Abstract
Host immunity represents a complex array of factors that evolved to provide protection against potential pathogens. While many factors regulate host immunity, glycan binding proteins (GBPs) appear to play a fundamental role in orchestrating this process. In addition, GBPs also reside at the key interface between host and pathogen. While early studies sought to understand GBP glycan binding specificity, limitations in the availability of test glycans made it difficult to elucidate a detailed understanding of glycan recognition. Recent developments in glycan microarray technology revolutionized analysis of GBP glycan interactions with significant implications in understanding the role of GBPs in host immunity. In this review, we explore different glycan microarray formats with a focus on the impact of these arrays on understanding the binding specificity and function of GBPs involved in immunity.
Introduction
Infectious disease typically represents a breach in host immunity by distinct pathogenic organisms. As individual microbes can pose unique threats, host immunity often deploys specialized factors uniquely tailored to effectively combat potential pathogens. Although many factors regulate this process, a number of glycan binding proteins (GBPs) appear to play a fundamental role in orchestrating host immunity following pathogen exposure [1,2]. In addition, innate and acquired factors also directly engage pathogens through recognition of a diverse range of microbial glycans [1]. Pathogens themselves also utilize GBPs to facilitate host attachment, which not only contributes to host tropism, but also often reflects a fundamental virulence factor required for host invasion [1]. As a result, host and microbial glycans and the GBPs that recognize them represent a key interface in host immunity.
As GBPs regulate fundamental aspects of host immunity and also directly engage pathogens, understanding these interactions can provide important insight into host immunity and infectious disease. However, glycan ligands of GBPs represent complex post-translational modifications that often require the coordinated efforts of many different enzymes [3]. As a result, early studies designed to probe GBP glycan interactions utilized relatively simple sugar substrates [4]. While these studies provided significant insight into the general binding properties of various GBPs [4], the potential impact of subtle glycan alterations remained enigmatic. To address this, many investigators began developing larger libraries of complex carbohydrates to provide a more detailed analysis of GBP glycan interactions [4]. In this review, we will examine the utilization of large libraries of glycans in a variety of glycan microarray formats to understand GBP glycan interactions involved in immunity.
Traditional glycan microarrays
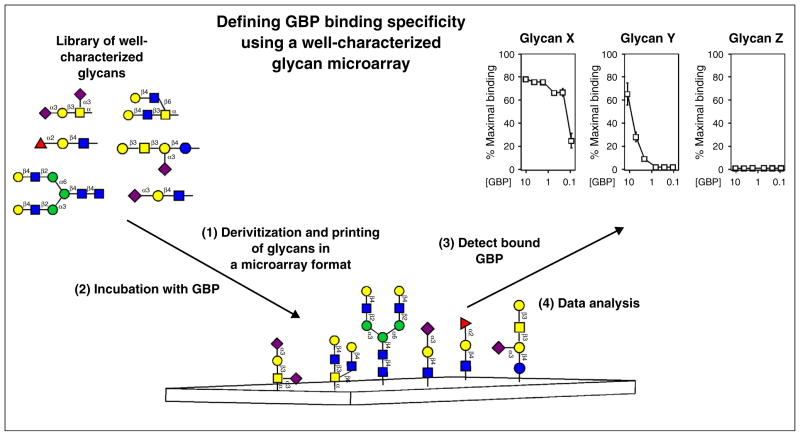
To overcome limitations in the diversity and complexity of glycan test libraries used to evaluate GBP glycan interactions, several groups began to generate larger libraries of glycans harvested from well-characterized glycoproteins or directly synthesized using chemical synthesis or combinatorial chemoenzymatic approaches [5–7]. These libraries contain glycans representative of naturally occurring glycans, as well as uniquely modified chimeric glycans that possessed common core features with alterations not typically found in nature [5,6,8]. While many studies utilized a variety of approaches to interrogate these types of glycan libraries [4], it soon became expedient to use microarray formats to accommodate a growing number of glycans and limited quantities of test GBPs available for interrogation. Furthermore, this approach requires very small amounts of test glycan, thus reducing the quantity of glycan needed to generate large glycan libraries. Using well-characterized glycan microarrays in this manner, careful examination of bound and unbound ligands following microarray analysis provides unique insight into the specificity of a particular GBP with detailed information regarding the impact of glycan modification on GBP recognition (Figure 1) [9].
Figure 1.
Utilization of defined glycan microarrays to elucidate GBP specificity. Libraries of well-characterized glycans generated by release of defined glycans from glycoproteins, other natural sources or by chemical or chemoenzymatic synthesis are used to populate well-defined glycan microarrays. Structures reflect naturally occurring glycans and modifications of glycans not typically found in nature. Glycan libraries undergo derivatization with a functional coupling moiety, followed by printing in a microarray format to generate the glycan microarray. GBPs are incubated with the glycan microarrays over different concentrations and detected by fluorescence emission if directly labeled or by a similarly labeled suitable secondary detecting agent. While many approaches can be taken to analyze glycan array data, examination of GBP binding over a variety of concentrations for individual glycans is shown.
Shotgun glycan microarrays
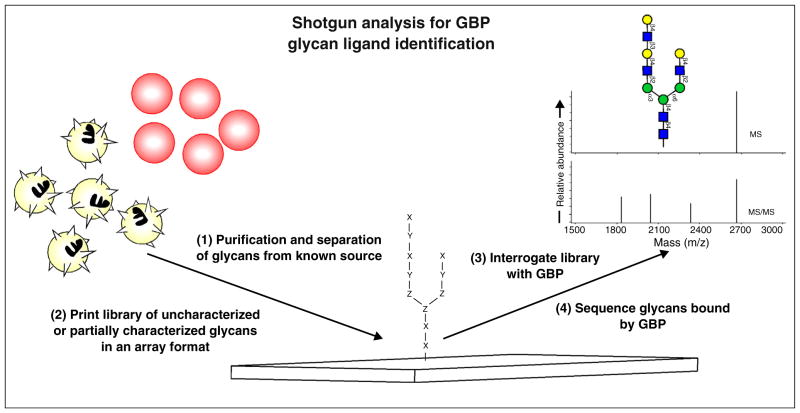
While well-defined glycan microarrays provide important insight regarding the specificity of GBP glycan interactions, synthetic or other limitations may reduce the overall breadth of a particular library. Furthermore, as the entire glycome of pathogens and hosts remains undefined, actual glycan ligands for a particular GBP may not be represented on a particular glycan microarray. To overcome this limitation, recent approaches generated large libraries of test glycans directly from natural sources [10•,11] (Figure 2). While this approach does not lend itself to similar analysis of bound and unbound glycans using chemically defined targets, it does provide the potential to characterize actual glycan ligands isolated from native sources for a particular GBP [10•,11]. Characterization of glycan ligands using this approach also enables prioritization of native glycans for sequencing, thus facilitating a functional approach to glycomic analysis [10•] (Figure 2). Thus, the shotgun glycan microarray represents a true functional glycomic approach where none of the glycan structures are known before their interrogation with biologically relevant GBPs [12]. Designer microarrays, on the other hand, incorporate structurally characterized naturally occurring glycans in addition to modifications of these glycans designed to elucidate detailed binding specificities of GBPs [12,13].
Figure 2.
Using shotgun glycan microarrays to identify native glycan ligands for GBPs. Glycans released from natural sources, such as red blood cells or neutrophils shown in the schematic, are derivatized with a functional linker, subjected to multidimensional chromatography and printed in a microarray format. Following interrogation of the library with GBP, glycan fractions isolated following multidimensional chromatography that correspond to GBP bound ligands are subjected to mass spec glycan sequencing to identify native glycans recognized by GBP.
Microbial glycan microarrays
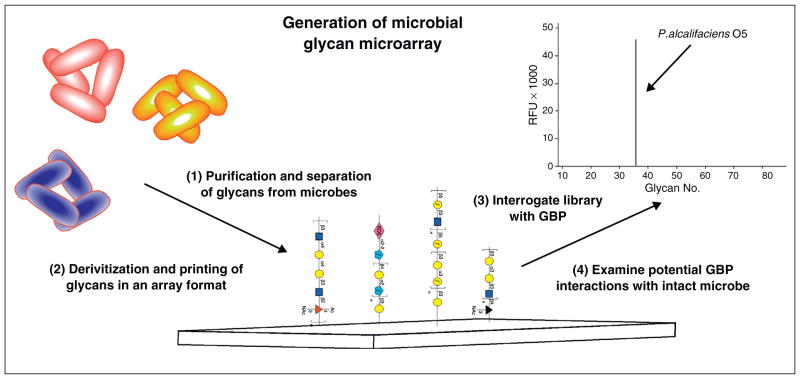
Although glycan microarrays largely based on mammalian glycans continue to provide significant insight into host GBP interactions with pathogens [13–17], the glycan antigens of pathogens themselves represent a broad range of structures that incorporate diverse monosaccharides not typically found in the mammalian glycome. As a result, only limited information can be obtained following interrogation of host GBPs that recognize potential pathogens using conventional glycan microarray approaches. In order to provide a more representative repertoire of the complex glycans that decorate various pathogens, recent microarrays utilized synthetic microbial glycans or glycans harvested directly from known microbes [18–21] (Figure 3). Utilization of microbial glycans in this way provides a unique platform of structurally diverse microbial glycans to interrogate the specificity of host factors directly involved in host pathogen interactions.
Figure 3.
Using microbial glycans to examine host pathogen interactions. Glycans, in particular lipopolysaccharide O antigens, released from well-characterized microbes, undergo derivatization and printing in a microarray format. GBPs with putative pathogen recognition activity or sera are incubated with the microarray to examine potential interactions with microbial glycans. Positive interactions are confirmed by examining GBP or sera with intact microbe.
Using glycan microarrays to understand GBPs involved in immune regulation
Although glycan array analysis has been conducted on a variety of GBPs with various roles in immune regulation, array analysis of the galectin family provides a unique example of the utility of using defined and shotgun microarrays when seeking to elucidate GBP binding specificity. While early studies suggested that galectins primarily recognize β-galactoside containing glycans [4], the impact of β-galactose or other glycan modifications on galectin recognition remained difficult to define, partly due to the apparent promiscuity of galectin family members toward a wide variety of β-galactoside containing glycans. Examination of galectins on the glycan microarray over a range of concentrations began to provide significant insight into the glycan binding preferences used by individual members of the galectin family to engage leukocytes [22–25]. These results demonstrated that individual galectins display a high degree of specificity toward terminal or internal lactosamine within polylactosamine (polyLacNAc) glycans. Differences in lactosamine recognition can differentially impact the binding of individual family members following terminal sialic acid modifications with significant implications on galectin-mediated signaling [23,24,26]. These studies also suggested that tandem repeat galectins, such as Gal-8, might exist as a dimer, demonstrating that glycan microarrays can provide unique insight into the quaternary structures and domains of galectins responsible for signaling [23–25,27,28,29•]. Examination of galectin interactions with naturally occurring glycans on a shotgun glycan array extended these previous findings and demonstrated that galectins display significant preference for N glycans with extended polyLacNAc sequences [30]. Analysis of cell surface glycan binding largely corroborated these results [23,31].
Similar utilization of glycan microarrays provided significant insight into the binding specificity of other GBPs known to regulate host immunity, such as siglecs and C-type lectins. For example, while recognition of sialic acid became a defining feature of siglec family members, the potential impact of sialic acid presentation and modification of penultimate monosacchrides remained relatively unknown. Using the glycan microarray, Bochner and colleagues discovered that siglec 8 specifically recognizes 6′-sulfo-sLex, among over 40 other sialylated glycans present on the microarray, which suggested that siglec 8 displays exquisite specificity for unique sialic acid presentation [32]. Similar utilization of the glycan microarray demonstrated that murine homologs of human macrophage galactose-type lectin (MGL), designated MGL1 and MGL2, display distinct binding preferences for Lex or Lea and N-acetylgalactosamine, respectively [33].
Utilization of glycan microarrays to elucidate pathogen interactions with host glycans
Analysis of pathogen GBP binding specificity using glycan microarrays can also offer significant insight into pathogen interaction with host glycans. Influenza provides one of the most classic examples of viral interactions that depend on distinct host glycans for infectivity. While sialic acid recognition by the influenza hemagglutinin (HA) represents a fundamental step in virion attachment and subsequent infection, array analysis provided significant insight into the binding specificity of different strains, including the impact of HA mutations on glycan recognition [34,35]. Although glycan microarrays with defined glycan structures can provide significant insight into the binding specificity of HA, a recent study suggested that the HA binding specificity obtained from currently defined glycan microarrays failed to accurately predict the actual glycans required for replication in the lung [36]. As a result, utilization of shotgun glycan arrays containing glycans harvested from natural sources will likely be critical in developing a complete picture of HA glycan interactions [10•], illustrating the utility of using combined glycan array approaches when seeking to elucidate GBP specificity.
Similar to influenza, utilization of glycan microarrays provided important information regarding the glycan binding specificities of other pathogen GBPs. For example, glycan microarray analysis identified GD1a as a glycan ligand for adenovirus 37 (Ad37), a common cause of keratoconjunctivitis [37••]. A subsequent study using a similar approach identified GM2 as a key ligand for serotype 1 reovirus [38]. Similar utilization of various glycan microarrays enabled identification of glycan ligands for other viral GBPs, including SV40, parvovirus and the HA of parainfluenza [39–41]. Analogous approaches identified sialyl-lactosamine glycans as ligands for TgMIC1, a Toxoplasma gondi GBP adhesion protein [42], while unique DNA conjugated glycan microarrays examined the impact of galactose or fucose presentation on Pseudomonas aeruginosa adhesion lectin 1 or IIL binding, respectively [43,44]. While many of these studies employed recombinant GBPs, examination of intact Helicobacter pylori on glycan microarrays facilitated the identification of sialic acid binding adhesion protein, SabA [45].
Glycan microarrays in the evaluation of host interactions with pathogens
In addition to examining pathogen interactions with host glycans, the potential specificity of host immune responses to particular glycans can also be examined using glycan microarrays. Examination of sera isolated from healthy human volunteers demonstrated that antibodies possess the ability to react with many different glycan structures [46]. However, following exposure to a microbe, very specific anti-glycan antibodies can be observed. For example, analysis of sera isolated from patients with lyme disease using shotgun glycan microarrays provided unique insight into the glycan binding specificity of sera for rare GD1b-lactone epitopes [10•]. Microarray studies also demonstrated that monoclonal antibodies against β-1,3 glucan possess the capacity to provide protection against fungal infection [47]. Similarly, recent studies described the binding specificity of various neutralizing anti-HIV antibodies, including a very broad and potent class of neutralizing antibodies that recognize Man8GlcNAc2 and Man9GlcNAc2 glycans [48••].
Microbial glycan microarrays have likewise been employed to examine serological reactivity of individuals following microbial infection using glycan antigens isolated from different species and strains of Salmonella [19]. Utilization of similar microarray approaches demonstrated that anti-dextran antibodies can display significant cross-reactivity between microbial and mammalian glycans [49]. Similar array platforms demonstrated the utility of pathogen carbohydrates in the serological diagnosis of Burkholderia pseudomallei and Burkholderia mallei infections [20]. Development of glycopeptide arrays bearing a variety of Tn antigen-possessing glycopeptides demonstrated that individuals infected with Cryptosporidium parvum developed significant and specific seroreactivity toward distinct Tn glycopeptides [21].
Utilization of glycan microarrays not only facilitated the detection of serological specificity toward microbes, but also provided insight into the binding specificity of innate immune GBPs that directly engage pathogens. Utilization of ‘designer’ microarray formats provided significant insight into the minimal β-glucan polymer length needed for efficient Dectin-1 glycan engagement [13]. Similarly, mycobacterial glycan microarrays provided unique insight into the impact of mannose polymer length in the binding affinity of DC SIGN for phosphatidylinositol mannoside precursors of complex mycobacterial glycolipids [18]. Recent utilization of glycan microarrays also identified various high mannose structures as ligands for GBPs that may inhibit or facilitate HIV transmission [14,16].
While many innate immune GBPs primarily recognize unique microbial determinants, recent studies using glycan microarrays demonstrated that several innate immune GBPs appear to preferentially bind mammalian carbohydrate blood group antigens. As ABO(H) blood group antigen expression in blood group positive individuals prevents anti-blood group antibody formation, these results suggested that these innate immune GBPs, in particular galectin-4 (Gal-4) and galectin-8 (Gal-8), may be uniquely poised to fill this gap in adaptive immunity. Indeed, Gal-4 or Gal-8 not only specifically recognized blood group expressing microbes, but also induced direct microbial killing [29•]. These results provide the first example of innate immunity against molecular mimicry. Future studies using analogous approaches with highly defined glycans isolated from pathogens will not only provide unique insight into host immune recognition of pathogen glycans, but also may enable elucidation of the key binding determinants required for these interactions (Figure 3).
Using glycan microarrays to discover GBPs
While glycan microarrays continue to provide significant insight into the binding specificity of known GBPs, recent advances may facilitate the identification of previously unrecognized GBPs. For example, while most glycan microarray approaches examine the binding specificity of previously derivatized GBPs, recent advances may allow detection of non-derivatized proteins, such as GBPs [50•]. This approach would be predicted to eliminate the potential influence of derivatization on GBP glycan recognition. In addition, removing the requirement for derivatization may facilitate the detection of previously unknown GBPs isolated from various cellular sources following glycan microarray analysis, followed by utilizing this information to isolate and characterize bound GBPs.
Conclusions
Given the involvement of GBPs in the regulation of host immunity and facilitation of host pathogen interactions, understanding GBP glycan specificity represents a fundamental step in understanding immunity. Before the advent of glycan microarrays, work by many investigators identified and characterized salient features of many GBPs. However, the development and utilization of glycan microarrays provides unprecedented ability to understand the complexity of GBP binding specificity and function. Expansion of existing glycan microarray formats and development of novel technologies will continue to increase our understanding of GBP function, with significant insight into host immunity.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.van Kooyk Y, Rabinovich GA. Protein–glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:93–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 2.Cerliani JP, Stowell SR, Mascanfroni ID, Arthur CM, Cummings RD, Rabinovich GA. Expanding the universe of cytokines and pattern recognition receptors: galectins and glycans in innate immunity. J Clin Immunol. 2011;31:10–21. doi: 10.1007/s10875-010-9494-2. [DOI] [PubMed] [Google Scholar]
- 3.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5:1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- 4.Cummings RD, Esko JD. Principles of glycan recognition. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. NY: Cold Spring Harbor; 2009. [PubMed] [Google Scholar]
- 5.Fukui S, Feizi T, Galustian C, Lawson AM, Chai W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate–protein interactions. Nat Biotechnol. 2002;20:1011–1017. doi: 10.1038/nbt735. [DOI] [PubMed] [Google Scholar]
- 6.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disney MD, Seeberger PH. The use of carbohydrate microarrays to study carbohydrate–cell interactions and to detect pathogens. Chem Biol. 2004;11:1701–1707. doi: 10.1016/j.chembiol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD. Human galectin-1 recognition of poly-n-acetyllactosamine and chimeric polysaccharides. Glycobiology. 2004;14:157–167. doi: 10.1093/glycob/cwh018. [DOI] [PubMed] [Google Scholar]
- 9.Smith DF, Song X, Cummings RD. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 2010;480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- 10•.Song X, Lasanajak Y, Xia B, Heimburg-Molinaro J, Rhea JM, Ju H, Zhao C, Molinaro RJ, Cummings RD, Smith DF. Shotgun glycomics: a microarray strategy for functional glycomics. Nat Methods. 2011;8:85–90. doi: 10.1038/nmeth.1540. In this study, the ability to harvest and separate a wide variety of glycans obtained from natural sources is described. This approach offers a unique ability to not only rapidly expand the repertoire of glycans on a microarray, but also affords the possibility of examining native glycans for various glycan binding proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Mishra S, Song X, Lasanajak Y, Bradley KC, Tappert MM, Air GM, Steinhauer DA, Halder S, Cotmore S, Tattersall P, et al. Functional glycomic analysis of human milk glycans reveals the presence of virus receptors and embryonic stem cell biomarkers. J Biol Chem. 2012;287:44784–44799. doi: 10.1074/jbc.M112.425819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DF, Cummings RD. Application of microarrays for deciphering the structure and function of the human glycome. Mol Cell Proteomics. 2013;12:902–912. doi: 10.1074/mcp.R112.027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodriguez E, Campanero-Rhodes MA, Costa J, Gordon S, Brown GD, Chai W. Ligands for the beta-glucan receptor, dectin-1, assigned using designer microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281:5771–5779. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- 14.Ratner DM, Seeberger PH. Carbohydrate microarrays as tools in HIV glycobiology. Curr Pharm Des. 2007;13:173–183. doi: 10.2174/138161207779313650. [DOI] [PubMed] [Google Scholar]
- 15.Calarese DA, Lee HK, Huang CY, Best MD, Astronomo RD, Stanfield RL, Katinger H, Burton DR, Wong CH, Wilson IA. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci U S A. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams EW, Ratner DM, Bokesch HR, McMahon JB, O’Keefe BR, Seeberger PH. Oligosaccharide and glycoprotein microarrays as tools in HIV glycobiology: glycan-dependent gp120/protein interactions. Chem Biol. 2004;11:875–881. doi: 10.1016/j.chembiol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 17.McFeeters RL, Xiong C, O’Keefe BR, Bokesch HR, McMahon JB, Ratner DM, Castelli R, Seeberger PH, Byrd RA. The novel fold of scytovirin reveals a new twist for antiviral entry inhibitors. J Mol Biol. 2007;369:451–461. doi: 10.1016/j.jmb.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boonyarattanakalin S, Liu X, Michieletti M, Lepenies B, Seeberger PH. Chemical synthesis of all phosphatidylinositol mannoside (PIM) glycans from Mycobacterium tuberculosis. J Am Chem Soc. 2008;130:16791–16799. doi: 10.1021/ja806283e. [DOI] [PubMed] [Google Scholar]
- 19.Blixt O, Hoffmann J, Svenson S, Norberg T. Pathogen specific carbohydrate antigen microarrays: a chip for detection of Salmonella O-antigen specific antibodies. Glycoconj J. 2008;25:27–36. doi: 10.1007/s10719-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 20.Parthasarathy N, DeShazer D, England M, Waag DM. Polysaccharide microarray technology for the detection of Burkholderia pseudomallei and Burkholderia mallei antibodies. Diagn Microbiol Infect Dis. 2006;56:329–332. doi: 10.1016/j.diagmicrobio.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimburg-Molinaro J, Priest JW, Live D, Boons GJ, Song X, Cummings RD, Mead JR. Microarray analysis of the human antibody response to synthetic Cryptosporidium glycopeptides. Int J Parasitol. 2013;43:901–907. doi: 10.1016/j.ijpara.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poland PA, Rondanino C, Kinlough CL, Heimburg-Molinaro J, Arthur CM, Stowell SR, Smith DF, Hughey RP. Identification and characterization of endogenous galectins expressed in Madin Darby canine kidney cells. J Biol Chem. 2011;286:6780–6790. doi: 10.1074/jbc.M110.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stowell SR, Arthur CM, Slanina KA, Horton JR, Smith DF, Cummings RD. Dimeric galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem. 2008;283:20547–20559. doi: 10.1074/jbc.M802495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsson S, Oberg CT, Carlsson MC, Sundin A, Nilsson UJ, Smith D, Cummings RD, Almkvist J, Karlsson A, Leffler H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology. 2007;17:663–676. doi: 10.1093/glycob/cwm026. [DOI] [PubMed] [Google Scholar]
- 26.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of th1, th2 and th-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 27.Earl LA, Bi S, Baum LG. Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology. 2011;21:6–12. doi: 10.1093/glycob/cwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, Karmakar S, Mehta P, Dias-Baruffi M, McEver RP, Cummings RD. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem. 2009;284:4989–4999. doi: 10.1074/jbc.M808925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, Ju T, Molinaro RJ, Rivera-Marrero C, Xia B, Smith DF, et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med. 2010;16:295–301. doi: 10.1038/nm.2103. Screening of various innate immune proteins using glycan microarrays identified a series of innate immune factors capable of providing immune protection against molecular mimicry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem Biol. 2009;16:36–47. doi: 10.1016/j.chembiol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karmakar S, Stowell SR, Cummings RD, McEver RP. Galectin-1 signaling in leukocytes requires expression of complex-type n-glycans. Glycobiology. 2008;18:770–778. doi: 10.1093/glycob/cwn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. Glycan array screening reveals a candidate ligand for siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 33.Singh SK, Streng-Ouwehand I, Litjens M, Weelij DR, Garcia-Vallejo JJ, van Vliet SJ, Saeland E, van Kooyk Y. Characterization of murine MGL1 and MGL2 C-type lectins: distinct glycan specificities and tumor binding properties. Mol Immunol. 2009;46:1240–1249. doi: 10.1016/j.molimm.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Childs RA, Palma AS, Wharton S, Matrosovich T, Liu Y, Chai W, Campanero-Rhodes MA, Zhang Y, Eickmann M, Kiso M, Hay A, et al. Receptor-binding specificity of pandemic influenza a (H1N1) 2009 virus determined by carbohydrate microarray. Nat Biotechnol. 2009;27:797–799. doi: 10.1038/nbt0909-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens J, Blixt O, Chen LM, Donis RO, Paulson JC, Wilson IA. Recent avian H5N1 viruses exhibit increased propensity for acquiring human receptor specificity. J Mol Biol. 2008;381:1382–1394. doi: 10.1016/j.jmb.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walther T, Karamanska R, Chan RW, Chan MC, Jia N, Air G, Hopton C, Wong MP, Dell A, Malik Peiris JS, Haslam SM, et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9:e1003223. doi: 10.1371/journal.ppat.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Angstrom J, Hedenstrom M, Eriksson TL, Frangsmyr L, Rinaldi S, Willison HJ, et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011;17:105–109. doi: 10.1038/nm.2267. This study illustrates the utility of having defined glycan microarrays when seeking to elucidate in fine detail the actual glycan ligands required for viral attachment. These finding not only provide biochemical evidence of the glycan ligand, but also suggest potential therapeutic strategies based on glycan microarray findings. [DOI] [PubMed] [Google Scholar]
- 38.Reiss K, Stencel JE, Liu Y, Blaum BS, Reiter DM, Feizi T, Dermody TS, Stehle T. The GM2 glycan serves as a functional coreceptor for serotype 1 reovirus. PLoS Pathog. 2012;8:e1003078. doi: 10.1371/journal.ppat.1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song X, Yu H, Chen X, Lasanajak Y, Tappert MM, Air GM, Tiwari VK, Cao H, Chokhawala HA, Zheng H, Cummings RD, et al. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J Biol Chem. 2011;286:31610–31622. doi: 10.1074/jbc.M111.274217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amonsen M, Smith DF, Cummings RD, Air GM. Human parainfluenza viruses hPiV1 and hPiV3 bind oligosaccharides with alpha2–3-linked sialic acids that are distinct from those bound by H5 avian influenza virus hemagglutinin. J Virol. 2007;81:8341–8345. doi: 10.1128/JVI.00718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campanero-Rhodes MA, Smith A, Chai W, Sonnino S, Mauri L, Childs RA, Zhang Y, Ewers H, Helenius A, Imberty A, Feizi T. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J Virol. 2007;81:12846–12858. doi: 10.1128/JVI.01311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garnett JA, Liu Y, Leon E, Allman SA, Friedrich N, Saouros S, Curry S, Soldati-Favre D, Davis BG, Feizi T, Matthews S. Detailed insights from microarray and crystallographic studies into carbohydrate recognition by microneme protein 1 (mic1) of Toxoplasma gondii. Protein Sci. 2009;18:1935–1947. doi: 10.1002/pro.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chevolot Y, Zhang J, Meyer A, Goudot A, Rouanet S, Vidal S, Pourceau G, Cloarec JP, Praly JP, Souteyrand E, Vasseur JJ, et al. Multiplexed binding determination of seven glycoconjugates for Pseudomonas aeruginosa lectin I (PA-IL) using a DNA-based carbohydrate microarray. Chem Commun (Camb) 2011;47:8826–8828. doi: 10.1039/c1cc12428e. [DOI] [PubMed] [Google Scholar]
- 44.Gerland B, Goudot A, Pourceau G, Meyer A, Dugas V, Cecioni S, Vidal S, Souteyrand E, Vasseur JJ, Chevolot Y, Morvan F. Synthesis of a library of fucosylated glycoclusters and determination of their binding toward Pseudomonas aeruginosa lectin B (PA-IIL) using a DNA-based carbohydrate microarray. Bioconjug Chem. 2012;23:1534–1547. doi: 10.1021/bc2006434. [DOI] [PubMed] [Google Scholar]
- 45.Walz A, Odenbreit S, Mahdavi J, Boren T, Ruhl S. Identification and characterization of binding properties of Helicobacter pylori by glycoconjugate arrays. Glycobiology. 2005;15:700–708. doi: 10.1093/glycob/cwi049. [DOI] [PubMed] [Google Scholar]
- 46.von Gunten S, Smith DF, Cummings RD, Riedel S, Miescher S, Schaub A, Hamilton RG, Bochner BS. Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. J Allergy Clin Immunol. 2009;123:1268.e15–1276.e15. doi: 10.1016/j.jaci.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torosantucci A, Chiani P, Bromuro C, De Bernardis F, Palma AS, Liu Y, Mignogna G, Maras B, Colone M, Stringaro A, Zamboni S, et al. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE. 2009;4:e5392. doi: 10.1371/journal.pone.0005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. In this work, antibodies with potent and broad neutralizing activity against HIV were screened using a glycan microarray. This approach identified potential carbohydrate ligands as epitopes for these promising antibodies with clear implications in the development of future vaccines and therapeutics against HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Liu S, Trummer BJ, Deng C, Wang A. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat Biotechnol. 2002;20:275–281. doi: 10.1038/nbt0302-275. [DOI] [PubMed] [Google Scholar]
- 50•.Kirk JT, Brault ND, Baehr-Jones T, Hochberg M, Jiang S, Ratner DM. Zwitterionic polymer-modified silicon microring resonators for label free biosensing in undiluted human plasma. Biosensors & Bioelectronics. 2013;42:100–105. doi: 10.1016/j.bios.2012.10.079. These authors describe the development of novel biosensor approach that enables detection of native proteins. This will likely greatly facilitate the development of glycan microarrays that may serve as unique platforms in the discovery of GBPs. [DOI] [PMC free article] [PubMed] [Google Scholar]