Abstract
Glycan binding proteins (GBPs) possess the unique ability to regulate a wide variety of biological processes through interactions with highly modifiable cell surface glycans. While many studies demonstrate the impact of glycan modification on GBP recognition and activity, the relative contribution of subtle changes in glycan structure on GBP binding can be difficult to define. To overcome limitations in the analysis of GBP-glycan interactions, recent studies utilized glycan microarray platforms containing hundreds of structurally defined glycans. These studies not only provided important information regarding GBP–glycan interactions, but have also resulted in significant insight into the binding specificity and biological activity of the galectin family. We will describe the methods used when employing glycan microarray platforms to examine galectin–glycan binding specificity and function.
Keywords: Glycan binding protein (GBP), Galectin, Glycan microarray, GBP–glycan interactions
1 Introduction
Members of the galectin family of carbohydrate binding proteins have been implicated in a wide variety of biological processes including roles in cell signaling, development, and immunity [1–3]. While galectins initially reside within the cytosol where they can regulate various intracellular processes [4–6], most documented activities of galectin family members reflect modulation of cellular behavior following release from cells, where they recognize and crosslink highly modifiable cell surface carbohydrates [3, 7, 8]. Although all galectins share a common affinity for β-galactose terminating carbohydrate structures, variations within the carbohydrate-binding domains of individual galectin family members can lead to differential binding following β-galactose modification [9–11]. Thus, knowledge of the specific binding preferences of individual galectins can provide significant insight into the function and regulatory activity of this protein family.
Various methods have been employed to determine the binding preferences of galectins. Early studies utilized simple monosaccharides, typically in the setting of hemagglutination inhibition assays, to understand general binding preferences of glycan binding proteins (GBPs), including individual members of the galectin family [12–14]. These early studies suggested that galectins typically recognize terminal β-galactoside-containing glycans, although the fine specificity for these ligands and the potential impact of β-galactose modification appeared to differ between individual family members [14, 15]. Subsequent studies utilized a variety of biochemical assays, including isothermal calorimetry, fluorescence polarization, surface plasmon resonance, and frontal affinity chromatography [10, 11, 16–18]. These approaches often used expanded libraries of glycans, which not only contributed to a greater understanding of galectin-carbohydate binding properties, but also provided significant insight into the thermodynamics of galectin–glycan interactions [17, 18].
In an effort to expand on previous findings [12–14, 19], several groups began to generate larger libraries of test glycans [20–22]. These libraries typically contained combinations of naturally occurring and uniquely modified glycans designed to specifically elucidate the binding specificity of GBPs [20, 21, 23–25]. Although these libraries can be utilized in a variety of formats, glycan microarrays allow interrogation of hundreds of structurally distinct glycans while employing small amounts of target glycan and test GBPs [10, 18, 26, 27]. As synthetic limitations may reduce the overall breadth of a particular glycan library, recent strategies also generated glycan microarrays from glycans directly harvested from natural sources [24, 25, 28–34]. Using a combined approach of different glycan microarray strategies, significant insight into galectin–glycan binding specificity and overall biological function can be obtained [11, 16, 35–39].
In this work we will discuss the methods used for examining galectin-glycan binding specificity using a glycan microarray format. The potential limitations and impact of galectin concentration on glycan microarray results and analysis will also be explored. Given the utility of this tool, not only in elucidating the binding specificity, but also in facilitating the discovery of previously unrecognized biological activities of individual members of the galectin family [11, 37, 39], additional studies using various glycan microarray approaches will likely continue to provide significant insight into the biochemical and biological roles of galectins and other GBPs.
2 Materials
2.1 Galectin Preparation
1. Lactosyl-sepharose (Sigma-Aldrich).
2. Isopropyl β- D -1-thiogalactopyranoside (IPTG) (Thermo Scientific).
3. Phosphate Buffered Saline (PBS) (Hyclone).
4. Lactose (Fisher).
5. β-mercaptoethanol (βME) (Fisher).
6. CelLytic B-II Bacterial Cell Lysis/Extraction reagent (Sigma).
7. Complete Mini EDTA-free Protease Inhibitor cocktail tablets (Roche).
8. Lysozyme (Sigma Aldrich).
9. RNase (Thermo Scientific).
10. DNase (Thermo Scientific).
11. Sodium Azide (Sigma Aldrich).
12. Tris Base.
13. Glycine.
14. Sodium Dodecyl Sulfate.
15. Loading buffer (Thermo Scientific).
16. Broad Range STD Molecular Weight Markers (Santa Cruz).
17. 4–20 % or 16 % Tris-Glycine Gel (10 Well) (Life Technologies).
18. Coomassie staining solution (Life Technologies).
19. Methanol.
20. Glacial Acetic Acid.
21. 1.7 mL snap cap microcentrifuge tube (Sigma-Aldrich).
22. 2 L Erlenmeyer flask.
23. 50 mL Erlenmeyer flask.
24. 1 L centrifuge bottles.
Special Equipment
25. Centrifuge.
26. Sonicator.
27. Fraction collector (BioRad).
28. Gel apparatus including voltage source.
Buffers
29. Lysis Buffer = (for 1 L of culture) Use 10 mL of CelLytic B-II Bacterial Cell Lysis/Extraction reagent, 14 mM β-Mercaptoethanol (βME), one Complete Mini EDTA-free Protease Inhibitor cocktail tablet, Lysozyme (1 mg), RNase with a stock of 10 mg/mL (10 μL) and DNase with a stock of 10 mg/mL (10 μL).
30. Wash Buffer = (1 L) 1× PBS with 14 mM βME.
31. Elution Buffer = (1 L) 1× PBS, 14 mM βME and 100 mM Lactose (36 g).
32. Column Storage Buffer = (total volume 500 mL) 1× PBS w/0.02 % Azide, 14 mM βME.
33. 1× Electrophoresis Buffer = 3 g Tris Base, 14.4 g Glycine and 1 g Sodium Dodecyl Sulfate in total volume of 1 L dH2O.
34. Destain Solution = 40 % Methanol and 10 % Glacial Acetic Acid in dH2O.
2.2 Galectin Derivatiztion
1. PD10 column (GE Healthcare).
2. PBS (Hyclone).
3. NHS-LC-Biotin (Thermo-Scientific).
4. Lactose (Fisher).
5. β-mercaptoethanol (Fisher).
6. Lactosyl-sepharose (Fisher).
2.3 Glycan Array Screening
1. Glycan printed slides (NIH Consortium for Functional Glycomics Core D), printed on the side of the slide with the white etched bar code and black marks—DO NOT TOUCH THIS AREA.
2. Cover slips, 24 × 50 (Fisher scientific).
3. Humidified Slide processing chambers (Fisher), or homemade system made with Petri Dish, with wet paper towels in the bottom of the chamber.
4. 100 mL Coplin jars for slide washing.
5. Tris–HCl.
6. NaCl.
7. CaCl2.
8. MgCl2.
9. Potassium Phosphate Monobasic.
10. dH2O.
11. BSA (Fisher scientific).
12. Alexa Fluor-488-Streptavidin (Invitrogen).
13. Tween-20 (EMD Biosciences).
Special Equipment
14. ProScanArray Scanner (Perkin Elmer).
Buffers
15. TSM = 20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2.
16. TSM Wash Buffer (TSMW) = TSM Buffer + 0.05 % Tween-20.
17. TSM Binding Buffer (TSMBB) = TSM buffer + 0.05 % Tween 20 + 1 % BSA.
3 Methods
3.1 Galectin Preparation
1. In a laminar flow hood pour 10 mL of autoclaved LB Media into a sterile 50 mL Erlenmeyer flask or other appropriate container. Add 10 μL of Ampicillin (stock 50 μg/mL) to media (see Note 1).
2. Remove the previously prepared and validated glycerol stock of bacteria transformed with the appropriate galectin expression vector from the −80 °C freezer and place on ice. Place a loopful of bacteria into the LB/Amp media to inoculate starter culture.
3. Place cover on flask loosely and incubate overnight at 37 °C, shaking at 250 revolutions per minute (rpm).
4. Prepare a 2 L Erlenmeyer flask containing 1 L of LB media by adding 20 g of LB Broth powder in 1 L of dH2O in the flask. Mix well and then autoclave on a 30 min liquid cycle to sterilize. Following autoclave cycle, remove flask and allow it to cool to room temperature.
5. Place the 2 L Erlenmeyer flask containing 1 L LB media into the incubator/shaker and allow it to warm to 37 °C before proceeding.
6. Once media is warmed, move media to a culture hood and add 1 mL of Ampicillin (stock concentration 50 μg/mL) to the 1 L of LB media in each flask (see Note 1).
7. Add 10 mL of starter culture to the flask containing 1 L of sterile LB media and antibiotic to inoculate cultures.
8. Place cap on flask loosely and incubate shaking for 2–2.5 h at 37 °C and 250 rpm.
9. Check the cultures OD at 600 nm (OD600) using visible light approximately every 30 min. Blank with autoclaved sterile LB Media. When OD600 of each culture reaches between 0.45 and 0.50, induce the bacteria in each culture to express the recombinant galectin by addition of 0.36 g of IPTG to each flask (see Note 2).
10. Continue IPTG induced growth for 4–5 h at 37 °C and 250 rpm (see Note 3).
11. Collect bacteria by pouring 1 L of culture into 2 (500 mL in each) clean 1 L plastic centrifuge bottles. Spin down @ 4,200 × g for 30 min at 4 °C.
12. Pour off the supernatant and place pelleted bacteria at −80 °C. (Pellet can be stored at −80 °C for up to 2 weeks).
13. Thaw pellets on ice. Add 5 mL of Lysis Buffer to each bottle and resuspend pellets (homogenization). Let resuspension sit at RT for 30 min or at 37 °C for 15 min (see Note 4).
14. Pool the resuspended pellets in a 250 mL plastic centrifuge bottle on ice and sonicate the pellets two to three times for 20 s per cycle (see Note 5).
15. Spin the lysate @ 13,000 × g/4 °C for 30 min.
16. Collect the supernatant without disrupting the pelleted cell debris and place on ice.
Purification
17. Prepare the Lactosyl Sepharose column by washing with 10 column volumes of wash buffer (see Note 6).
18. Apply the supernatant to the column (see Note 7).
19. Collect the supernatant flow through (Sample Flow Through) (see Note 8).
20. Wash the column with 10 more column volumes of Wash Buffer (see Note 9).
21. Elute the recombinant protein by preparing 3 column volumes of Elution Buffer and adding it to the column.
22. Begin to collect 1–2 mL fractions immediately following addition of Elution Buffer.
23. Take 10 μL from each fraction and dilute tenfold (i.e. 10 μL of each fraction in 90 μL Elution Buffer). Read the OD of each dilution at OD at 280 nm (OD280) using a spectrophotometer. Blank with Elution Buffer.
24. Calculate protein concentration using the following equation: OD280 × 10 × extinction coefficient ratio (see Note 10).
25. Identify the peak fractions (the three to five fractions with the highest concentration of protein as determined by OD280).
26. Prepare samples for an SDS PAGE using 10 μL of the “Start Material” and “Sample Flow Through” and the volume that equals 2 μg of total protein for each of the “Peak Fractions”. Use 7.5 μL of 4× Loading Buffer. Bring up the total load volume to 30 μL with dH2O. Load 30 μL into each well.
27. Use Broad Range STD Molecular Weight Markers (Total load volume should be 10 μL).
28. Over a Bunsen burner, allow a pot of dH2O to come to a boil. Place samples in the water and boil for 10 min.
29. Set up gel apparatus and fill with Electrophoresis Buffer.
30. Spin down samples in a table top centrifuge for 5 s before loading on Gel.
31. Load samples into a 4–20 % or 16 % Tris-Glycine Gel (10 Well).
32. Perform SDS-PAGE at 125 V for about 1.5 h.
33. Remove the gel from gel apparatus and stain the gel with Coomassie Stain for 30 min then de-stain for 2 h with de-stain solution. Verify the “Peak Fractions” are the appropriate molecular weight. Take a picture of the gel and dry.
34. Once the protein identity is verified by SDS PAGE, pool all of the fractions that contain galectin protein. Examine protein content by measuring the OD280 of each fraction. This is typically done by diluting each fraction tenfold (i.e. 10 μL of each fraction in 90 μL PBS) followed by examination of the OD280. Blank with Elution Buffer.
35. Calculate protein concentration using following equation: OD280 × 10 × extinction coefficient ratio (see Note 10).
36. Make 500 μL aliquots of the purified galectin protein in 1.7 mL microcentrifuge tubes. Store aliquots at −80 °C.
3.2 Galectin Biotinylation
1. To remove βME, prepare a PD10 column for gel filtration by equilibration with 5 column volumes of cold PBS (pH 7.4) (see Note 11).
2. Thaw frozen stock aliquots of galectins at 4 °C.
3. Add 1 mL of recombinant galectin solution (containing Elution Buffer) to the PBS re-equilibrated PD10 column and collect 0.5 mL fractions.
4. Following complete penetration of the galectin solution into the PD10 column, add 2 mL cold PBS.
5. Continue to collect 0.5 mL fractions while adding additional PBS as needed to elute all protein and prevent the column from drying.
6. To determine which fractions may have galectin protein, examine protein content by measuring the OD280 of each fraction. This is typically done by diluting each fraction tenfold (i.e. 10 μL of each fraction in 90 μL PBS) followed by examination of the OD280 (see Note 10).
7. Once positive fractions are identified, pool galectin containing fractions and reevaluate the OD280 to determine the final concentration of pooled galectin (see Note 12).
8. Add lactose in PBS at a final concentration of 100 mM to facilitate the maintenance of galectin activity during the labeling procedure (see Note 13).
9. Add NHS biotin at the concentration recommended by the manufacturer followed by incubation at RT for 1 h or at 4 °C for 2 h (see Note 14).
10. Remove Lactose and nonreacted NHS biotin by passing the reaction mixture over a new PD10 column, re-equilibrated with cold PBS, using the same approach as outlined in steps 1–7 above.
11. Once pooled biotinylated galectin fractions are collected and evaluated for concentration, add βME at a final concentration of 14 mM to sustain galectin activity for the duration of the additional purification steps and actual experiment (see Note 15).
12. To separate potentially inactive galectin from active galectin, pass pooled biotinylated galectin over a lactosyl-sepharose column re-equilibrated in PBS containing 14 mM βME.
13. Wash the column with 10 column volumes of PBS containing 14 mM βME.
14. Elute bound protein with PBS containing 14 mM βME and 100 mM lactose. Collect 0.5 mL fractions as soon as the elution buffer is added to the column.
15. Evaluate galectin concentration within each collected fraction by diluting each fraction tenfold in PBS containing βME and 100 mM lactose (i.e. 10 μL of each fraction in 90 μL PBS). Be sure to use PBS with βME and 100 mM lactose as a baseline measure of OD280.
16. Once protein-positive fractions are identified, pool galectin containing fractions and reevaluate the OD280 to determine the final galectin concentration.
17. The degree of biotin incorporation can be evaluated by commonly employed mass spectrometric analysis, western blot analysis using HRP-streptavidin or by examining whether engagement of cell surface ligands by biotinylated galectin can be detected using FITC-streptavidin using standard flow cyto-metric analysis (see Note 16).
18. Once biotinylation is documented on active recombinant galectin, these proteins can then be analyzed on the glycan microarray.
3.3 Microarray Probing with Biotinylated Galectin
1. Make TSM, TSMW, and TSMBB or bring them to room temperature if they have made previously and stored at 4 °C.
2. Prepare 100 μL of sample by diluting biotin-labeled galectin protein in TSMBB with 14 mM βME added if needed to maintain galectin activity (see Note 17).
3. Remove slide(s) from desiccator and hydrate by placing in a glass Coplin staining jar containing 100 mL of TSMW for 5 min.
4. Remove excess liquid from slide by setting the slide upright on a paper towel to drain the liquid off.
5. Carefully apply 70 μL of sample made in step 2 above close to the left edge of the slide in between the black marks found on the slide surface.
6. Slowly lower the cover slip onto the slide and gently remove any bubbles trapped between the slide and cover slip by soft tapping with a pipette tip. Be sure the cover slip stays between the black marks on the slide since this is the portion of the slide where glycans are printed.
7. Incubate slide in a humidified tray in the dark for 1 h at RT.
8. After 1 h incubation, remove cover slip by turning the slide onto its side and allowing the cover slip to slip off into the glass trash/biohazard trash.
9. To wash, dip the slide four times into 100 mL of first TSMW and then TSM in Coplin Jars.
10. Set the slide upright on a paper towel to remove excess TSM buffer.
11. Add 70 μL of Streptavidin-AlexaFluor-488 and apply cover slip as outlined above in step 6 and incubate in a humidified tray in the dark for 1 h at RT.
12. After 1 h incubation, remove cover slip by turning the slide onto its side and allowing the cover slip to slip off into the glass trash/biohazard trash.
13. To wash, dip the slide four times into 100 mL of first TSMW followed by TSM and then dH2O in Coplin Jars.
14. Spin slide in slide centrifuge for ~15 s to remove excess water.
15. Scan in a fluorescent Scanner and obtain mean fluorescent intensities for each glycan binding event on the array (see Note 18).
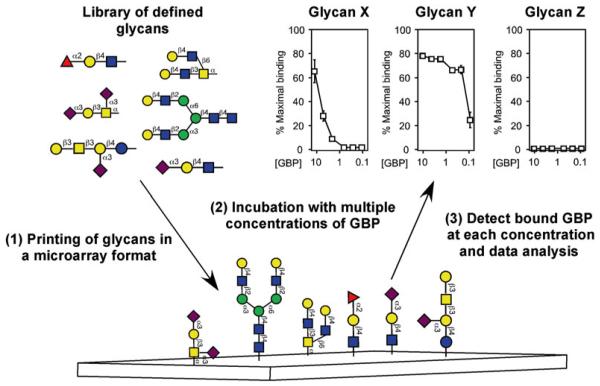
Fig. 1.
Utilization of defined glycan microarrays to elucidate GBP specificity. Libraries of well-characterized glycans generated by release of defined glycans from glycoproteins or other natural sources or by chemical or chemoenzymatic synthesis are used to populate well-defined glycan microarrays. Structures refl ect naturally occurring glycans and modifications of glycans not typically found in nature. Glycan libraries undergo derivatization with a functional coupling moiety, followed by printing in a microarray format to generate the glycan microarray. GBPs are incubated with the glycan microarrays over different concentrations and detected by fl uorescence emission if directly labeled or by a similarly labeled suitable secondary detecting agent. While many approaches can be taken to analyze glycan array data, examination of GBP binding over a variety of concentrations for individual glycans is shown. This research was originally published in the Journal of Biological Chemistry [37] with permission from the American Society for Biochemistry and Molecular Biology
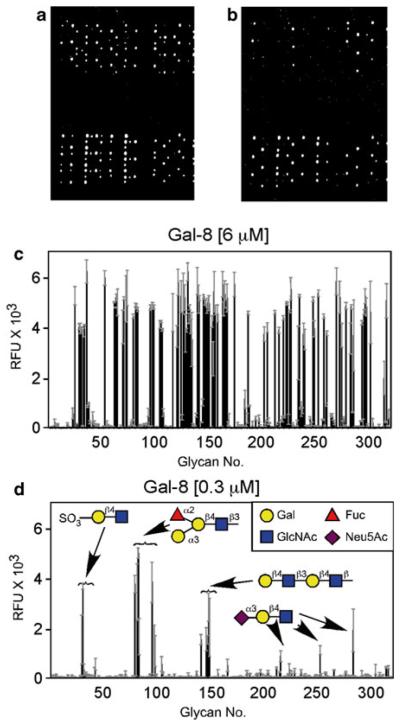
Fig. 2.
Galectins interact with glycan microarray in a concentration-dependent manner. Gal-8 recognizes distinct classes of glycans. (a and b) Examination of the glycan microarray followed incubation of the glycan microarray with 6 μM Gal-8 (a) or 0.3 μM Gal-8 (b). (c and d) Glycan microarray data obtained following incubation with 6 μM Gal-8 (c) or 0.3 μM Gal-8 (d). d inset: Legend of symbols for monosaccharides. This research was originally published in the Journal of Biological Chemistry [37] with permission from the American Society for Biochemistry and Molecular Biology
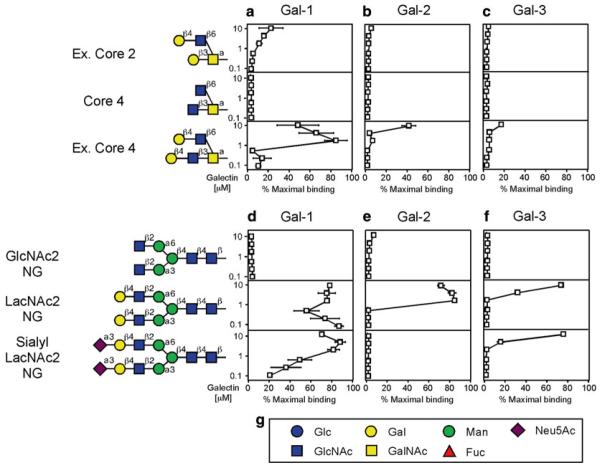
Fig. 3.
Examination of galectins over a range of concentrations can provide a relative affinity for individual ligands on the glycan microarray. Trivial names followed by the structures of each glycan tested are shown. Recognition of each representative glycan is displayed as the percent bound when compared with the highest bound ligand for each respective galectin tested in this study. Glycan recognition of O-glycans is shown for Gal-1 (a), Gal-2 (b), and Gal-3 (c). Glycan recognition of N-glycans is shown for Gal-1 (d), Gal-2 (e), and Gal-3 (f). (g) Legend of symbols for monosaccharides. This research was originally published in the Journal of Biological Chemistry [16] with permission from the American Society for Biochemistry and Molecular Biology
Acknowledgments
This work was supported in part by grants from the National Blood Foundation, American Society of Hematology and Hemophilia of Georgia to S.R.S.
4 Notes
Use the appropriate antibiotic selection protocol based on the antibiotic resistance gene encoded in the expression vector for each recombinant galectin.
IPTG can be dissolved directly into 10 mL of sterile autoclaved media for each 1 L culture. Use the appropriate inducing agent and concentration as outlined by the manufacture for the expression vector used to generate recombinant galectin.
While induction at 0.4–0.5 OD600 followed by 4–5 h of continued incubation yields optimal galectin production for most galectins, variations may occur depending on expression vector, galectin stability, and other expression parameters. In these cases, optimal conditions should be empirically determined.
Use of bacterial lytic reagent when seeking to isolate galectin-2 will results in loss of galectin-2 activity. Instead use PBS when isolating galectin-2. It should also be noted that several methods of bacterial lysis have been published and employed by our lab. Each appears to yield relatively similar overall protein amounts.
Although this may help bacterial lysis and appears to increase the ultimate protein yield, this step is not absolutely required when using the Bacterial lytic reagent.
Assign separate lactosyl sepharose columns for the purification of individual galectin family members and/or mutants to avoid potential contamination between galectins when isolating each protein.
Save some of the supernatant to be analyzed by SDS-PAGE as the “Start Material”.
Some of the “Sample Flow Through” will also be analyzed by SDS-PAGE.
Collect the “Wash Flow Through” in case the column capacity is exceeded. Keep the “Wash Flow Through” separate from the “Sample Flow Through”.
To extrapolate the protein concentration from the OD 280 nm values, use the extinction coefficient for the particular galectin being examined to calculate actual concentration in mg/mL. The following websites http://www.basic.northwestern.edu/biotools/proteincalc.html and http://web.expasy.org/protparam/protparam-doc.html, offer explanation and assistance in calculating the extinction coefficient and using this calculation to determine the actual concentration of a given protein in mg/mL, including caveats as to how these numbers may differ from the actual number. As methods of calculating the extinction coefficient only provide estimates, alternative approaches can be used to empirically determine these values. These include using a Bradford assay to calculate protein concentration or simply reequilibrating the recombinant protein directly into water, lyophilizing, weighing, and then resuspending in the buffer of choice followed by empirically determining the extinction coefficients for a particular galectin family member.
βME can readily inactivate NHS biotin and therefore significantly reduce the efficacy of galectin biotin incorporation.
Reaction efficiency is typically a function of the concentration of the biotin labeling reagent and the protein. We typically label galectins at a concentration of >1 mg/mL to optimize labeling efficiency. If the final concentration is not sufficient, galectins can be readily concentrated using centricon concentrating devices (Millipore) according to the manufacturer’s protocol.
The biotin and galectin reaction mixture will pass over a PD10 column at least once more after the labeling reaction is completed. Since some galectin can be lost with each pass over a column, it is important to start with sufficient galectin to achieve the required amount of end product needed to conduct the experiment.
Many different labeling strategies can be employed when seeking to provide a method of detecting recombinant galectin binding. However, we have found that biotinylation is the least likely to induce inactivation of the galectins following derivatization. In contrast, several galectins readily loose carbohydrate binding activity following direct labeling with fluorescent adducts. As a result, care should be taken to determine the potential impact of galectin derivatization on carbohydrate binding activity. The impact of the reactive label and galectin concentrations and the duration of the labeling reaction, as well as the type of label should be empirically evaluated to assess the potential impact of the label on galectin activity. Partially denatured, yet labeled, galectin can result in nonspecific binding on array platforms and therefore result in false positive hits that may significantly impact the interpretation of the apparent binding preferences for an individual galectin.
Not all galectins are equally sensitive to oxidative inactivation. For example, Gal-3 does not appear to display significant sensitivity to oxidative inactivation in the absence of reducing agents. In contrast, Gal-1, Gal-2, and Gal-7 can display significant loss of activity over time. Other galectins can also lose activity in the absence of reducing conditions, albeit at a reduce rate compared to Gal-1. As a result, the requirement of βME to maintain galectin activity should be empirically determined for each galectin following the labeling procedure.
While mass spectrometric analysis and western blot approaches may provide a general sense of the degree of protein biotinylation and the passage of the protein over a column would in theory allow isolation of active protein, examination of cell surface binding by flow cytometry simultaneously tests whether the galectin is biotinylated and retains gross carbohydrate binding properties.
Cy5-labeled Streptavidin for aligning subarray grids for analysis can be added as a separate step on the slide before adding galectin.
Although glycan microarrays allow rapid examination of GBP–glycan interactions on a single platform, several considerations should be taken when using this approach to study GBP binding specificity. For example, while microarray presentation of glycans fixed on a solid support may be analogous to glycans similarly fixed on a cell surface, they still reflect artificial presentation of potential ligands. As a result, potential alterations in printing density, presentation, coupling formats, and methods of GBP binding detection can impact the overall binding specificity of a particular GBP. Furthermore, some GBPs, such as selectins, exhibit complex interactions with glycan ligands that also include the peptide backbone and other posttranslational modifications [31, 40]. In these situations, more complex libraries of glycopeptides can be used to evaluate the potential influence of non-glycan moieties on GBP–glycan interactions [31, 40].
In addition to potential differences in glycan presentation between individual glycan microarrays and cell surface glycans, very few arrays possess well-defined concentrations of the glycans actually coupled to the array. As a result, differences in GBP binding to unique glycans can also be significantly influenced by variation in glycan coupling efficiency. To reduce the impact of this limitation, analysis of GBP binding over a variety of concentrations can reduce the impact of alterations in printing by providing apparent Kd values toward individual glycans [16, 37]. Although GBPs may be in equilibrium with many different glycans on a microarray and therefore limit the overall accuracy of this approach, evaluating the binding of GBPs at different concentrations still appears to reduce bias based on alterations in printing efficiency. This is especially important when Bmax values between individual glycans differ significantly [16, 37] (Figs. 1, 2, and 3). Regardless of the microarray approach used, validation of findings using alternative methods, including evaluation of cell surface glycans, provides a useful strategy to insure that array findings reflect real interactions with native glycan ligands [11, 16, 19, 37, 39].
References
- 1.Cerliani JP, Stowell SR, Mascanfroni ID, Arthur CM, Cummings RD, Rabinovich GA. Expanding the universe of cytokines and pattern recognition receptors: galectins and glycans in innate immunity. J Clin Immunol. 2011;31(1):10–21. doi: 10.1007/s10875-010-9494-2. doi: 10.1007/s10875-010-9494-2. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DN, Barondes SH. God must love galectins; he made so many of them. Glycobiology. 1999;9(10):979–984. doi: 10.1093/glycob/9.10.979. [DOI] [PubMed] [Google Scholar]
- 3.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12(5):616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572(2–3):263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 5.Dias-Baruffi M, Stowell SR, Song SC, Arthur CM, Cho M, Rodrigues LC, Montes MA, Rossi MA, James JA, McEver RP, Cummings RD. Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle. Glycobiology. 2009;20(5):507–520. doi: 10.1093/glycob/cwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakahara S, Raz A. On the role of galectins in signal transduction. Methods Enzymol. 2006;417:273–289. doi: 10.1016/S0076-6879(06)17019-6. doi: 10.1016/S0076-6879(06)17019-6. [DOI] [PubMed] [Google Scholar]
- 7.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9(6):593–601. doi: 10.1038/ni.f.203. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 8.Cerri DG, Rodrigues LC, Stowell SR, Araujo DD, Coelho MC, Oliveira SR, Bizario JC, Cummings RD, Dias-Baruffi M, Costa MC. Degeneration of dystrophic or injured skeletal muscles induces high expression of galectin-1. Glycobiology. 2008;18(11):842–850. doi: 10.1093/glycob/cwn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19(7–9):433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- 10.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, Kasai K. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572(2–3):232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson S, Oberg CT, Carlsson MC, Sundin A, Nilsson UJ, Smith D, Cummings RD, Almkvist J, Karlsson A, Leffler H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology. 2007;17(6):663–676. doi: 10.1093/glycob/cwm026. doi: 10.1093/glycob/cwm026. [DOI] [PubMed] [Google Scholar]
- 12.Teichberg VI, Silman I, Beitsch DD, Resheff G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci U S A. 1975;72(4):1383–1387. doi: 10.1073/pnas.72.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi G, Teichberg VI. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981;256(11):5735–5740. [PubMed] [Google Scholar]
- 14.de Waard A, Hickman S, Kornfeld S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J Biol Chem. 1976;251(23):7581–7587. [PubMed] [Google Scholar]
- 15.Pritchett TJ, Brossmer R, Rose U, Paulson JC. Recognition of monovalent sialosides by influenza virus H3 hemagglutinin. Virology. 1987;160(2):502–506. doi: 10.1016/0042-6822(87)90026-2. [DOI] [PubMed] [Google Scholar]
- 16.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283(15):10109–10123. doi: 10.1074/jbc.M709545200. doi: 10.1074/jbc.M709545200. M709545200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad N, Gabius HJ, Sabesan S, Oscarson S, Brewer CF. Thermodynamic binding studies of bivalent oligosaccharides to galectin-1, galectin-3, and the carbohydrate recognition domain of galectin-3. Glycobiology. 2004;14(9):817–825. doi: 10.1093/glycob/cwh095. doi: 10.1093/glycob/cwh095. [DOI] [PubMed] [Google Scholar]
- 18.Brewer CF. Thermodynamic binding studies of galectin-1, -3 and -7. Glycoconj J. 2004;19(7–9):459–465. doi: 10.1023/B:GLYC.0000014075.62724.d0. doi: 10.1023/B:GLYC.0000014075.62724.d0. [DOI] [PubMed] [Google Scholar]
- 19.Karmakar S, Stowell SR, Cummings RD, McEver RP. Galectin-1 signaling in leukocytes requires expression of complex-type N-glycans. Glycobiology. 2008;18(10):770–778. doi: 10.1093/glycob/cwn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukui S, Feizi T, Galustian C, Lawson AM, Chai W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat Biotechnol. 2002;20(10):1011–1017. doi: 10.1038/nbt735. doi: 10.1038/nbt735. nbt735 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101(49):17033–17038. doi: 10.1073/pnas.0407902101. doi: 10.1073/pnas.0407902101. 0407902101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Disney MD, Seeberger PH. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem Biol. 2004;11(12):1701–1707. doi: 10.1016/j.chembiol.2004.10.011. doi: 10.1016/j.chembiol.2004.10.011. S1074-5521(04)00312-6 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology. 2004;14(2):157–167. doi: 10.1093/glycob/cwh018. [DOI] [PubMed] [Google Scholar]
- 24.Arthur CM, Cummings RD, Stowell SR. Using glycan microarrays to understand immunity. Curr Opin Chem Biol. 2014;18C:55–61. doi: 10.1016/j.cbpa.2013.12.017. doi: 10.1016/j.cbpa.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stowell SR, Arthur CM, McBride R, Berger O, Razi N, Heimburg-Molinaro J, Rodrigues JP, Noll AJ, von Gunten S, Smith DF, Knirel YA, Paulson JC, Cummings RD. Microbial glycan microarrays define key features of host-microbial interactions. Nat Chem Biol. 2014;10(6):470–6. doi: 10.1038/nchembio.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric galectin-1 binds with high affinity to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J Biol Chem. 2005;280(7):5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- 27.Sorme P, Kahl-Knutson B, Wellmar U, Nilsson UJ, Leffler H. Fluorescence polarization to study galectin-ligand interactions. Methods Enzymol. 2003;362:504–512. doi: 10.1016/S0076-6879(03)01033-4. doi: 10.1016/S0076-6879(03)01033-4. S0076687903010334 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Song X, Lasanajak Y, Xia B, Heimburg-Molinaro J, Rhea JM, Ju H, Zhao C, Molinaro RJ, Cummings RD, Smith DF. Shotgun glycomics: a microarray strategy for functional glycomics. Nat Methods. 2011;8(1):85–90. doi: 10.1038/nmeth.1540. doi: 10.1038/nmeth.1540. nmeth.1540 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Mishra S, Song X, Lasanajak Y, Bradley KC, Tappert MM, Air GM, Steinhauer DA, Halder S, Cotmore S, Tattersall P, Agbandje-McKenna M, Cummings RD, Smith DF. Functional glycomic analysis of human milk glycans reveals the presence of virus receptors and embryonic stem cell biomarkers. J Biol Chem. 2012;287(53):44784–44799. doi: 10.1074/jbc.M112.425819. doi: 10.1074/jbc.M112.425819. M112.425819 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodriguez E, Campanero-Rhodes MA, Costa J, Gordon S, Brown GD, Chai W. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281(9):5771–5779. doi: 10.1074/jbc.M511461200. doi: 10.1074/jbc.M511461200. M511461200 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Song X, Heimburg-Molinaro J, Dahms NM, Smith DF, Cummings RD. Preparation of a mannose-6-phosphate glycan microarray through fluorescent derivatization, phosphorylation, and immobilization of natural high-mannose N-glycans and application in ligand identification of P-type lectins. Methods Mol Biol. 2012;808:137–148. doi: 10.1007/978-1-61779-373-8_9. doi: 10.1007/978-1-61779-373-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song X, Yu H, Chen X, Lasanajak Y, Tappert MM, Air GM, Tiwari VK, Cao H, Chokhawala HA, Zheng H, Cummings RD, Smith DF. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J Biol Chem. 2011;286(36):31610–31622. doi: 10.1074/jbc.M111.274217. doi: 10.1074/jbc.M111.274217. M111.274217 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knirel YA, Gabius HJ, Blixt O, Rapoport EM, Khasbiullina NR, Shilova NV, Bovin NV. Human tandem-repeat-type galectins bind bacterial non-betaGal polysaccharides. Glycoconj J. 2014;31(1):7–12. doi: 10.1007/s10719-013-9497-3. doi: 10.1007/s10719-013-9497-3. [DOI] [PubMed] [Google Scholar]
- 34.Geissner A, Anish C, Seeberger PH. Glycan arrays as tools for infectious disease research. Curr Opin Chem Biol. 2014;18C:38–45. doi: 10.1016/j.cbpa.2013.11.013. doi: 10.1016/j.cbpa.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, Karmakar S, Mehta P, Dias-Baruffi M, McEver RP, Cummings RD. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem. 2009;284(8):4989–4999. doi: 10.1074/jbc.M808925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180(5):3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 37.Stowell SR, Arthur CM, Slanina KA, Horton JR, Smith DF, Cummings RD. Dimeric galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem. 2008;283(29):20547–20559. doi: 10.1074/jbc.M802495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poland PA, Rondanino C, Kinlough CL, Heimburg-Molinaro J, Arthur CM, Stowell SR, Smith DF, Hughey RP. Identification and characterization of endogenous galectins expressed in Madin Darby canine kidney cells. J Biol Chem. 2011;286(8):6780–6790. doi: 10.1074/jbc.M110.179002. doi: 10.1074/jbc.M110.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, Ju T, Molinaro RJ, Rivera-Marrero C, Xia B, Smith DF, Cummings RD. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med. 2010;16(3):295–301. doi: 10.1038/nm.2103. doi: 10.1038/nm.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leppanen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275(50):39569–39578. doi: 10.1074/jbc.M005005200. doi: 10.1074/jbc.M005005200. M005005200 [pii] [DOI] [PubMed] [Google Scholar]