating to the eyes, nose, and upper respiratory track. PFA is a known human carcinogen and a suspected reproductive hazard.
Embed the samples in paraffin, cut 5 µm sections at the level of the optic nerve head and mount them on glass slides.
Stain the deparaffinized sections with 0.1% acid hematoxylin solution for 5 min and dip the slides two times in distilled water after dipping the slides in hydrochloric acid mix (2 mL 25% HCl in 250 mL distilled water) and ammonia mix (2 mL 25% ammonia in 250 mL distilled water). Stain the sections with eosin G aqueous solution 0.5% for 3 min after development of the hematoxylin staining by tap water for at least 10 min.
Mount the dehydrated slides in acrylic resin mounting medium and observe the slides under the light microscope.
6. Immunohistochemistry for the MC Activation
Heat the deparaffinized sections in antigen retrieval buffer (Tris-EDTA + 0.05% non-ionic detergent, pH 9.0) for 3 min in an appropriate steamer or a microwave for 10 - 15 min and wash three times with TBS for 5 min each.
Circle the sections with a silicone pen and add 100 µL blocking solution (TBS + 10% goat normal serum + 1% bovine serum albumin, pH 7.6) at room temperature for 1 h.
Stain with anti-glial fibrillary acidic protein (GFAP) rabbit polyclonal antibody and with anti-glutamine synthetase (GS) mouse monoclonal antibody, both in a 1:200 dilution (40 µL per sample). Incubate the slide in a humidified chamber at 4 °C overnight. Wash three times with TBS + 0.1 % Tween 20 for 5 min each.
Finish visualization with the appropriate secondary antibodies. This protocol used a goat anti-rabbit IgG H&L green secondary antibody for GFAP and a goat anti-mouse IgG H&L bright red for GS, both in a 1:500 dilution at room temperature for 1 h.
Mount the slides with mounting medium containing DAPI and observe the slides under the fluorescence microscope.
Representative Results
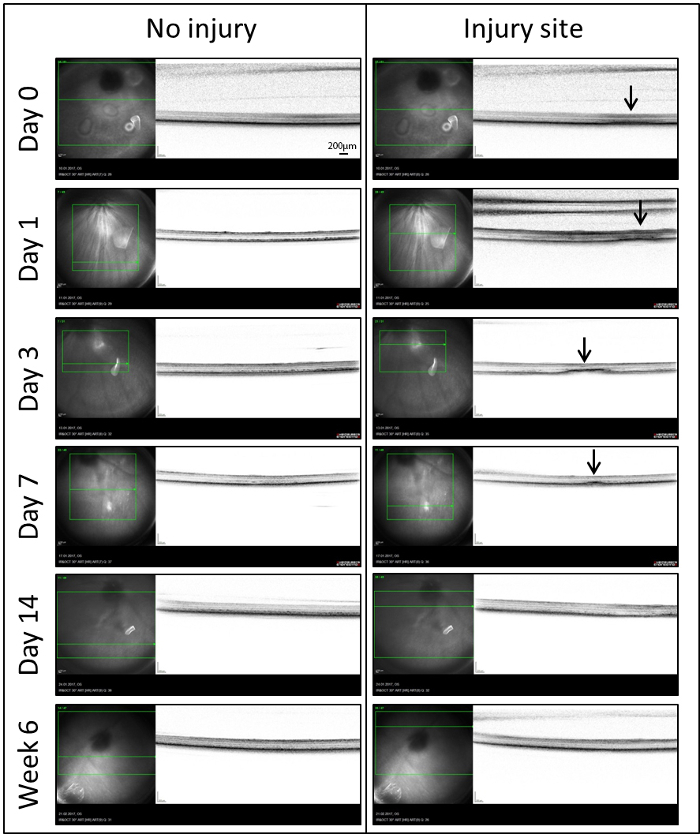
Real-time OCT: In order to analyze the role of MCs in retinal repair, we used a laser injury model inducing a well-delineated zone of damage in the zebrafish retina. The site of damage was imaged by means of in vivo OCT for the first time (Day 0) within 60 minutes following the injury (Figure 3). To compensate for the optics of the fish eye, a custom-made contact lens was placed on the cornea. Immediately after laser treatment, a diffuse hyper-reflective signal was localized to the outer retina (Arrows). It extended from the retinal pigmented epithelium (RPE) to the outer plexiform layer (OPL). A similar signal was also detectable on day 1. After day 3, this diffuse signal became more organized and dense. It was consistently seen in the outer nuclear layer (ONL) extending into the photoreceptor layer. Following the first week (Day 7), there was a significant decrease in average lesion size and only a small hyper-reflective signal was detected. Starting from day 14 until the latest time point investigated (Week 6), the laser spots were no longer visible in IR and OCT images.
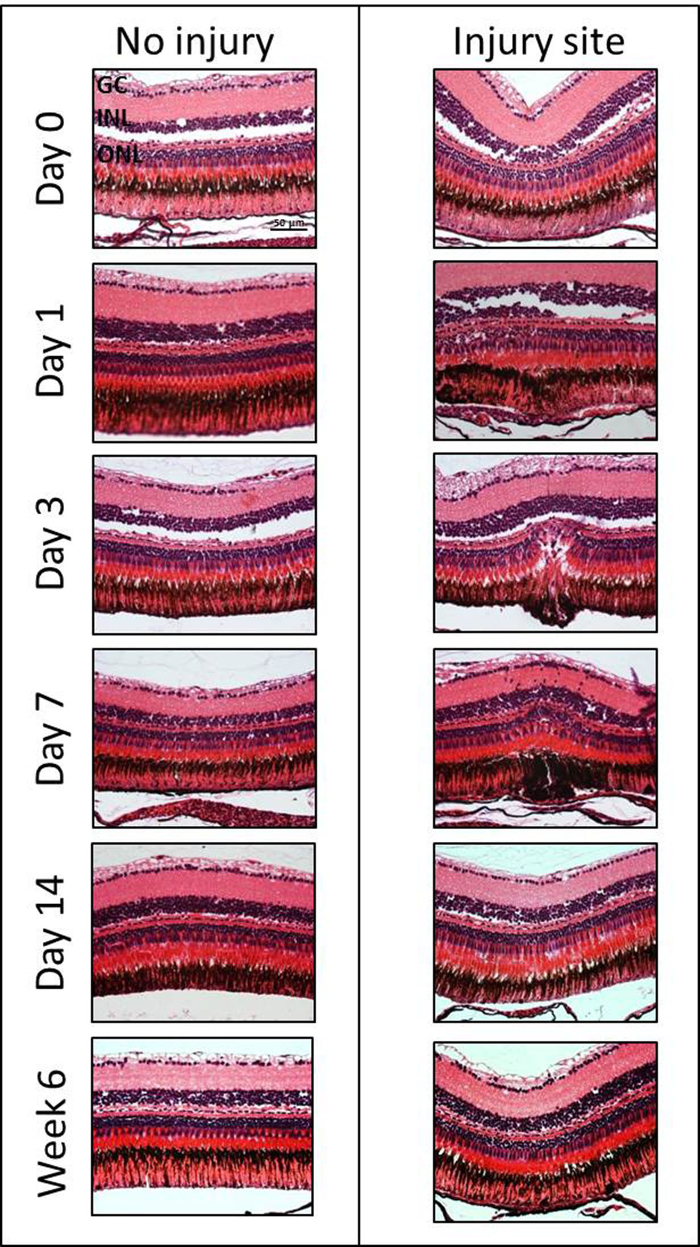
Histological Assessment of the Retinal Degeneration/Regeneration: In order to investigate the extent and kinetics of retinal degeneration/regeneration, H&E staining was employed at different time points after damage induction (Day 0, 1, 3, 7, 14 and Week 6) (Figure 4). Experiments were performed on three eyes of three fish. However, no statistical analysis was performed as the goal of this manuscript is to demonstrate the method. Subtle changes in the inner nuclear layer (INL) and ONL can be seen immediate post-laser (e.g., slight edema in the ONL and reduced intercellular space in the INL) within 60 minutes following laser treatment. The degeneration was followed for 6 weeks after the induction of laser injury. Morphologic changes were consistently observed after 1 day with disorganization of the PRs and with a cavity formation in the ONL and in the subretinal space. Indeed, there was a loss of nuclei within the ONL in the damage area between days 1 and 7. The maximum PR loss was found on day 3. Starting from 14 days to 6 weeks, the outer retina had re-established its normal morphology.
Glial Involvement During Retinal Degeneration/Regeneration: To assess MC activation at different time points (Day 0, 3, 14) following the induction of retinal degeneration, we performed immunohistochemical analyses for the glial cell markers GS and GFAP (Figure 5). Experiments were again performed on three eyes of three fish without quantitative analysis. GS plays an important role in controlling the level of extracellular glutamate and is considered to be exclusively expressed in MC soma and its processes19,20. GFAP is upregulated during ageing and when the retina is damaged or stressed. It is localized in the MC end-feet and processes21. Weak GFAP expression was also found in the inner part of the uninjured-control retina labelling other glial cell types, e.g., astrocytes. The autofluorescence of the PR outer segments also provided a signal in the ONL but this could be clearly distinguished. The GFAP signal was upregulated at day 3 post-injury. Thereby, analyses performed at day 3 after the laser treatment showed GFAP-positive MCs only within the injury site in the ONL of the retina. From day 14, the regeneration was largely complete and the GFAP signal was downregulated to baseline level in the damage area. Unlike GFAP there was no considerable change in the level of GS expression. However, there might have been a localized downregulation of GS at the site of the injury.
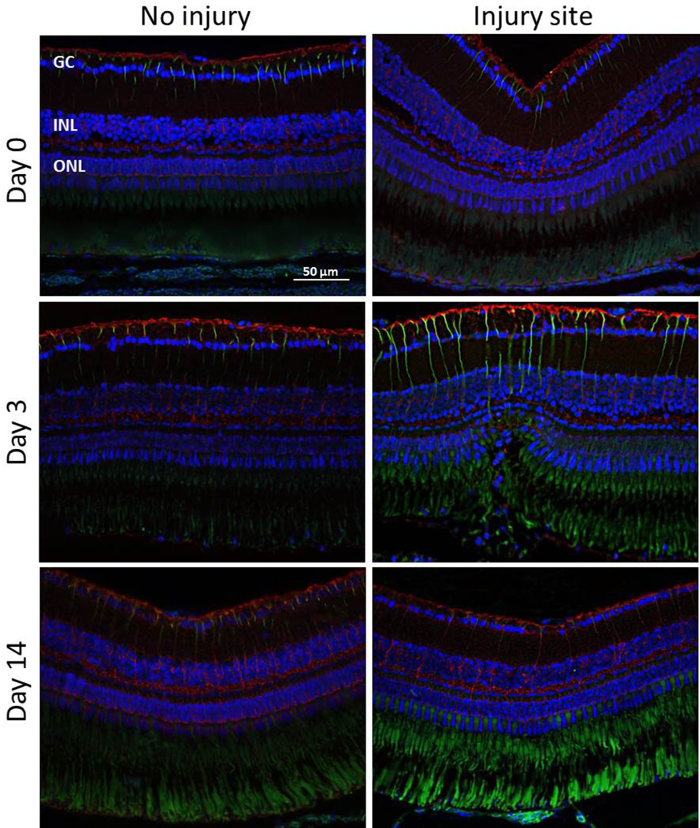
Figure 1: Setup for the induction of the laser injury of zebrafish retina and following OCT analysis. (A) Arrangement of the laser system before treatment. (A.1) Zebrafish placed on silicone pin holder with fundus contact lens in place just prior to application of the laser beam. (B) Arrangement of the OCT system before imaging. (B.1) Zebrafish placed before 78D slit lamp lens before the detection of the damage sites by means of OCT. Please click here to view a larger version of this figure.
Figure 2: Setup for acquiring the IR and OCT images. (A) Screenshot of the software window during imaging. IR and OCT modes are depicted. (B) Overview of the panel used to acquire the images. Please click here to view a larger version of this figure.
Figure 3: IR (left) and OCT (right) images of the injured and uninjured sites of retina in the same eye at different time points (Days 0, 1, 3, 7, 14 and Week 6) after laser-induced retinal degeneration. In the IR images, a green box indicates the portion of the retina that has be analyzed and the green line shows the location of the OCT images on the retina. Arrows indicate sites of the laser spots detected as hyper-reflective signals in the OCT. Scale bar = 200 µm. Please click here to view a larger version of this figure.
Figure 4: H&E staining of the injured and the contralateral uninjured zebrafish eye at different time points (Days 0, 1, 3, 7, 14 and Week 6) after laser-induced retinal degeneration. GC, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Figure 5: Immunohistochemical detection of MC activation. GFAP (green) and GS (red) staining in the injured and uninjured zebrafish retina at different time points (Days 0, 3, 14) after laser-induced retinal degeneration. The green signal in the ONL is due to the autofluorescence of the PR outer segments. Cell nuclei are stained with DAPI (blue). Scale bar = 50 µm. Please click here to view a larger version of this figure.
Discussion
Retinal regeneration/degeneration in the zebrafish has been investigated by different approaches such as cytotoxin-mediated cell death22, mechanical injury23, and thermal injury24. We employed a 532 nm diode laser to damage the zebrafish retina. Thereby, our model offers several advantages. For instance, we rapidly created a well-defined area of injury localized in the outer retina, specifically in the PRs layer. Furthermore, this experimental set-up can be modified to produce larger areas of damage to study other biological processes, for example, by damaging Bruch's membrane to induce choroidal neovascularization. There is minimal collateral damage and the timing of the regenerative response can be modulated precisely and reproducibly.
To follow the phenotypical changes over time, we employed the Heidelberg Spectralis system and the Heidelberg Eye Explorer software. Thus, fundus IR imaging was found to be especially useful in zebrafish. Unlike fundus autofluorescence (AF), which shows low contrast between the laser spot and the surrounding area, the damaged sites are easier to localize by IR imaging. This allows us to detect changes and to monitor regeneration in vivo, which would not be possible with the AF mode. As previously reported25, OCT can be used to monitor degenerative/regenerative processes in zebrafish retina. In our model, retinal laser injury was detected as a hyper-reflective band in the ONL (Figure 3), which is similar to what has been reported in mammals26. During regeneration, the hyper-reflective signal decreased until it totally disappeared at day 14.
The OCT images were correlated with morphological changes seen within the laser lesions (Figure 4). The diffuse hyper-reflective signal in OCT images (Day 0) represents the post-laser changes seen in the INL of zebrafish euthanized within 1 hour following laser treatment. The subtle and well-delineated hyper-reflective signal detected at day 3 corresponds to the cavity formation in the ONL due to the rod loss. Starting from day 14, both OCT and histological analyses confirm that the outer retina had re-established its normal morphology.
In the present study, we also evaluated the MC response during retinal regeneration (Figure 5). Many groups have already investigated the activation of glial cells, particularly of MCs, as an initial regenerative response to damage. More specifically, they suggested that pathophysiological MC activation may induce MCs to adopt stem cell characteristics, providing an endogenous source of new functioning cell types that can be integrated into damaged areas of the retina27. As expected, we found that retinal laser injury stimulated MC activation as indicated by the upregulation of GFAP. Indeed, increased GFAP expression was remarkably detected at 3 days post-injury and was restricted to the damage area. Starting from day 14, the regeneration was largely complete and the GFAP signal was downregulated to the baseline level in the damage area.Thus, we have demonstrated that MCs play an active role in retinal reorganization, and potentially regeneration, following injury.
In conclusion, the laser-induced retinal degeneration/regeneration model is a versatile tool to produce rapid and focal damage to the zebrafish retina and the following regeneration can be visualized by non-invasive OCT imaging. Additionally, we were able to show that laser-retinal degeneration is accompanied by MC activation and that the ensuing gliosis is reversed upon regeneration. However, a detailed investigation of the involved cell types (e.g., microglia, macrophages) and pathways needs to be performed. Critical modifications may be needed, especially live tracking of the MCs to better understand how they behave during the regenerative process (e.g., migration from their original location in the INL into the outer retina, especially in the PRs layer) and their differentiation towards PR cells. Alternatively, light damage paradigms could be used to induce widespread and consistent loss of both rod and cone PRs28. However, the advantage of the described model is a more defined injury area where one can study local interactions between degenerating photoreceptors and activated MCs.
In general, we believe that the model will help to better understand degenerative/regenerative processes in the sensory retina and may enable the comparison of these developments with the mammalian system. It could also be used to study the influence of the innate immune system and effects of neuroactive substances. In the future, one might be able to use these results to modify the degenerating human visual system.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Martin Zinkernagel, MD, PhD and Miriam Reisenhofer, PhD for her scientific input at establishing the model and Federica Bisignani for her excellent technical assistance.
References
- Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv. Ophthalmol. 2006;51(4):316–363. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Stefano Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis Pigmentosa: Genes and Disease Mechanisms. Curr Genomics. 2011;12(4):238–249. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34(5):1659–1676. [PubMed] [Google Scholar]
- Strettoi E. A Survey of Retinal Remodeling. Front Cell Neurosci. 2015;9:494. doi: 10.3389/fncel.2015.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Wang DY, Chan WM, Tam PO, Baum L, Lam DS, Chong KK, Fan BJ, Pang CP. Gene mutations in retinitis pigmentosa and their clinical implications. Clin Chim Acta. 2005;351(1-2):5–16. doi: 10.1016/j.cccn.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Pierce EA. Pathways to photoreceptor cell death in inherited retinal degenerations. BioEssays. 2001;23:605–618. doi: 10.1002/bies.1086. [DOI] [PubMed] [Google Scholar]
- Tackenberg MA, Tucker BA, Swift JS, Jiang C, Redenti S, Greenberg KP, Flannery JG, Reichenbach A, Young MJ. Muller cell activation, proliferation and migration following laser injury. Mol. Vis. 2009. pp. 1886–1896. [PMC free article] [PubMed]
- Newman E, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996;19(8):307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Kubota R, Hokoc JN, Moshiri A, McGuire C, Reh TA. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002;134:31–41. doi: 10.1016/s0165-3806(01)00287-5. [DOI] [PubMed] [Google Scholar]
- Zhao TT, Tian CY, Yin ZQ. Activation of Müller cells occurs during retinal degeneration in RCS rats. Adv Exp Med Biol. 2010;664:575–583. doi: 10.1007/978-1-4419-1399-9_66. [DOI] [PubMed] [Google Scholar]
- DiCicco RM, Bell BA, Kaul C, Hollyfield JG, Anand-Apte B, Perkins BD, Tao YK, Yuan A. Retinal Regeneration Following OCT-Guided Laser Injury in Zebrafish. Invest Ophthalmol Vis Sci. 2014;55(10):6281–6288. doi: 10.1167/iovs.14-14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta J, Saszik S. The zebrafish as a model visual system. Int. J. Dev. Neurosci. 2001. pp. 621–629. [DOI] [PubMed]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26(23):6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Ashutosh PJ, Roesch K, Cepko CL. Development and neurogenic potential of Müller gial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28(4):249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Ahmad I. Unlocking the Neurogenic Potential of Mammalian Müller Glia. Int J Stem Cells. 2016;9(2):169–175. doi: 10.15283/ijsc16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Granato M, Nüsslein-Volhard C. Keeping and raising zebrafish. In: Nüsslein-Volhard C, Dahm R, editors. Zebrafish: A Practical Approach. IRL Press; 2002. pp. 7–38. [Google Scholar]
- Riepe RE, Norenburg MD. Müller cell localisation of glutamine synthetase in rat retina. Nature. 1977;268(5621):654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J Neurosci Res. 1995;42(1):131–143. doi: 10.1002/jnr.490420115. [DOI] [PubMed] [Google Scholar]
- Bignami A, Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979;28(1):63–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- Sherpa T, Fimbel SM, Mallory DE, Maaswinkel H, Spritzer SD, Sand JA, Li L, Hyde DR, Stenkamp DL. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68(2):166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA, Carney LH. Cell mosaic patterns in the native and regenerated inner retina of zebrafish: implications for retinal assembly. J Comp Neurol. 2000;416(3):356–367. [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TJ, Davis DH, Vance JE, Hyde DR. Spectral-domain optical coherence tomography as a noninvasive method to assess damaged and regenerating adult zebrafish retinas. Invest Ophthalmol Vis Sci. 2012;53(6):3126–3138. doi: 10.1167/iovs.11-8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinzer S, Saeger M, Hesse C, Portz L, Kleemann S, Schlott K, Brinkmann R, Roider J. Correlation with OCT and histology of photocoagulation lesions in patients and rabbits. Acta Ophthalmol. 2013;91(8):e603–e611. doi: 10.1111/aos.12188. [DOI] [PubMed] [Google Scholar]
- Wan J, Zheng H, Chen ZL, Xiao HL, Shen ZJ, Zhou GM. Preferential regeneration of photoreceptor from Müller glia after retinal degeneration in adult rat. Vision Res. 2008. pp. 223–234. [DOI] [PubMed]
- Thomas JL, Thummel R. A novel light damage paradigm for use in retinal regeneration studies in adult zebrafish. J Vis Exp. 2013. p. e51017. [DOI] [PMC free article] [PubMed]


