Abstract
Electrospun fibers (EFs) have been widely used in a variety of therapeutic applications; however, they have only recently been applied as a technology to prevent and treat sexually transmitted infections (STIs). Moreover, many EF technologies focus on encapsulating the active agent, relative to utilizing the surface to impart biofunctionality. Here we describe a method to fabricate and surface-modify poly(lactic-co-glycolic) acid (PLGA) electrospun fibers, with the potent antiviral lectin Griffithsin (GRFT). PLGA is an FDA-approved polymer that has been widely used in drug delivery due to its outstanding chemical and biocompatible properties. GRFT is a natural, potent, and safe lectin that possesses broad activity against numerous viruses including human immunodeficiency virus type 1 (HIV-1). When combined, GRFT-modified fibers have demonstrated potent inactivation of HIV-1 in vitro. This manuscript describes the methods to fabricate and characterize GRFT-modified EFs. First, PLGA is electrospun to create a fiber scaffold. Fibers are subsequently surface-modified with GRFT using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS)chemistry. Scanning electron microscopy (SEM) was used to assess the size and morphology of surface-modified formulations. Additionally, a gp120 or hemagglutinin (HA)-based ELISA may be used to quantify the amount of GRFT conjugated to, as well as GRFT desorption from the fiber surface. This protocol can be more widely applied to fabricate fibers that are surface-modified with a variety of different proteins.
Keywords: Bioengineering, Issue 128, Electrospun fibers, sexually transmitted infections, human immunodeficiency virus, surface-modification, microbicide, Griffithsin, poly(lactic-co-glycolic acid)
Introduction
The use of EFs as a topical delivery platform has the potential to significantly reduce STIs. Currently, there are over 36 million people living with HIV, with over two million new cases of reported in 2015 alone1,2. Additionally, herpes simplex virus type 2 (HSV-2) infection affects hundreds of millions of people worldwide and has been shown to enhance the acquisition of HIV by 2 - 5 fold3. Due to this relationship between HSV-2 infection and HIV acquisition, there is significant interest in developing new active agents that provide simultaneous protection against multiple STIs. Moreover, the development of new vehicles to improve the delivery of these antiviral agents offers the potential to further enhance protective and therapeutic potency. Toward this goal, EFs have been investigated as a new delivery platform to reduce the prevalence of HIV-1 and HSV-2 infections.
During the past two decades, EFs have been extensively used in the fields of drug delivery and tissue engineering4. Often, biocompatible polymers are selected to easily translate to therapeutic applications. To fabricate polymeric EFs, the selected polymer is dissolved in an organic solvent or aqueous solution, depending on the degree of polymer hydrophobicity5. Active agents of interest are then added to the solvent or aqueous solution prior to the electrospinning process. The polymer solution is then aspirated into a syringe and slowly ejected while subject to an electrical current. This process typically results in polymer fibers with sheet or cylindrical macrostructures (Figure 1), and fiber diameters ranging from the micro- to nano-scale6. For most therapeutic applications, active agents are incorporated within the fibers during the electrospinning process and are released from the fiber via diffusion and subsequent fiber degradation. The rate of degradation or release may be altered by using different types of polymers or polymer blends to establish a desired release profile, imparting unique chemical and physical properties7, and promoting the encapsulation of virtually any compound. As such, EFs have proven beneficial to the delivery of small molecule drugs and biological agents including proteins, peptides, oligonucleotides, and growth factors6,8,9.

In the field of STI prevention, EFs have been recently used to incorporate and provide sustained- or inducible-release of antiviral agents10,11,12,13,14,15,16,17,18,19. In one of the earliest studies, pH-responsive fibers were developed to release active agents in response to environmental changes within the female reproductive tract (FRT), as an on-demand method of protection against HIV-111. Since, other studies have investigated polymer blends comprised of polyethylene oxide (PEO) and poly-L-lactic acid (PLLA), to evaluate the tunable release of antiviral and contraceptive agents for HIV-1 prevention and contraception in vitro12. Additional studies have demonstrated the feasibility of EFs to provide the following: prolonged release of small molecule antivirals14, strong and flexible mechanical properties20, 3-D delivery architectures21, inhibition of sperm penetration12, and the ability to merge with other delivery technologies13. Finally, previous work has evaluated polymeric fibers for the sustained-delivery of antiviral agents against common co-infective viruses, HSV-2 and HIV-114. In this study, polymer fibers provided complementary activity to antiviral delivery by retaining their structure for up to 1 month and providing a physical barrier to viral entry. From these results, it was observed that EFs may be used to both physically and chemically hinder virus infection.
While tunable release properties make polymeric EFs an attractive delivery platform for microbicide delivery, EFs have been developed in other applications to serve as surface-modified scaffolds7. EFs have been used to mimic the morphology of the extracellular matrix (ECM), often acting as scaffolds to improve cellular regeneration22, and enhance their utility in tissue engineering23,24. Fibers comprised of polymers such as poly-ε-caprolactone (PCL) and PLLA have been surface-modified with growth factors and proteins after electrospinning to impart ECM-like properties including increased cellular adhesion and proliferation25,26. Additionally, antimicrobial surface-modified EFs have been evaluated to prevent the growth of specific pathogenic bacteria27,28. Due to this versatility and the ability to induce biological effects, EF technology continues to expand across a variety of fields to provide multi-mechanistic functionality. Yet, despite their utility in a diversity of applications, surface-modified fibers have only recently been explored in the microbicide field29.
In parallel with the development of new delivery technologies to prevent and treat STIs, novel biological therapeutics have been developed. One of the most promising microbicide candidates is the adhesive antiviral lectin, GRFT30. Originally derived from a species of red algae, GRFT has demonstrated activity as a potent inhibitor of HIV, HSV-2, SARS, as well as Hepatitis C virus31,32,33,34,35,36. In fact, among biologically-based inhibitors, GRFT has the most potent anti-HIV activity, inactivating HIV-1 almost immediately upon contact30, while maintaining stability and activity in the presence of culture media from vaginal microbes for up to 10 days37. More recently, a 0.1% GRFT gel was shown to protect mice against intravaginal HSV-2 challenge, making it a promising candidate for the first line of protection against both HSV-2 and HIV-132,38. For HIV specifically, GRFT inhibits infection by physically binding gp120 or terminal mannose N-linked glycan residues on viral envelope surfaces to prevent entry38,39,40,41,42. This inhibition is highly potent, with IC50s approaching 3 ng/mL43. In addition to inhibiting HIV infection, studies have also shown that GRFT protects against HSV-2 infection by inhibiting the cell-to-cell spread of the virus32. In all cases, GRFT has been shown to be adhesive to viral particles, while demonstrating high resistance to denaturation. Last, GRFT has demonstrated synergistic activity with combinations of Tenofovir (TFV) and other antivirals44, making it feasible and likely beneficial to co-administer with EFs. The potent properties of GRFT make it an excellent biologically-based antiviral candidate, in which delivery may be enhanced with EF technology.
Utilizing this knowledge of the adhesive and innate antiviral properties of GRFT, a polymeric fiber scaffold was designed, that integrates these properties to provide the first layer of virus entry inhibition29. Finding inspiration in the way that cervicovaginal mucus hinders virus transport primarily through mucoadhesive mucin interactions, we hypothesized that by using EFs as a scaffold and covalently modifying the surface with GRFT, a high density of surface-conjugated GRFT would debilitate and inactivate virus at its entrypoint45,46,47. Here EFs were developed as a stationary scaffold to provide a protein-based, viral adhesive-inactivating barrier platform. We sought to combine the potent antiviral properties of GRFT with a biocompatible, modifiable, and durable polymer platform, to create a novel virus "trap."
To achieve these goals, fibers comprised of PLGA were electrospun, and EDC-NHS chemistry was used to subsequently modify the EF surface with GRFT. PLGA served as a model polymer due to its extensive use in electrospinning48, combined with its biocompatibility and cost-effectiveness. Additionally, surface modification exploits the large surface area of EFs, and provides a useful alternative that can be combined with encapsulation to maximize fiber utility49. Unlike traditional encapsulation methods where only a portion of GRFT is available (and only transiently present in the FRT), surface modification may enable GRFT to maintain maximum bioactivity during the entire duration of treatment. Furthermore, the incorporation of hydrophilic compounds such as proteins, by traditional electrospinning methods, may result in lower encapsulation efficiencies and loss of protein activity50. Therefore, GRFT surface-modified fibers may offer a promising alternative delivery method that can be used alone or in combination with electrospinning to enhance protection against STI infection.
Protocol
1. Preparation and Fabrication of the Electrospun Fiber Scaffold
CAUTION: All work with solvents or polymer solutions should be performed in a chemical fume hood. Refer to material safety datasheet of each reagent before starting the protocol.
To electrospin a 3 mL 15% w/w PLGA polymer solution, weigh 720 mg of 50:50 poly(lactic-co-glycolic acid) (PLGA; 0.55 to 0.75 dL/g, 31-57 kDa) into a 10 mL scintillation vial. The volume of the solution is based on the typical batch size used in current studies. NOTE: The polymer mass to add to a given volume of solvent must be calculated by first determining the density of the solvent used to dissolve the polymer. The density of the solvent Hexafluoro-2-propanol (HFIP) is 1.59 g/mL. Thus, the weight of solvent, based on a volume of 3.0 mL HFIP needed, is 4.8 g (3.0 mL x 1.59 g/mL). For a 15% w/w fraction of PLGA to HFIP, 720 mg PLGA must be added to 3.0 mL HFIP (0.15 x 4,800 mg = 720 mg). The advantage of using a % w/w polymer/solution, rather than % w/v, is that this provides a defined weight of the final solution. This defined weight enables more accurate solvent replacement, in the case of solvent evaporation during step 1.3.
Add 3.0 mL HFIP to the glass scintillation vial containing PLGA (from step 1.1) using a serological glass pipette. Cover the vial with plastic film, then measure and record the vial mass.
Incubate the polymer suspension overnight at 37 °C to ensure complete dissolution of the polymer. If any solvent evaporates, decreasing the vial mass, add HFIP until the vial reaches its original mass in step 1.2.
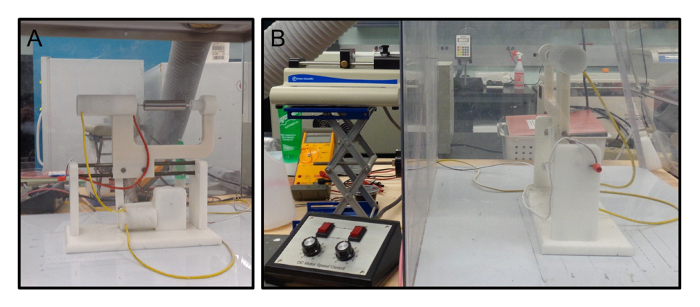
After incubation, prepare the electrospinning apparatus (Figure 2A). Although a mandrel of any size may be used, here a rotating 25 mm outer-diameter stainless steel mandrel was used as the collector. NOTE: A larger mandrel diameter will decrease the fiber thickness, given the same volume of electrospinning solution.
Aspirate the polymer solution into a 3 mL syringe.
Connect a blunt 18-gauge, ½ inch needle tip to the syringe and dispense the excess solution (typically 0.25 mL) to remove empty headspace in the needle tip.
Place the syringe on a syringe pump and set the instrument flow rate to 2.0 mL/h. NOTE: This flow rate was previously optimized based on polymer viscosity for this formulation.
Connect the power source to the syringe needle and electrospin the polymer solution using a voltage of +27 kV. The distance between the needle and collector should be set to approximately 25 cm (Figure 2B). CAUTION: The electrospinning process creates a solvent vapor. Use a fume hood or an enclosed apparatus (Figure 2) to remove the harmful vapor.
Once the entire solution is electrospun, turn off the power source and allow the mandrel to spin for an additional 30 min to fully evaporate solvent.
Turn off the rotating mandrel collector, and use a razor blade to cut the fiber from the mandrel. Use the blade to gently peel the fiber from the mandrel.
Collect the electrospun PLGA fiber into a labeled Petri dish, and place in a desiccatorovernight to remove residual solvent.
2. Surface-modification of Fibers with GRFT
Prepare solutions of phosphate-buffered saline (PBS) and 2-(N-morpholino)ethanesulfonic acid (MES buffer). Prepare PBS by dissolving 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.24 g KH2PO4 in 1 L of ultrapure water. Similarly, dissolve 19.52 g MES (free acid, MW 195.2) and 29.22 g NaCl in 1 L of ultrapure water, to prepare MES buffer. Ensure the final pH of each solution is between 7.2 - 7.5 and 5.0 - 6.0, respectively, using a pH meter.
- Prepare individual working solutions of EDC (2 mM) and NHS (5 mM).
- Remove the EDC and NHS from the freezer and allow them to equilibrate to room temperature before weighing.
- Weigh 4 mg of EDC into a 1.5 mL microcentrifuge tube.
- Weigh 6 mg of NHS into another microcentrifuge tube.
- Add 1 mL MES buffer to each tube. Vortex both tubes vigorously to ensure the reagents are fully dissolved.
Prepare a solution of hydroxylamine by weighing 70 mg into a 50 mL conical centrifuge tube.
Add 20 mL PBS to the hydroxylamine and vortex to dissolve.
Mass out an appropriate amount of PLGA fiber into a 15 mL conical centrifuge tube. Typically, 75 mg of fiber is used for each reaction batch.
Add 8 mL of MES buffer to the 15 mL tube.
Add 1 mL each of the EDC and NHS solutions prepared earlier to the tube. The final volume of the solution should be 10 mL. The final concentrations of EDC and NHS should be 0.4 mg/mL and 0.6 mg/mL, respectively.
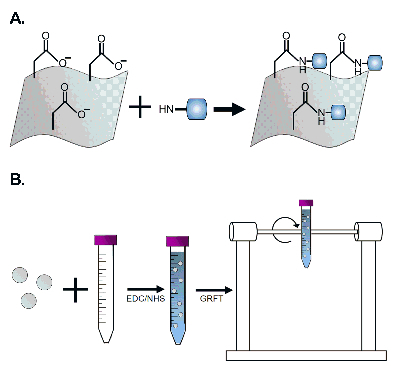
Close and seal the 15 mL tube with plastic film and place on a rotator to allow the solution to be gently inverted for 15 min at room temperature (Figure 3B). This step activates the carboxyl groups on the polymer to allow for covalent modification with the GRFT protein (Figure 3A).
After activation, carefully quench the reaction by adding 14 µL of β-mercaptoethanol to the tube. Invert the tube several times to ensure complete mixing. CAUTION: β-mercaptoethanol is highly toxic and should only be used in a chemical fume hood.
Discard the supernatant and rinse the PLGA fiber twice, with 10 mL of PBS, to remove any remaining β-mercaptoethanol.
After rinsing, add an appropriate amount of GRFT stock solution to the tube. For example, a 5 nmol GRFT/mg fiber would require 6.35 µL of GRFT stock solution (from a 10 mg/mL stock) per mg of fiber. Thus a 75 mg fiber sample would require 476.25 µL of 10 mg/mL GRFT stock solution. NOTE: GRFT fibers with theoretical loadings of 0.05, 0.5, and 5 nmol GRFT per mg fiber were fabricated.
Add enough PBS to bring the final volume to 8 mL, close and invert the tube to ensure thorough mixing.
Seal the tube with plastic film and place on a rotor again, this time for 2 h.
After the 2 h incubation, quench the reaction by adding 2 mL of hydroxylamine solution into the 15 mL centrifuge tube. Per manufacturer instructions, the final concentration of hydroxylamine during the quenching reaction should be 0.7 mg/mL.
Mix the solution well and discard the supernatant. Rinse the surface-modified PLGA fiber twice with 10 mL ultrapure water to remove any unconjugated GRFT.
Transfer the fiber to a Petri dish and place inside of a desiccator until the fiber is completely dry. Transfer the Petri dish to 4 °C for storage.
3. SEM Characterization of GRFT Surface-modified Fibers
Place a strip of double-sided carbon tape on an SEM specimen mount. Label the bottom of the specimen mount with the sample identifying information using a permanent marker.
Cut three samples from one surface-modified fiber and place them on separate specimen mounts. The thickness of each sample is approximately 0.5 mm.
Sputter coat the samples using electron-induced particle deposition from a gold plate. Sputter coat for 90 s, at 2.4 kV. NOTE: The sputter coat time may vary depending on equipment parameters, including voltage and amperage.
Image the samples at 8 kV with a magnification ranging from 1,000 to 5,000X.
4. Extraction of GRFT from Surface-modified Fibers
Mass out 2 mg of fiber in triplicate into 1.5 mL microcentrifuge tubes.
Add 1 mL dimethyl sulfoxide (DMSO) to the tube, then vortex and incubate for 1 min at room temperature to completely dissolve the fiber.
After incubation, dilute a 10 µL aliquot of the DMSO-fiber solution from step 4.2, at least 100-fold in Tris-EDTA (TE) buffer (pH = 8.0).
Store samples at -20 °C until loading characterization with ELISA.
5. Measuring GRFT Desorption from Fibers
To assess the amount of GRFT released or desorbed from the fiber, weigh 5 - 10 mg of surface-modified fiber and place in a microcentrifuge tube. Record the fiber mass in each tube.
Add 1 mL of an appropriate solution that mimics the physiological environment (e.g., PBS, TE buffer, simulated vaginal fluid (SVF), etc.) to each sample.
Incubate the samples for 1 h on a rotating shaker at 200 rpm, 37 °C.
After incubation, remove approximately 1 mL of TE buffer containing the desorbed GRFT from the vial, and aliquot to cluster tubes. Store at -20 °C until protein quantification.
Transfer the sample to a new microcentrifuge tube, add 1 mL of fresh buffer solution to the fiber within the microcentrifuge tube, and incubate until the next time point.
Typical time points used to measure release include: 1, 2, 4, 6, 8, 24, 48, 72 h, and 1 wk. Negligible desorption was observed in these studies after 4 h.
6. Quantification of GRFT Extraction and Desorption via ELISA
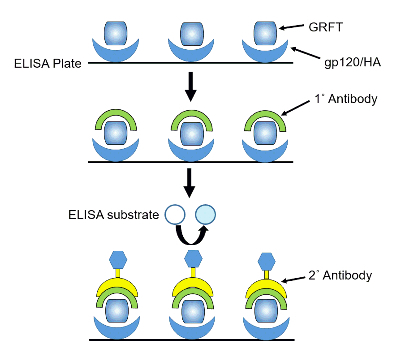
Coat a 96-well ELISA plate with 0.1 mL of HA (10 µg/mL) per well as previously described51. Seal the plate with plastic film and incubate overnight at 4 °C (Figure 4).
Remove the coating buffer, and add 0.3 mL blocking buffer (2 - 3% bovine serum albumin (BSA) in PBS with 0.05% polysorbate 20) to each well. Incubate the plate for at least 2 h at room temperature.
After incubation, rinse the plate 3 times with 1x PBS with 0.1% polysorbate 20 (PBS-P). After rinsing, dispense 0.1 mL of sample, standard, or PBS as negative control into the respective wells. Incubate the plate again for 1 h at room temperature.
Rinse the plate 3 times again with PBS-P. After rinsing, add 0.1 mL of primary antibody (goat anti-GRFT antiserum) into each well and incubate for at least 1 h at room temperature. Typically, the primary antibody solution is diluted by 1:10,000 in PBS.
After incubating the samples with primary antibody rinse the plates again 3 times with PBS-P. Add 0.1 mL of secondary antibody (horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG) to each well and incubate for 1 h at room temperature. The secondary antibody solution is diluted by 1:10,000 in PBS.
Wash the plate 3 times. Add 0.1 mL TMB 2-peroxidase substrate to each well. Monitor color development (approximately 2 min), then add 0.1 mL H2SO4 (1 N) to quench the reaction. Read plate at 450 nm on a plate reader.
Average the background OD values (wells which only receive PBS), and subtract this from the experimental groups.
Representative Results
Fiber morphology has a significant effect on the ability of surface-modified EFs to provide protection against viruses. Although electrospinning is a convenient and straightforward procedure, non-optimized polymer formulations may result in irregular fiber morphology (Figure 5B-C). Alterations in electrospinning conditions that result in the formation of beaded or amorphous mat-like morphologies, are often caused by solvent-polymer incompatibility, low polymer viscosity, flow rate, or other electrospinning conditions. The resulting variations in fiber structure can result in different release profiles for drug-incorporated fibers or inconsistencies in conjugation efficacy, altering the fibers ability to physically or chemically hinder virus penetration. The PLGA EFs fabricated using the described electrospinning conditions should result in distinct fiber morphologies with diameters ranging between 1.5 and 2.8 µm (Figure 6). To determine fiber morphology and diameter, EFs should be examined with SEM prior to other characterization or modification steps.
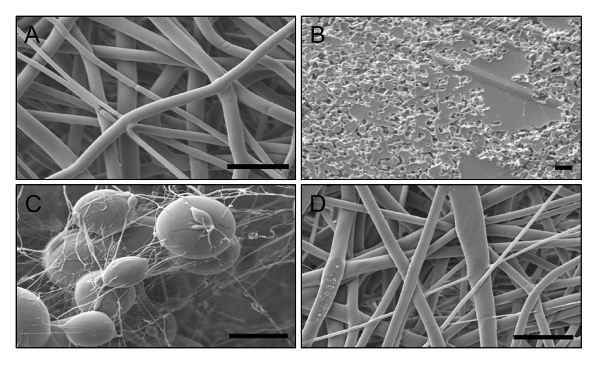
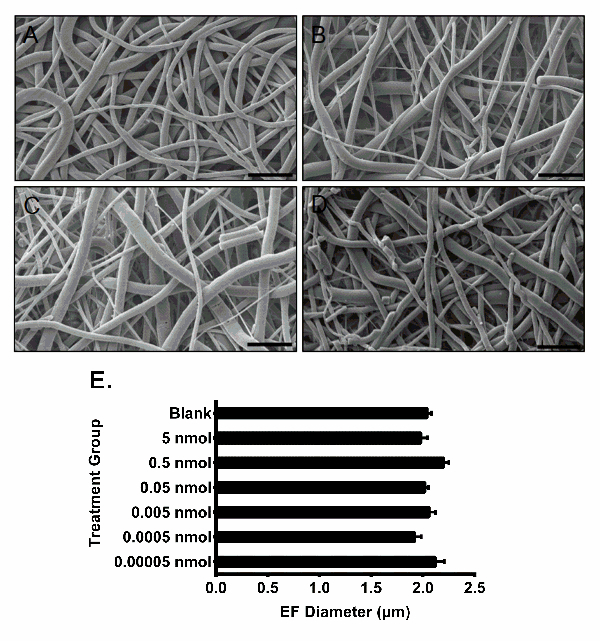
SEM images of blank, 0.05, 0.5, and 5 nmol GRFT fibers showed no significant differences in fiber morphology (Figure 6A-D), indicating that GRFT modification has no effect on fiber morphology. To determine the average diameter of each EF formulation, a minimum of 50 random measurements were taken per field of view from the SEM images. The average diameters of the EF formulations were measured and calculated in ImageJ, as shown in Figure 6E. All EF formulations had similar average diameters around 1.9 µm, demonstrating the consistency of unmodified fiber fabrication process across batches.
To determine the amount of GRFT conjugated to the EFs, GRFT-EFs were dissolved in DMSO, followed by a 100-fold dilution in TE buffer, to extract GRFT from the fiber. The quantity of GRFT conjugated per mg of fiber was quantified using ELISA. For each modification density (0.05, 0.5, and 5 nmol GRFT per mg fiber), ten replicates were evaluated. For the 5, 0.5, and 0.05 nmol GRFT/mg EF modifications, each EF had 373, 165, and 42 ng GRFT per mg of EF, resulting in conjugation efficiencies of 0.6, 4.2, and 6.9%, respectively. These results demonstrate that GRFT-EFs conjugated with higher theoretical surface-modification density, result in more GRFT conjugated to the fiber (Figure 7A). However, there was an inverse correlation with the resulting conjugation efficiency29.
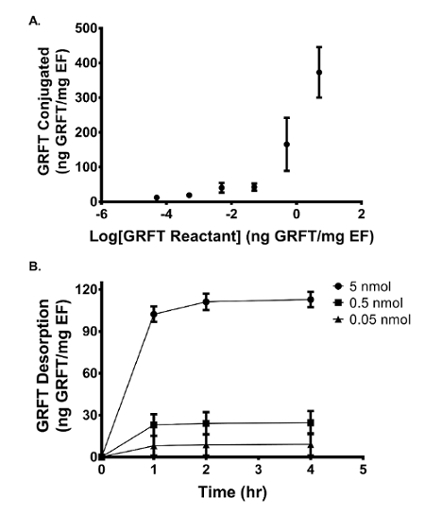
To assess the amount of GRFT covalently conjugated to the fiber surface relative to that adsorbed, GRFT-EFs were incubated in SVF to determine the amount of GRFT released. Within the first 4 h, 113, 25, and 10 ng of GRFT per mg EF was detected in SVF for the 5, 0.5, and 0.05 nmol theoretical modification concentrations, respectively. These values correspond to 30%, 41%, and 24% of the amount of GRFT conjugated to 5, 0.5, and 0.05 nmol GRFT-EFs. After 4 h, negligible GRFT was detected in the release eluate for all three formulations. GRFT release after 1, 2, and 4 h is shown in Figure 7B. Taken together, these data indicate that the majority of GRFT is covalently bound to the EFs, and that the surface-adsorbed GRFT is released within the first 4 h.
Figure 1: The macroscale morphology of electrospun fibers. The electrospun fibers shown were fabricated using a 4 mm (cylinder) and 25 mm (sheet) diameter mandrel, respectively. Please click here to view a larger version of this figure.
Figure 2: Electrospinning apparatus. (A) The collecting mandrel where the liquid jets of polymer deposit, and (B) the full electrospinning setup. Please click here to view a larger version of this figure.
Figure 3: Schematic of EF modification with GRFT using EDC-NHS chemistry. (A) Carboxyl groups on the PLGA EF react with NHS in the presence of EDC to form amine-reactive esters, which will subsequently form stable amide bonds with the primary amines of GRFT. (B) Two milligram fiber disks or pieces are cut and incubated in 8 mL of MES buffer with 2 mL of EDC/NHS reagent and rotated for 15 min. Please click here to view a larger version of this figure.
Figure 4: Schematic illustrating GRFT quantification using ELISA. A 96-well immunoplate is coated with gp120 or HA to capture and immobilize GRFT. Primary antibodies against GRFT, secondary antibodies linked to horseradish peroxidase, and ELISA substrate are sequentially added to quantify GRFT. Please click here to view a larger version of this figure.
Figure 5: Effects of solvent choice on PLGA EF morphology. (A) The 15% w/w PLGA EFs in HFIP displayed desirable thread-like morphology. (B) The 15% w/w PLGA EFs in chloroform and dimethylformamide, failed to form due to non-optimal solvent choice or polymer concentration (viscosity). (C) The 15% w/w and (D) 20% w/w PLGA EFs in TFE demonstrate the importance of polymer viscosity. Beads formed in the formulation with lower polymer concentration (C), while increasing the polymer concentration (solvent viscosity) resulted in well-defined fiber morphology (D). Note, Figure 5B was taken at a lower magnification to show the mat-like morphology. Scale bars = 10 µm. Please click here to view a larger version of this figure.
Figure 6: SEM images and fiber diameters of bare and GRFT-modified EFs. (A) Bare PLGA EFs and PLGA EFs surface-modified with (B) 0.05 nmol, (C) 0.5 nmol, and (D) 5 nmol of GRFT per mg of fiber. Scale bars = 10 µm. (E) Diameters of unmodified and GRFT-EFs. Error bars represent the mean ± SEM. No statistical difference was observed between the diameters of unmodified and GRFT-modified fibers. Figure 6E has been adapted from Grooms et al.29 Please click here to view a larger version of this figure.
Figure 7: Quantity of GRFT conjugated to and desorbed from GRFT-modified EF. (A) The quantity of GRFT conjugated to each mg of EF fiber increases with increased GRFT reactant concentration. (B) The quantity of GRFT released from each mg of fiber is shown for the 0.05, 0.5, and 5 nmol formulations after 1, 2, and 4 h incubation in SVF. Error bars represent the mean ± SEM. This figure has been adapted from Grooms et al.29 Please click here to view a larger version of this figure.
Discussion
Due to their porous structures and large surface areas, EFs have found a variety of applications in healthcare, one of which includes serving as therapeutic delivery vehicles. Drugs and other active agents can be incorporated within EFs for tunable delivery, while biologics and chemical ligands can be conjugated to the fiber surface for cell-specific targeting52 or biosensing53. Here the fabrication of GRFT surface-modified PLGA EFs, as a delivery scaffold to prevent HIV infection, is described. GRFT-EFs were synthesized by electrospinning, providing the advantages of low cost and high production rate relative to other fiber production methods49,53.
Critical Steps in the Protocol The formation of EFs is critically dependent on the properties of polymer solution, in particular the solution or solvent viscosity54. The factors that affect the viscosity of a polymer solution include polymer molecular weight, polymer concentration, and the type of solvent used. The solution or solvent viscosity is typically adjusted by changing the ratio of polymer to solvent, to obtain the desired polymer concentration. With each fabrication, the volume must be maintained during overnight incubation to maintain the proper polymer-to-solvent ratio (viscosity). At sufficiently high polymer concentration, the polymer molecules entangle in the solution during the electrospinning process to produce fibers. During the electrospinning process, a bead will form at the spinneret tip and if there is sufficient polymer entanglement, liquid jets will erupt from this point at a critical voltage and accelerate in whip-like fashion toward the collecting mandrel55. Solvent evaporation will then lead to jet thinning, producing threads of fiber as they deposit on the collecting mandrel. Once synthesized, EFs should be analyzed by SEM to verify proper morphology and consistent fiber diameter. The presence of beaded EFs may be the result of low solution viscosity56, exceedingly high applied voltage57, polymer feed rate58, or a combination of all three factors. If this is observed, polymer concentration should be increased and the applied voltage and feed rate should be adjusted, to attain fiber-like morphology.
To conjugate proteins to the EF surface, here PLGA carboxyl groups were reacted using EDC-NHS carbodiimide crosslinking chemistry59. During the modification process, fibers are briefly incubated with EDC in the presence of NHS which results in the conversion of carboxylates into semi-stable, amine-reactive esters. During the two-step conjugation process, it is critical that the buffers used during each step have the optimal pH, noted in the manufacturer's instructions, to ensure maximum conjugation efficiency. The half-life of NHS esters ranges from four to five hours at neutral pH and dramatically decreases in more basic conditions60. Thus, the first reaction should be performed in MES buffer at pH 5 - 6 and the activated fibers should then be transferred to a PBS buffer (pH 7.2 - 7.5) for subsequent and immediate reaction with GRFT. It is also important that EDC is inactivated by the addition of 2-mercaptoethanol and sufficiently rinsed from the fibers after carboxylate activation. This will help prevent protein activation by EDC and self-crosslinking during the second reaction, which may reduce conjugation efficiency.
Modifications and Troubleshooting Although our previous work demonstrated the efficacy of a variety of GRFT-modified fibers against HIV-1 infection, certain process alterations may be considered to customize EF morphology or to improve GRFT (or other protein) conjugation efficiency or fiber yield29. In particular, the EF surface area may be increased by decreasing the fiber diameter, to enable a greater surface area for conjugation. Previous studies have shown that reducing polymer concentration and viscosity produces smaller fiber diameter61,62. However, this approach is limited by the formation of beaded fibers when the concentration is below the threshold value. To decrease fiber diameter without changing solution viscosity, dual-solvent systems can be utilized to reduce the surface tension, or salts may be added to increase solution conductivity56,57. Both methods will enable greater stretching of the electrospinning jets which may produce smaller fiber diameters. In addition, lower molecular weight polymers may be used to fabricate smaller diameter fibers. Decreasing fiber diameter also provides the added advantage of generating smaller pores, potentially making EFs more effective as a barrier to virus penetration, for microbicide-based applications63. Finally, it was observed that humidity can affect EF yield. At higher humidity, the yield tends to decrease due to the formation of fibers with unusual macro-morphology. Installing a humidity control system within the electrospinning chamber could therefore facilitate producing EFs with consistent yields, if this presents a challenge to fabrication.
To improve GRFT conjugation efficiency, investigating the selection and location of functionalizable groups may also be considered. For example, if the primary amines of the protein of interest are located near the interior of the three-dimensional protein structure, steric hindrance may prevent activated carboxyl groups on EFs from interacting with primary amines, thereby decreasing the likelihood of the reaction. This challenge may be overcome by amino acid substitution of the protein to generate a primary amine group closer to the protein surface. However, as GRFT and other proteins depend on the specific activity of its binding sites for functionality, alterations in protein conformation should be thoroughly tested in functional assays prior to conjugation.
Limitations A major limitation of GRFT surface modification is the potential for low conjugation efficiency using carbodiimide crosslinking chemistry. If the antiviral protein of interest has a high IC50, a low conjugation efficiency may not provide sufficient protection (in these applications) against virus infection. However, for GRFT (or other modified) EFs, proteins and active agents that are not covalently bound to EFs may still adsorb to the surface. These adsorbed agents offer the potential to complement the activity of conjugated GRFT, by transiently increasing the localized concentration available for virus binding. In this example, desorbed GRFT may bind to HIV-1 virions that do not directly contact EFs, and may provide an alternative mechanism of protection with the first 4 h of (pericoital) administration. Thus, both conjugated and surface-adsorbed GRFT may contribute to providing uniform protection against STIs.
Despite the protection conferred from both protein conjugation and desorption, other surface-modification strategies with potentially higher efficiency might be pursued. For example, PLGA terminated with amines instead of carboxyl groups could be used to conjugate activated GRFT. Alternatively, a different surface-modification strategy may be utilized to improve EF-protein conjugation efficiency. Nanofibrous membranes composed of EFs have been functionalized with avidin via carbodiimide crosslinking chemistry64. Biotinylation or addition of a Strep-tag (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys) through recombination may enable the modified protein to form strong and extremely stable non-covalent interaction with avidin EFs. While non-covalent, the avidin-biotin linkage is the strongest non-covalent bond, with femtomolar affinity, likely resulting in stable conjugation to the fiber surface. For any surface-modification strategies, steric hindrance should be considered to maximize conjugation efficiency.
Lastly, we envision that surface-modified EFs will offer an alternative strategy to active agent encapsulation to provide complementary modes of protection. One consideration with combining encapsulation and surface-modification technologies on a single EF platform will be reducing the premature release of active agents during the surface-modification process. For surface-modification reactions that require relatively long incubation time in aqueous solution, a significant percentage of the active agents loaded could be lost due to polymer hydrolysis.
Significance of the Method with Respect to Existing and Alternative Methods In previous work, we observed that unmodified blank EFs were able to inhibit HIV infection by ~38% when placed in a transwell insert above infectible cells29. This observation combined with the biocompatibility, web-like microstructure, and tortuosity of EFs, in parallel with the observed potent antiviral and adhesive properties of GRFT, prompted the development of the surface-modified EFs described here.
Relative to other delivery technologies currently employed in microbicide applications, EFs have a wide range of potential applications due to their architecture and capacity for customization. In other work, the fibrous morphology of EFs has enabled them to deliver active agents, and to mimic the ECM, making them suitable scaffolds for tissue engineering. EFs have also been surface-modified to enhance biocompatibility and enhance sustained-release65,66.
To enhance biocompatibility or deliver agents that function through specific binding activity, numerous methods exist that allow for the attachment of compounds to the surfaces of EFs such as plasma treatments, wet chemical methods, and surface graft polymerizations50. In the case of GRFT which specifically bind to the viral glycoproteins of HIV-1, the wet chemical method of EDC-NHS is the most optimal due to the ability to penetrate fibers deep within the mesh while also preserving GRFT functionality50. GRFT immobilized to EFs can then be delivered in a durable formulation to the FRT and provide immediate protection against HIV infection.
Compared to the existing strategy of encapsulating therapeutics within EFs to inhibit STIs, covalent conjugation offers the distinct advantage of increasing the potential avidity between GRFT and HIV virions. By immobilizing GRFT to EFs through surface-modification, highly localized concentrations of GRFT can be achieved, which increase the opportunity for multivalent binding with HIV. In addition, EF-immobilized GRFT, relative to free GRFT in solution, may prevent the depletion of the GRFT pool by hindering cell internalization. Moreover, due to the unique mechanism of action of GRFT as a stable and adhesive entry inhibitor, covalent surface conjugation enables surface-modified EFs to provide a physical barrier to virus penetration, in addition to potent antiviral properties.
Future Applications of this Method The utilization of surface-modification for delivery presents the opportunity to integrate multiple active agents within a single EF platform. In the future, we seek to develop a multipurpose technology (MPT) where a variety of active agents may be encapsulated within and conjugated to EFs. These MPTs may be designed to confer protection against a wider range of pathogens by incorporating therapeutics with different mechanisms of action. By utilizing both the surface and interior of EFs to deliver active agents with different mechanisms of action, the potential of EFs to protect against virus infection will be maximized.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We are grateful to the Jewish Heritage Fund for Excellence for funding this research. We thank Dr. Stuart Williams II for generously providing usage of the electrospinning system. We also thank Dr. Kenneth Palmer for providing us with Griffithsin. We additionally thank Dr. Nobuyuki Matoba and his lab for training us in the GRFT ELISA work.
References
- Gottlieb SL, et al. Toward global prevention of sexually transmitted infections (STIs): the need for STI vaccines. Vaccine. 2014;32:1527–1535. doi: 10.1016/j.vaccine.2013.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fact sheet November 2016. 2016. Available from: http://www.unaids.org/en/resources/fact-sheet.
- Freeman EE, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Prog Polym Sci. 2015;46:1–24. [Google Scholar]
- Hu X, et al. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63:2223–2253. [Google Scholar]
- Son YJ, Kim WJ, Yoo HS. Therapeutic applications of electrospun nanofibers for drug delivery systems. Arch. Pharmacal Res. 2014;37:69–78. doi: 10.1007/s12272-013-0284-2. [DOI] [PubMed] [Google Scholar]
- Ji W, et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011;28:1259–1272. doi: 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 2013;100:9–16. doi: 10.1016/j.antiviral.2013.09.022. Suppl. [DOI] [PubMed] [Google Scholar]
- Huang C, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33:962–969. doi: 10.1016/j.biomaterials.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS One. 2012;7:49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for tunable drug release: using displacement of air to control delivery rates. J. Am. Chem. Soc. 2012;134:2016–2019. doi: 10.1021/ja211148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniagyei SE, et al. Evaluation of poly(lactic-co-glycolic acid) and poly(dl-lactide-co-epsilon-caprolactone) electrospun fibers for the treatment of HSV-2 infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;72:238–251. doi: 10.1016/j.msec.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, et al. Electrospun polystyrene fibers for HIV entrapment. Polym. Advan. Technol. 2014;25:827–834. [Google Scholar]
- Carson D, Jiang Y, Woodrow KA. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm. Res. 2016;33:125–136. doi: 10.1007/s11095-015-1769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SF, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release. 2015;220:584–591. doi: 10.1016/j.jconrel.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball C, Woodrow KA. Electrospun solid dispersions of Maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrob. Agents Chemother. 2014;58:4855–4865. doi: 10.1128/AAC.02564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int. J. Nanomedicine. 2014;9:2967–2978. doi: 10.2147/IJN.S61664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CM, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Cai S, Xu H, Jiang Q, Yang Y. Novel 3D electrospun scaffolds with fibers oriented randomly and evenly in three dimensions to closely mimic the unique architectures of extracellular matrices in soft tissues: fabrication and mechanism study. Langmuir. 2013;29:2311–2318. doi: 10.1021/la304414j. [DOI] [PubMed] [Google Scholar]
- Li MY, et al. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26:5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2010;11:014108. doi: 10.1088/1468-6996/11/1/014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Advan. Technol. 2010;21:77–95. [Google Scholar]
- Vaidya P, Grove T, Edgar KJ, Goldstein AS. Surface grafting of chitosan shell, polycaprolactone core fiber meshes to confer bioactivity. J Bioact Compat Pol. 2015;30:258–274. [Google Scholar]
- Rim NG, et al. Mussel-inspired surface modification of poly(L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloid Surface B. 2012;91:189–197. doi: 10.1016/j.colsurfb.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Yao C, Li XS, Neoh KG, Shi ZL, Kang ET. Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J Membrane Sci. 2008;320:259–267. [Google Scholar]
- Kangwansupamonkon W, Tiewtrakoonwat W, Supaphol P, Kiatkamjornwong S. Surface Modification of Electrospun Chitosan Nanofibrous Mats for Antibacterial Activity. J Appl Polym Sci. 2014;131 [Google Scholar]
- Grooms TN, et al. Griffithsin-Modified Electrospun Fibers as a Delivery Scaffold To Prevent HIV Infection. Antimicrob. Agents Chemother. 2016;60:6518–6531. doi: 10.1128/AAC.00956-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emau P, et al. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J. Med. Primatol. 2007;36:244–253. doi: 10.1111/j.1600-0684.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- Meuleman P, et al. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 2011;55:5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B, et al. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013;87:6257–6269. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe BR, et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishag HZ, et al. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2013;158:349–358. doi: 10.1007/s00705-012-1489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferir G, et al. Combinations of griffithsin with other carbohydrate-binding agents demonstrate superior activity against HIV Type 1, HIV Type 2, and selected carbohydrate-binding agent-resistant HIV Type 1 strains. AIDS Res. Hum. Retroviruses. 2012;28:1513–1523. doi: 10.1089/aid.2012.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, et al. The Griffithsin Dimer Is Required for High-Potency Inhibition of HIV-1: Evidence for Manipulation of the Structure of gp120 as Part of the Griffithsin Dimer Mechanism. Antimicrob Agents Ch. 2013;57:3976–3989. doi: 10.1128/AAC.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouokam JC, et al. Investigation of griffithsin's interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One. 2011;6:22635. doi: 10.1371/journal.pone.0022635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulaei T, et al. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure. 2010;18:1104–1115. doi: 10.1016/j.str.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C, et al. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Ziolkowska NE, et al. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure. 2006;14:1127–1135. doi: 10.1016/j.str.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska NE, et al. Crystallographic, thermodynamic, and molecular modeling studies of the mode of binding of oligosaccharides to the potent antiviral protein griffithsin. Proteins. 2007;67:661–670. doi: 10.1002/prot.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe BR, et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferir G, Palmer KE, Schols D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology. 2011;417:253–258. doi: 10.1016/j.virol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. U. S. A. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile P, Chiono V, Carmagnola I, Hatton PV. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int J Mol Sci. 2014;15:3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repanas A, Andriopoulou S, Glasmacher B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J Drug Deliv Sci Tec. 2016;31:137–146. [Google Scholar]
- Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Barton C, Kouokam JC, Hurst H, Palmer KE. Pharmacokinetics of the Antiviral Lectin Griffithsin Administered by Different Routes Indicates Multiple Potential Uses. Viruses. 2016;8 doi: 10.3390/v8120331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka K, Gouma P, Simon S. Electrospun biocomposite nanofibers for urea biosensing. Sensor Actuat B-Chem. 2005;108:585–588. [Google Scholar]
- Ramakrishna S, et al. Electrospun nanofibers: solving global issues. Mater Today. 2006;9:40–50. [Google Scholar]
- Liu X, et al. Electrospinnability of Poly Lactic-co-glycolic Acid (PLGA): the Role of Solvent Type and Solvent Composition. Pharm. Res. 2017;34:738–749. doi: 10.1007/s11095-017-2100-z. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Kundu SC. Electrospinning: A fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Fong H, Chun I, Reneker DH. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585–4592. [Google Scholar]
- Zong XH, et al. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43:4403–4412. [Google Scholar]
- Rodoplu D, Mutlu M. Effects of Electrospinning Setup and Process Parameters on Nanofiber Morphology Intended for the Modification of Quartz Crystal Microbalance Surfaces. J Eng Fiber Fabr. 2012;7:118–123. [Google Scholar]
- Grabarek Z, Gergely J. Zero-Length Crosslinking Procedure with the Use of Active Esters. Anal Biochem. 1990;185:131–135. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- Staros JV, Wright RW, Swingle DM. Enhancement by N-Hydroxysulfosuccinimide of Water-Soluble Carbodiimide-Mediated Coupling Reactions. Anal Biochem. 1986;156:220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- Tan SH, Inai R, Kotaki M, Ramakrishna S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer. 2005;46:6128–6134. [Google Scholar]
- Spasova M, Stoilova O, Manolova N, Rashkov I, Altankov G. Preparation of PLLA/PEG Nanofibers by Electrospinning and Potential Applications. J Bioact Compat Pol. 2007;22:62–76. [Google Scholar]
- Boland ED, et al. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005;1:115–123. doi: 10.1016/j.actbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Senecal A, Magnone J, Marek P, Senecal K. Development of functional nanofibrous membrane assemblies towards biological sensing. React Funct Polym. 2008;68:1429–1434. [Google Scholar]
- Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583–2589. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- Gupta D, Venugopal J, Mitra S, Giri Dev VR, Ramakrishna S. Nanostructured biocomposite substrates by electrospinning and electrospraying for the mineralization of osteoblasts. Biomaterials. 2009;30:2085–2094. doi: 10.1016/j.biomaterials.2008.12.079. [DOI] [PubMed] [Google Scholar]


