Abstract
Transurethral instillation can be used to deliver different solutions with active ingredients (e.g., drugs, chemicals, bacteria, and viruses) locally into the urinary bladder to either induce animal models of bladder pathologies or evaluate the effectiveness of intravesical treatments. Most rodent models of lower urinary tract (LUT) pathologies are induced in female mice due to ease of intravesical instillation of the substances via the female urethra. However, due to anatomical differences between the female and male LUT, transurethral instillation in a male mouse has been deemed a very challenging procedure, and it has not been previously described. In this manuscript, we provide a detailed description of how to prepare polyethylene (PE) tubing for subsequent insertion into the urethra of a male mouse. In addition, we discuss the ideal types of PE tubing to be used depending on the desired site of inoculation. Furthermore, we describe point by point how to prepare an animal for a successful transurethral instillation to avoid injury to the urethra and ensure the delivery of the solution to the desired location. The procedure is started by retracting the prepuce and the glans to expose the opening of the urethral meatus. Next, the glans are grasped by blunt non-crushing forceps to stabilize the penis and the PE tubing. The PE tubing is first inserted into the urethral meatus parallel to the animal body, then its angle is adjusted by tilting the catheter to maneuver it to follow the natural curvature of the urethra. This technique can be used to induced murine models of bladder pathologies and/or evaluate the effectiveness of intravesical treatments in male mice.
Keywords: Immunology, Issue 129, Transurethral instillation, male mouse, urinary bladder, lower urinary tract (LUT), rodent models of inflammation, Catheterization Procedure
Introduction
The transurethral instillation approach has been used in previous studies as one of the methods to create rodent models of bladder pathologies1,2,3,4 and can be used to evaluate the effectiveness of locally delivered treatments in mice. Although animal models cannot fully recapitulate human pathologies, identification of the underlying mechanisms in animal studies provides a better understanding of the human LUT disorders such as interstitial cystitis/bladder pain syndrome, neurogenic cystitis, autoimmune cystitis, and prostatic inflammation5.
The transurethral instillation procedure performed on an adult male mouse is more technically challenging than intravesical instillation in an adult female mouse6. The naturally curved anatomy of the male urethra along with its small diameter make it technically difficult to accomplish the transurethral insertion of a catheter. Therefore, detailed instructions for the transurethral induction of mouse urinary tract infection6 and LUT inflammation7,8,9,10,11,12,13,14,15 via bladder-inserted catheter were previously outlined for female mice only. This manuscript aims to provide a step-by-step description of the technique for transurethral instillation of substances in male mice including the video clips, images, and illustrations. Transurethral instillation procedures in an adult male mouse can be performed with variable inoculates similar to that previously described in the female mouse7 and merged with additional techniques such as electromyogram recordings of visceromotor responses (VMR)16.
Protocol
All the procedures with animals, including the method and duration of anesthesia, as well as postoperative care, were discussed with a veterinarian and approved by the Institutional Animal Care and Use Committee (IACUC) at the affiliated institution. In this manuscript, all procedures were performed with 8 to 15-week-old C57BL/6 male mice and the protocol follows the animal care guidelines approved by the IACUC at the University of Colorado.
1. Preparation of Tubing
NOTE: To instill the inoculum, a needle attached to the polyethylene (PE) tubing (Figure 1A) or a small gauge (<24 G) angiocatheter can be used (Figure 1G). The use of an angiocatheter requires no preparation other than sterilization with 70% ethanol (EtOH). The inoculum is the substance that is instilled/injected/deposited into the urinary tract. This can be a solution containing bacteria, virus, or a chemical. This is prepared ahead of time and per the research protocol. For example, if the study aims to evaluate the effects of E. coli infection on the lower urinary tract, the researcher will need to prepare the inoculum containing bacteria, which can be inoculated/instilled in the lower urinary tract.
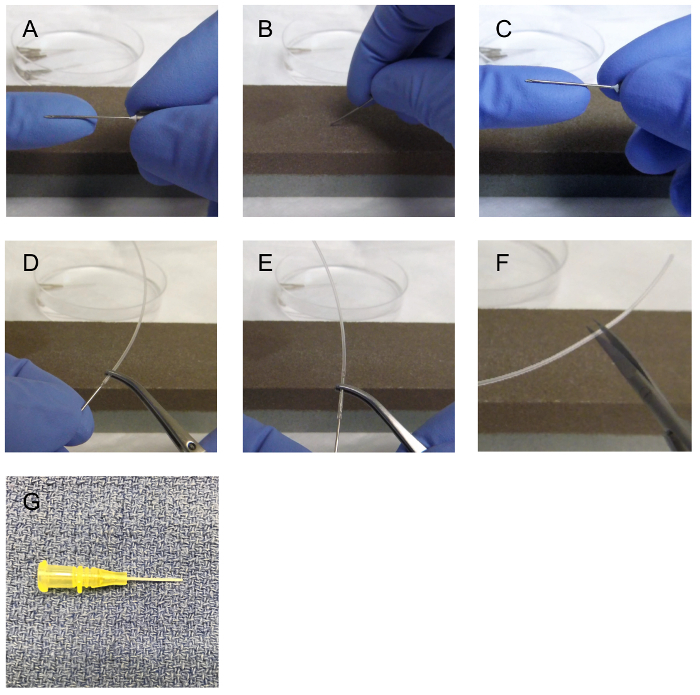
Preparation of the needle attached to PE tubing is simple and consists of the following: Choose the size of PE tubing based on the target organ and the age of an animal. For an animal younger than 12 weeks, use PE-10 tubing (outside diameter is 0.024 inches and inside diameter is 0.011 inches). PE-10 tubing is suggested to be applicable for (1) the primary seeding of inoculum in the anterior prostate (AP) and seminal vesicles (SV), or (2) the primary seeding of inoculum in the bladder of an animal younger than 12 weeks of age. PE tubing with a larger diameter of up to size PE 50 (outside diameter up to 0.038 inches and inside diameter up to 0.023 inches) can be used for the primary seeding of inoculum in the bladder of the older animals.
Cut approximately 2.5 inches of PE tubing.
- Select a needle that will fit the selected PE diameter to be used. A 27 G needle can fit into the lumen of PE-10 tubing, and a 22 G needle fits the lumen of PE-50 tubing.
- Prepare the needle prior to insertion into the PE tubing by rubbing the sharp edge of the needle on a commercially available oil stone (Figure 1A-B) to make it dull, or use a commercially available blunt needle.
Introduce the dull needle (Figure 1C) into the lumen of the PE tubing. Avoid damage to the lumen or entry into a false lumen by ensuring the needle remains in the center of lumen. (Figure 1D-E). If the PE tubing is damaged during insertion of the dull needle, cut the damaged end and restart the process.
Place the needle-attached PE tubing in 70% of EtOH for sterilization until its use for the procedure. Flush the 70% EtOH through the needle-PE tubing to sterilize the lumen and to ensure there is no leakage from the PE tubing from damage during the process of needle insertion. If there is leakage, discarded the needle-PE tubing. NOTE: The tip of the PE tubing needs to be smooth prior to catheterization. To avoid issues with introducing the PE tubing into the urethra, it is recommended to cut the tip of the PE tubing immediately prior to catheterization (Figure 1F). The suggested minimum length of the PE tubing is 1.5 inches, which includes the part surrounding the needle.
2. Catheterization Procedure
- Prior to induction of anesthesia, empty the bladder of mouse.
- Apply gentle pressure and massage to the lower abdomen of the mouse. These maneuvers typically lead to spontaneous voiding. A full bladder may result in the dilution or immediate leakage any instilled inoculum. NOTE: An additional option to ensure an empty bladder in a mouse is to deprive the animal of water for at least 1 hour before the start of the transurethral instillation procedure. Water deprivation should be approved by the institution as part of the animal protocol.
- Use an anesthesia machine with isoflurane flow (2%) to induce anesthesia.
- Once the animal is anesthetized, place it on a warm heating blanket and cover it with a blue pad with its nose inside a nosecone for the maintenance of continuous anesthesia.
- Confirm that the animal is under anesthesia by checking the pedal reflex (toe pinch).
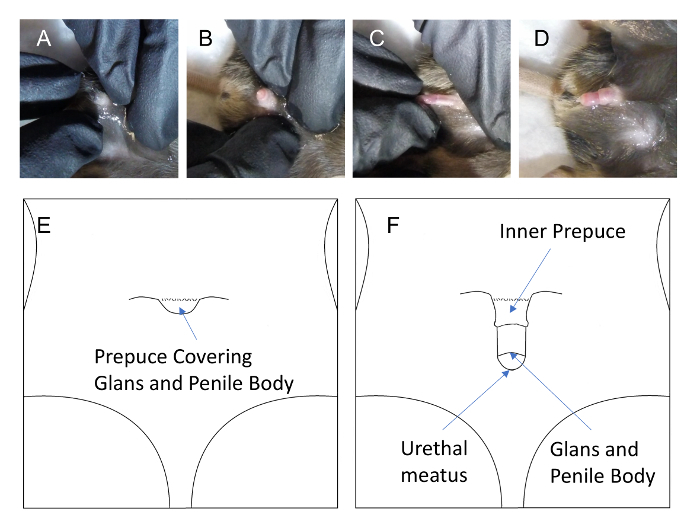
- Once the pedal reflex is absent, proceed by retracting the prepuce.
- Press the pubic region with both the thumb and index finger of one hand (Figures 2Aand 2B), and pull out the glands penis using the thumb and index finger of the other hand (Figure 2C). When this is accomplished correctly, the prepuce, glans penis, and penile body remain exposed. (Figure 2D and Figure 2F). Do not use forceps to pull out glands penis as this will result in damage to the tissue.
Lubricate the tip of the catheter and the glans with sterile surgical lubricant prior to insertion of catheter. This will facilitate insertion of the PE tubing. NOTE: Individually packaged squares of surgical lubricant, instead of one large tube, are recommended for each use to maintain sterile conditions.
Gently hold the tip of the penis using dull forceps and gently squeeze the glans to cause the urethral meatus to open.
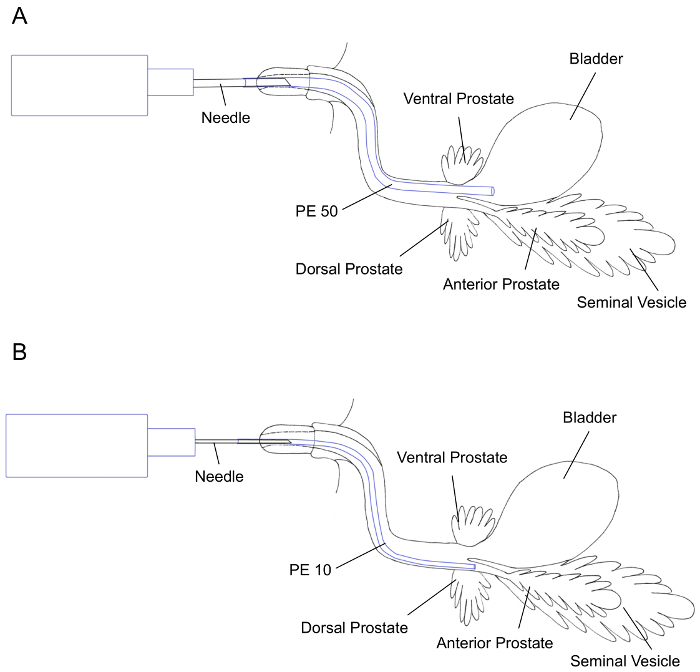
Hold the syringe with the needle-attached PE-tubing or PE tubing itself and insert the PE tubing through the urethral meatus. NOTE: A tripod grip of the syringe with the needle-attached PE-tubing or the PE tubing itself using the thumb, index, and middle fingers is recommended for stability. NOTE: The path of catheterization should be curved due to the natural curvature of the urethra surrounded by the glans penis and penile body in the adult male mouse as shown in Figure 3A and Figure 3B. The catheterized PE tubing may meet resistance during catheterization, therefore, it is recommended to adjust the angle by tilting the tubing up or down to help advance the PE tubing further to the target organ. If needed, the PE tubing can be slightly rotated to help it maneuver through the various sharp turns of urethra. When there is too much resistance, the tubing should be pulled back and then advance again with minimal pressure. Pulling the PE-tubing out completely and using more lubrication may be helpful.
- Perform instillation of the inoculum once the PE-tubing has reached the desired organ by pushing the needle plunger to instill the desired volume of the inoculum (5-200 µL volume is suggested).
- For instillation of the inoculum into the urinary bladder, advance the PE tubing until approximately 0.75 inches of PE tubing is inserted (Figure 3A). NOTE: For instillation of the inoculum in the AP and SV, generally, the inserted tubing meets resistance once it reaches the point of opening into the anterior prostate. By adjusting the angle by tilting the tubing up or down, the PE tubing will advance further until approximately 1 inch of PE tubing has been inserted (Figure 3B).
3. In Vivo Imaging of the Lower Urinary Tract and Kidneys
NOTE: The purpose of in vivo imaging in this manuscript is: (1) to confirm the spatial localization of the inoculum; and (2) to assess the incidence of reflux of the inoculum to the kidneys. For this purpose, either an anesthetized or euthanized mouse can be used.
- To visualize the lower urinary tract of an adult male mouse, perform lower abdominal midline laparotomy to expose the bladder, prostate, and seminal vesicles.
- Incise the skin in the midline from the pubic bone to just below the xiphoid process using scissors.
- Elevate the skin off the subcutaneous tissue.
- Identify the avascular linea alba located in the midline, and incise this to enter the peritoneal cavity where all the organs are located.
- Confirm the spatial localization by visualization of the blue dye or another suitable tracking agent.
Representative Results
Transurethral instillation of blue dye via PE 50 tubing resulted in instillation of the dye into the urinary bladder (Figure 4A), and via PE 10 tubing in colorization of the SV and AP (Figure 4B), respectively. To evaluate whether the performed transurethral instillation causes an instant reflux of the inoculum to the kidneys or not, the kidney and ureter were observed followed by instillation of 100 µL of the blue dye and fluorescent dye (Figure 4A-B). None of the instillation procedure using two different sizes of PE tubing and two different dyes resulted in colorization of the ureters or kidneys.
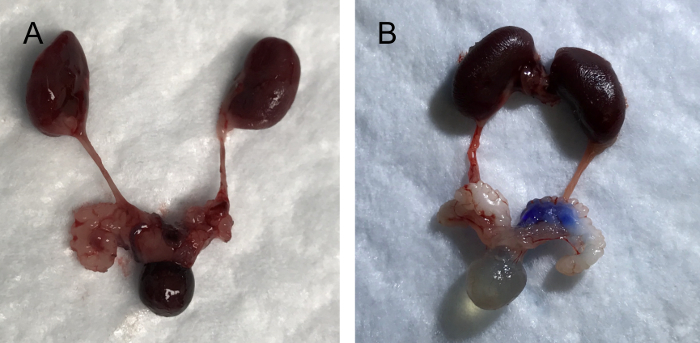
Figure 1:22 G needle preparation for insertion into polyethylene (PE) tubing 50. The sharp needle (A) is grinded against an oil stone (B) to dull the sharp edges (C). Once the needle is dull, the needle is inserted into the lumen of the PE tubing (D) and advanced over the needle with fine forceps (E). The tubing is then cut to approximately 1.5 inches (F). 24 G angiocath (G) can also be used. Please click here to view a larger version of this figure.
Figure 2: Male mouse prepuce covers the glans and urethral meatus (A), with slight traction of prepuce the glans can be exposed (B), and with slight traction of the glans (C), the entire prepuce can be retracted (D), thus allowing for easy access to the urethral meatus and urethra. Please click here to view a larger version of this figure.
Figure 3: Illustrations of lower urinary tract organs; urinary bladder, prostate, and seminal vesicle (SV) showing transurethral instillation using PE 50 tubing (A) and PE 10 tubing (B) for primary seeding of the urinary bladder and SV/anterior prostate, respectively. Please click here to view a larger version of this figure.
Figure 4: Images of upper and lower urinary tract organs; kidneys, urinary bladder, prostate, and seminal vesicle (SV) after transurethral instillation using PE 50 tubing (A) and PE 10 tubing (B). Please click here to view a larger version of this figure.
Discussion
This manuscript describes in detail a method of transurethral instillation in adult male mouse. Differential primary seeding area can be reached by using PE tubing of different diameters. PE-50 is recommended for successfully reaching the urinary bladder lumen, while a larger diameter PE-10 is used to reach the AP and SV (Figure 3A-B). In addition to the choice of size of PE tubing and target organ for inoculation, other factors, such as the volume of instilled inoculum, also play a role in the end location and distribution of the solution. Our experiments demonstrate that transurethral instillation of 100 µL of blue dye using either PE-10 or PE-50 does not trigger the instant reflux of the dye to the kidneys. However, the reflux of urine to the kidneys may happen during the post-instillation period and may result in kidney infection, as was previously shown in adult female mice8. Therefore, histological evaluation of the kidneys is highly recommended to confirm the absence of indirect contamination of the kidneys from transurethral instillations.
There are several important key steps to perform a successful transurethral instillation of the inoculum. First, the PE tubing should be of a sufficient length — at least 2.5 inches. This would allow for additional adjustments of the tubing length, if necessary, during the tubing preparation process, or following the transurethral instillation procedure. Multiple attempts and failures during step 9 of this protocol can cause a blockage of the inserted end of the PE tubing by body fluids. In this situation, the tip of the PE tubing should be cut out to have a clear tip for subsequent attempts. Once the tip of the PE tubing is cut, the surgical lubricant should be re-applied. Second, a proper grip of either the syringe or PE tubing itself does increase the success rate. Third, a sufficient length of the PE tubing should be inserted and the prepuce, glans penis, and penile body should be rigidly lined up without hand grip during the process of catheterization. Otherwise, backflow of the inoculum may happen when the syringe plunger is pushed.
Better outcomes for the transurethral instillation procedure can be a result of practice accompanied by avoiding damage to the urethra and target organ during the procedure. Performance outcomes can be validated by using colored dyes or fluorescent dyes as the procedure to confirm primary localization of the inoculum and any signs of reflux to the kidneys.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors do not have any acknowledgements.
References
- Lee S, Yang G, Bushman W. Prostatic inflammation induces urinary frequency in adult mice. PLoS One. 2015;10(2):e0116827. doi: 10.1371/journal.pone.0116827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L, Hutson PR, Bushman W. Prostatic inflammation induces fibrosis in a mouse model of chronic bacterial infection. PLoS One. 2014;9(6):e100770. doi: 10.1371/journal.pone.0100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkahwaji JE, Zhong W, Hopkins WJ, Bushman W. Chronic bacterial infection and inflammation incite reactive hyperplasia in a mouse model of chronic prostatitis. Prostate. 2007;67(1):14–21. doi: 10.1002/pros.20445. [DOI] [PubMed] [Google Scholar]
- Boehm BJ, Colopy SA, Jerde TJ, Loftus CJ, Bushman W. Acute bacterial inflammation of the mouse prostate. Prostate. 2012;72(3):307–317. doi: 10.1002/pros.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn. 2011;30(5):673–682. doi: 10.1002/nau.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai KH, Thathireddy A, Hsieh MH. Transurethral induction of mouse urinary tract infection. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]
- Bjorling DE, Wang ZY, Boldon K, Bushman W. Bacterial cystitis is accompanied by increased peripheral thermal sensitivity in mice. J Urol. 2008;179(2):759–763. doi: 10.1016/j.juro.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WJ, Gendron-Fitzpatrick A, Balish E, Uehling DT. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect Immun. 1998;66(6):2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis P, Charrua A, Avelino A, Cruz F. Intravesical resiniferatoxin decreases spinal c-fos expression and increases bladder volume to reflex micturition in rats with chronic inflamed urinary bladders. BJU Int. 2004;94(1):153–157. doi: 10.1111/j.1464-4096.2004.04855.x. [DOI] [PubMed] [Google Scholar]
- Cayan S, et al. Botulinum toxin type A may improve bladder function in a rat chemical cystitis model. Urol Res. 2003;30(6):399–404. doi: 10.1007/s00240-002-0291-0. [DOI] [PubMed] [Google Scholar]
- Jerde TJ, Bjorling DE, Steinberg H, Warner T, Saban R. Determination of mouse bladder inflammatory response to E. coli lipopolysaccharide. Urol Res. 2000;28(4):269–273. doi: 10.1007/s002400000114. [DOI] [PubMed] [Google Scholar]
- Saban MR, et al. LPS-sensory peptide communication in experimental cystitis. Am J Physiol Renal Physiol. 2002;282(2):F202–F210. doi: 10.1152/ajprenal.0163.2001. [DOI] [PubMed] [Google Scholar]
- Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166(2):1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- Saban MR, et al. Discriminators of mouse bladder response to intravesical Bacillus Calmette-Guerin (BCG) BMC Immunol. 2007;8:6. doi: 10.1186/1471-2172-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorling DE, et al. Acute acrolein-induced cystitis in mice. BJU Int. 2007;99(6):1523–1529. doi: 10.1111/j.1464-410X.2007.06773.x. [DOI] [PubMed] [Google Scholar]
- Sadler KE, Stratton JM, Kolber BJ. Urinary bladder distention evoked visceromotor responses as a model for bladder pain in mice. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]


