Abstract
Background
In 2011, 15.8% of eligible patients in the US were vaccinated against herpes zoster (HZ).
Purpose
To increase usage of the HZ vaccine by studying physicians’ knowledge, attitudes, practices, and perceived obstacles after interventions to overcome barriers.
Methods
General internal medicine (GIM) physicians were surveyed with a cross-sectional internet survey October to December 2011 before interventions to increase use of the HZ vaccine and 1 year later. Interventions included education, increasing availability at the medical center pharmacy, and electronic medical record reminders. Outcome measures included changes in knowledge, attitudes, and practices, and perceived barriers. McNemar chi square tests were used to compare the changes from the baseline survey for physicians who completed the follow up survey.
Results
Response rate for the baseline study was 33.5% (89/266) and for the follow-up 29.8% (75/252). 55 completed both surveys. There was a decrease from 57% at baseline to 40% at follow-up in the proportion of physicians who reported less than 10% of their patients were vaccinated. They were more likely to know the HZ annual incidence (30% baseline; 70% follow-up; p=0.02), and report having educational information for physicians (7% baseline; 27% follow-up; p=0.003). The top helpful intervention was nursing administration of the vaccine. Average monthly HZ vaccine usage in the affiliated outpatient pharmacy increased in the 10 months between surveys by 156% compared with the 3 months prior to the baseline survey.
Conclusions
Interventions implemented during the study led to an increase in physicians’ basic knowledge of the HZ vaccine and an increase in usage at the affiliated pharmacy.
Introduction
There are more than 1,000,000 cases of herpes zoster (HZ) in the United States (U.S.) annually.1–3 The incidence of HZ has been rising in the U.S. since the 1990s.2,4–7 One third of all people in the U.S. will get HZ with the highest incidence in people age 50s to 70s.2,5 The Shingles Prevention Study (SPS) demonstrated that a HZ vaccine reduces the burden of illness by 61%, the incidence of post-herpetic neuralgia (PHN) by 66.5%, and incidence of zoster by 51.3%.8 As a result, the U.S. Food and Drug Administration (FDA) and Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) approved the Zostavax vaccine (Merck & Co Inc, Whitehouse Station, New Jersey) for the prevention of HZ in 2006 for immunocompetent patients age 60 and above.9 The FDA extended the approval to patients age 50 to 59 in March 2011.10
Post-licensure studies have continued to demonstrate the efficacy and safety of the HZ vaccine.11–16 Despite this evidence, since licensure the uptake of zoster vaccination among eligible patients has been significantly less than other standard adult vaccines.1,17–20 CDC estimates show that only 15.8% eligible patients received the vaccine in 2011, up from 14.4% in 2010, 10% in 2009, and 6.7% in 2008.20,21 The 2011 rates were even lower in African Americans (7.9%) and Hispanics (8.0%).
Hurley et al. identified the top barriers for physicians as the perceived cost to patients, reimbursement difficulties, and the up-front cost of stocking the vaccine.18 Evidence shows a lack of strong physician recommendation continued to be a major barrier to patient adherence with national guidelines.18,19 The previously published baseline survey results show that only 66% of general internal medicine (GIM) physicians responded that HZ vaccination was an important clinical priority, and 48% reported that less than 10% of their patients received the HZ vaccine.22 The top barrier to vaccination was cost to patients; and lack of awareness of national recommendations varied by provider setting.
The purpose of the study was to use a follow up survey to measure whether institution wide interventions would increase the prescriptions of the HZ vaccine by GIM physicians at a major urban academic medical center 1 year following the baseline survey. The surveys were based on the work of Cabana et al., which described that to overcome barriers to physician adherence to evidence-based practice, there must first be a change in knowledge and then attitudes to finally affect behavior.23 Furthermore, we aimed to determine whether changes in use of the HZ vaccine depend on exposure to interventions by 4 different practice sites affiliated with the medical center. Interventions included education for GIM physicians through presentations and other conferences, education for patients advertised on plasma screens in the medical center, option for administration by nurse at pharmacy with a prescription at the outpatient pharmacy, and electronic medical record (EMR) health maintenance reminders and alerts.
Materials and Methods
From October 6 to December 12, 2011, SurveyMonkey (SurveyMonkey.com, LLC, Palo Alto, California) IRB approved surveys were sent to 266 valid faculty email addresses of all members of the Division of General Internal Medicine (DGIM) in the Department of Medicine of New York University Langone Medical Center (NYULMC). The same survey was sent to 252 valid DGIM faculty email addresses from October 1 to December 21, 2012 for the one year follow up. The follow up survey period was extended due to disruptions from Hurricane Sandy at the medical center between October 29 and November 26, 2012. Faculty members are required to have NYULMC email addresses, therefore all eligible physicians in the four practice settings described below were reached by email. 10 email reminders were sent to non-responders during the survey periods. At the end of the survey period, correct answers to the knowledge-based questions were emailed. There were no exclusion criteria.
Surveyed physicians practice in different outpatient settings included the Faculty Group Practice at NYULMC (FGP), voluntary private practices affiliated with NYULMC (Vol), outpatient clinics of the public Health and Hospitals Corporation at Bellevue Hospital and Gouverneur Healthcare Services (B/G), and the Primary Care Clinic at the Manhattan Veterans Affairs (VA). The FGP doctors are employees of NYULMC with a high percentage of insured patients; Vol physicians have private offices located throughout the area with access to the NYU Pharmacy; B/G is a safety net hospital system without the HZ vaccine on formulary at its pharmacy; and the VA hospital serves a large population of veterans with a well-developed EMR, and the HZ vaccine provided through its pharmacy.
The survey assessed the baseline knowledge, attitudes, practices, and perceived barriers regarding HZ and the vaccine against it. The survey used close-ended items to record physician background characteristics. Multiple choice questions and simple case vignettes were used to assess knowledge of the nature of HZ, vaccination safety, efficacy, guidelines, and procedures for obtaining the vaccine. Likert-type attitude items were included to further understand the priority of vaccination against HZ and perceived barriers to ordering and administering the vaccine. Close- and open-ended questions were included to measure current practices with the vaccine and experiences prescribing the vaccine to patients. Doses of the HZ vaccine administered and number of prescribers at the medical center outpatient pharmacy used by the physicians were monitored. All patients have access to this pharmacy, however, BG and VA have separate outpatient pharmacies not monitored during the study.
All analysis was conducted with SAS 9.3 (SAS Institute Inc., Cary, North Carolina). For respondents to both the baseline and follow up surveys, changes in responses from baseline to the follow up were evaluated using statistical tests for paired data. For the survey questions with qualitative responses, McNemar chi square tests were used to compare whether the distributions of the responses to each question were significantly different at two-sided alpha level of 0.05. For those questions with quantitative responses, Wilcoxon Signed Rank nonparametric tests were used to compare the distributions of responses. In these analyses, no adjustments for multiple questions were used.
For the follow up survey, we compared the distributions of the responses of the physicians from four practice sites (FGP, Vol, B/G, and VA). Fisher’s exact tests were used to investigate whether the distributions of the responses to each question were significantly different at two-sided alpha level of 0.05 among the four groups. For those questions with quantitative responses, Kruskal-Wallis nonparametric analysis of variance methods were used to compare the mean responses among the four groups. For those questions with statistically significant differences among groups of respondents, all pair-wise comparisons between groups were tested with a Bonferroni correction to adjust for the multiple comparisons (p value for statistical significance ≤ 0.0083). No adjustment for multiple questions was applied.
Results
Respondent Characteristics
The total response rate for the baseline survey was 33.5% (89/266) and for the follow up survey 29.8% (75/252), however, only 64 identified a primary working site to be included in the analysis. Table 1 summarizes the baseline characteristics for the 55 physicians who completed both surveys. There were minimal changes from the baseline survey to the follow-up survey with respect to the numbers of outpatients they saw annually, the percentages of their patients who filled prescriptions at the NYU Pharmacy, who were over 60 years of age, and number of HZ vaccine prescriptions last year (all p-values ≥0.090). There was no statistical difference among the sites in either survey regarding the percent that completed fellowship, and the majority (71 percent at preliminary and 74 percent in the follow up survey) did not complete fellowship.
Table 1.
Demographic and practice characteristics.
| Baseline Survey (n=55) |
Follow Up Survey (n=55) |
P value from McNemar Tests |
|
|---|---|---|---|
| Physician Characteristics | Number (%) | ||
| Male | 28 (51%) | 28 (51%) | N/A |
| Completed Residency 2000–2011 | 32 (58%) | 33 (60%) | 1.000 |
| Completed Fellowship | 17 (31%) | 16 (29%) | 1.000 |
| Practice Site | |||
| FGP | 12 (21%) | 12 (21%) | N/A |
| Vol | 11 (20%) | 11 (20%) | N/A |
| BG | 24 (44%) | 24 (44%) | N/A |
| VA | 8 (15%) | 8 (15%) | N/A |
| Practice sites does not administer the HZ vaccine | 12 (22%) | 14 (26%) | 0.727 |
| Medical Record System: | |||
| Paper | 4 (7%) | 4 (7%) | N/A |
| EPIC EMR | 8 (15%) | 8 (15%) | N/A |
| Other EMR | 43 (78%) | 43 (78%) | N/A |
| Practice Characteristics | Median, (range) | Median, (range) | |
| Percent time spent in outpatient care per physician | 70 (0–100) | 60 (0–100) | 0.856 |
| Number of outpatients seen annually per physician | 1000 (0–7500) | 1000 (0–6000) | 0.522 |
| Percentage of patients per physicians that fill prescriptions at the medical center Pharmacy | 0 (0–80) | 0 (0–90) | 0.524 |
| Percentage of patients over 60 years old per physician | 50 (2–100) | 45 (0–100) | 0.188 |
| Percentage of immununocompromised patients age 60 and above per physician | 20 (0–100) | 10 (0–100) | 0.108 |
| Number of HZ vaccine prescription last year per physician | 6 (0–600) | 5 (0–1000) | 0.090 |
N/A= McNemar Tests not applicable since the values did not change; FGP= Faculty Group Practice; Vol= Voluntary Physicians; BG= Bellevue and Gouverneur Hospitals; VA= Manhattan Veterans Affairs; HZ= Herpes zoster; EMR= Electronic medical record
There was a statistical difference in the estimated percent immunocompromised and therefore not eligible for vaccination patients among the four practice settings in both surveys, with the trend of BG physicians estimating having the most immunocompromised and Vol estimating to have the least (p-value=0.007 and p-value=0.002 for the preliminary and follow up surveys respectively). These trends were expected prior to conducting the survey. However, the differences among the groups did not change between the surveys (p-value=0.1).
Physician knowledge, attitudes, and practices regarding the HZ vaccine before and after interventions
Table 2 describes the knowledge, attitudes, and practices among the 55 physicians who responded to both surveys. Physicians tended to answer more knowledge based questions correctly in the follow up than the baseline (Table 2). Notably the proportion of physicians who answered the question regarding shingles incidence correctly (approximately 1,000,000 cases of shingles per year in the U.S) increased from 30% (6/20) to 70% (14/20) (p-value=0.02).
Table 2.
Physician Knowledge, Attitudes, and Practices Before and After Interventions among respondents of both surveys (N=55)
| Number/Number responding (%Agree) | ||
|---|---|---|
| Baseline Survey | Follow Up Survey | |
| Physician Knowledge | ||
| There are approximately 1,000,000 cases of shingles per year in the U.S.* | 6/20 (30) | 14/20 (70) |
| Injection site reactions are only significant adverse reactions in a randomized clinical triala,8 | 23/34 (68) | 26/34 (76) |
| Zostavax decreases the incidence of HZ by 50% | 20/33 (61) | 25/33 (76) |
| 30% of people will experience at least one episode of shingles in their lifetime | 20/37 (54) | 21/37 (57) |
| Zostavax vaccine reduces the incidence of post-herpetic neuralgia by about two-thirds | 23/26 (88) | 20/26 (77) |
| HZ vaccine is recommended by the CDC for all immunocompetent patients age 60 and over. | 33/40 (83) | 36/40 (90) |
| Acute zoster is often associated with pain that can interfere with normal activities | 44/47 (94) | 47/47 (100) |
| Physician Attitudes | ||
| HZ vaccine is too costly for patients | 30/37 (81) | 27/37 (73) |
| Ordering and administering HZ vaccine is too complicated | 22/36 (61) | 18/36 (50) |
| Storage requirements make the HZ vaccine difficult to use | 19/35 (54) | 20/35 (57) |
| Physicians have too much else to focus on with patients to recommend the HZ vaccine | 19/37 (51) | 12/37 (32) |
| Patients get vaccinated when their doctor recommends it | 28/36 (78) | 33/36 (92) |
| Preventive recommendations from organizations like the ACIP are an important determinant of my vaccination practices | 36/36 (100) | 36/36 (100) |
| HZ vaccinations are low because physicians are not aware of recommendations | 28/37 (76) | 26/37 (70) |
| HZ vaccination is an important clinical priority | 30/36 (83) | 25/36 (69) |
| Pneumococcal vaccination is an important clinical priority | 34/36 (94) | 34/36 (94) |
| Influenza vaccination is an important clinical priority | 35/36 (97) | 34/36 (94) |
| Physician Practices | ||
| Less than 10% of patients 60 and older receive the HZ vaccine | 17/30 (57) | 12/30 (40) |
| Greater than 75% of patients 65 and older receive the pneumococcal vaccine | 21/37 (57) | 21/37 (57) |
| Greater than 75% of patients 60 and older receive the influenza vaccine | 21/38 (55) | 19/38 (50) |
HZ= Herpes zoster; CDC= Centers for Disease Control and Prevention; ACIP= Advisory Committee on Immunization Practices of the CDC
Other choices included fever and generalized varicella-like rash.
P<0.05 for comparisons between surveys using McNemar’s Test for paired data
Among the top reported barriers to HZ vaccination, there was an 8% decrease in the number of physicians who reported cost to patients as a barrier to vaccination (81% (30/37) at baseline; 73% (27/37) at follow up), and a 6% decrease in the physicians who agreed that HZ vaccination rates were lower than should be because physicians are not aware of recommendations (76% (28/37) at baseline; 70% (26/37) at follow up) (Table 2). The proportion of physicians who responded that physicians have too much else to focus on with patients to recommend the HZ vaccine decreased (51% (19/37) at baseline; 32% (12/37) at follow up; p-value=0.06). This change was largely driven by fewer BG physicians agreeing with this barrier (67% (12/18) at baseline; 39% (7/18) at follow up; p-value=0.06).
The proportion of physicians reporting that less than 10% of their patients older than 60 years received HZ vaccines decreased from 57% (17/30) at baseline to 40% (12/30) at follow up. At the same time, the proportions of physicians reporting that more than 75% of their patients received pneumococcal vaccine was 57% (21/37) for both baseline and follow up surveys. Those reporting that more than 75% received influenza vaccines was 55% (21/37) at baseline and 50% (19/38) at follow up.
During the study period, monthly medical center pharmacy HZ vaccine prescriptions and number of physicians prescribing the HZ vaccine increased following implementation of key interventions (Figure 1). For example there was a 136% increase in HZ prescriptions in the 3 months prior to the baseline survey (average 47 (range 33–59)) compared to the 3 months prior to the follow up survey (average 111 (range 99–124)), and a 156% increase from the 3 months prior to the baseline survey compared with the 10 months between surveys (average 120 (range 93–159)).
Figure 1.
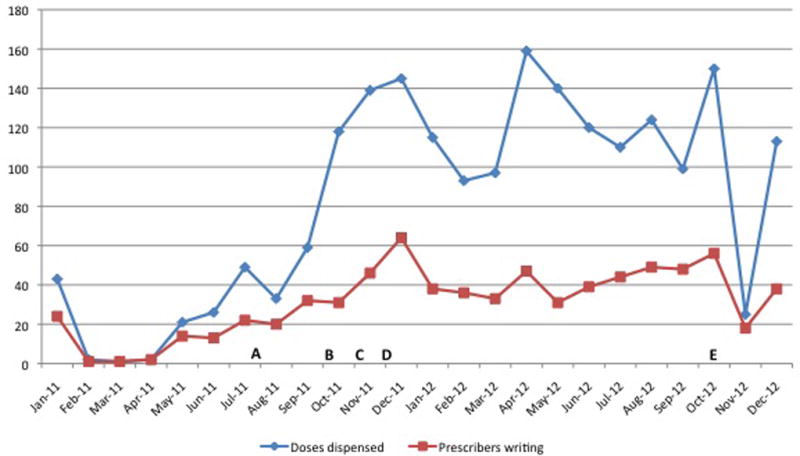
Monthly doses of the HZ vaccine dispensed at the NYU pharmacy and monthly number of prescribers of the HZ vaccine in 2011 and 2012. A) End of supply shortages; B) Dedicated HZ vaccine nurse provided in the pharmacy 3 days per week and patient education on plasma screens throughout the medical center; C) EPIC EMR reminders begin; D) NYU medicine grand rounds presentation.
Physician responses to interventions
The top “helpful” interventions reported among the 55 physicians who answered both surveys were administration of vaccine by a nurse after a prescription by physicians, making physicians aware of ACIP recommended immunization schedules, nurse-initiated prompting about vaccination, reminders in patient charts and having a pharmacy administer the vaccine with or without a physician prescription (Table 3). Particularly, VA physicians at follow up (90% (9/10)) were more likely to answer that having a pharmacy administer the HZ vaccine after a prescription by a physician would be helpful than at the baseline (p-value=0.06).
Table 3.
Responses to Interventions among respondents of both surveys (N=55)
| Responses/Number Responding (%) | ||
|---|---|---|
| Baseline Survey | Follow Up Survey | |
| Interventions Reported as “Helpful” | ||
| Nurse administration of the vaccine after prescription by physician | 37/42 (88) | 41/42 (98) |
| Making physicians aware of ACIP recommended immunization schedules | 38/43 (88) | 37/43 (86) |
| Nurse initiated prompting about vaccination with the patients | 37/43 (86) | 41/43 (95) |
| Reminders in patient charts | 32/42 (76) | 32/42 (76) |
| Pharmacy administration of the vaccine: | ||
| With physician prescription | 29/42 (69) | 33/42 (79) |
| Without physician prescription | 27/41 (66) | 30/41 (73) |
| Current Interventions in Place | ||
| Nurse administration of the vaccine after prescription by physician | 14/55 (25) | 20/55 (36) |
| Nurse initiated prompting about vaccination with the patients | 7/55 (13) | 9/55 (16) |
| Multi-pronged approach | 5/55 (9) | 11/55 (20) |
| Education for physicians about the severity of HZ** | 4/55 (7) | 12/55 (27) |
| Reminders in patient charts | 0/55 (0) | 0/55 (0) |
ACIP= Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention; HZ= Herpes zoster
P<0.05 for comparisons between surveys using McNemar’s Test
P<0.01 for comparisons between surveys using McNemar’s Test
Physicians at the follow up survey were more likely to respond that they already had nurses administer the vaccine after a prescription by the physician, nurse-initiated prompting about vaccination with the patients, multi-pronged interventions, and education for physicians about the severity of HZ in place (Table 3). Among the 55 physicians who answered both surveys, 27% (15/55) of physicians at follow up were significantly more likely to report having education for physicians about the severity of HZ compared with 7% (4/55) at baseline (p-value=0.003).
Physician knowledge, attitudes, and practices regarding the HZ by provider site
The 64 responses to the follow up survey were also compared across the four practice sites. Responses to knowledge based questions varied by question type and provider setting in the follow up survey (Table 4). FGP physicians were more likely to know that there are 1,000,000 cases of shingles per year in the U.S. than BG (80% and 23%, respectively; p-value=0.008). Physicians at all provider sites agreed that ACIP recommendations were important for HZ vaccination practices, however, over 60% of physicians at all sites agreed that vaccination rates are low because physicians are not aware of these recommendations. Additionally, both BG (86%) and Vol (100%) reported that the HZ vaccine was too costly more often than VA (14%) (both pairwise p-value<0.001). Furthermore, BG (71%) was more likely to report that ordering and administering HZ vaccine was too complicated compared with FGP (10%) (p-value=0.002). With regard to current vaccination practices BG (84%) was more likely to report less than 10% of patients receiving the HZ vaccine than FGP (0%) and VA (20%) (both pairwise p-value<0.001).
Table 4.
Physician knowledge, attitudes, and practices by provider setting among respondents on follow up survey (N=64)
| Number/ Number responding (%) Agree | ||||
|---|---|---|---|---|
| FGP | Voluntary | B/G | VA | |
| Physician Knowledge | ||||
| There are approximately 1,000,000 cases of shingles per year in the U.S.* | 8/10 (80) | 2/10 (20) | 7/30 (23) | 1/7 (14) |
| 30% of people will experience at least one episode of shingles in their lifetime | 7/10 (70) | 6/10 (60) | 10/30 (33) | 4/7 (57) |
| Zostavax decreases the incidence of HZ by 50% | 5/10 (50) | 6/10 (60) | 16/29 (55) | 4/7 (57) |
| Zostavax vaccine reduces the incidence of post-herpetic neuralgia by about two-thirds | 7/10 (70) | 6/10 (60) | 10/27 (37) | 2/5 (40) |
| Injection site reactions are only significant adverse reactions in a randomized clinical triala.8 | 7/10 (70) | 8/10 (80) | 13/30 (43) | 5/7 (71) |
| HZ vaccine is recommended by the CDC for all immunocompetent patients age 60 and over. | 9/10 (90) | 10/10 (100) | 20/29 (69) | 6/7 (86) |
| Acute zoster is often associated with pain that can interfere with normal activities | 10/10 (100) | 10/10 (100) | 29/30 (97) | 7/7 (100) |
| Physician Attitudes | ||||
| HZ vaccine is too costly for patients*** | 4/10 (40) | 10/10 (100) | 24/28 (86) | 1/7 (14) |
| Ordering and administering HZ vaccine is too complicated** | 1/10 (10) | 4/10 (40) | 20/28 (71) | 4/7 (57) |
| Storage requirements make the HZ vaccine difficult to use | 5/10 (50) | 3/10 (30) | 18/28 (64) | 6/7 (86) |
| Patients have health issues that take precedence | 5/10 (50) | 1/10 (10) | 11/28 (39) | 4/7 (57) |
| Patients get vaccinated when their doctor recommends it | 9/10 (90) | 7/10 (70) | 25/28 (89) | 7/7(100) |
| Preventive recommendations from organizations like the ACIP are an important determinant of my vaccination practices | 10/10 (100) | 10/10 (100) | 28/28 (100) | 7/7 (100) |
| HZ vaccinations are low because physicians are not aware of recommendations | 6/10 (60) | 6/10 (60) | 21/28 (75) | 7/7 (100) |
| HZ vaccination is an important clinical priority | 7/10 (70) | 9/10 (90) | 18/28 (64) | 5/7 (71) |
| Pneumococcal vaccination is an important clinical priority | 9/10 (90) | 10/10 (100) | 27/28 (96) | 6/7 (86) |
| Influenza vaccination is an important clinical priority | 10/10 (100) | 9/10 (90) | 26/27 (96) | 7/7 (100) |
| Physician Practices | ||||
| Less than 10% of patients 60 and older receive the HZ vaccine*** | 0/7 (0) | 0/7 (0) | 21/25 (84) | 1/5 (20) |
| Greater than 75% of patients 60 and older receive the pneumococcal vaccine | 4/8 (50) | 6/8 (75) | 15/26 (58) | 5/7 (71) |
| Greater than 75% of patients 60 and older receive the influenza vaccine | 5/8 (62) | 6/8 (75) | 9/26 (35) | 2/7 (29) |
FGP= Faculty Group Practice; Vol= Voluntary Physicians; BG= Bellevue and Gouverneur Hospitals; VA= Manhattan Veterans Affairs; HZ= Herpes zoster; CDC= Centers for Disease Control and Prevention; ACIP= Advisory Committee on Immunization Practices of the CDC
Other choices included fever and generalized varicella-like rash.
P<0.05 for comparisons by provider setting using Fischer’s Exact Test
P<0.01 for comparisons by provider setting using Fischer’s Exact Test
P<0.001 for comparisons by provider setting using Fischer’s Exact Test
Discussion
The HZ vaccine continues to be shown to be safe and efficacious since approval. A post-licensure study found efficacy for preventing HZ in persons 50–59 to be 69.8%11 and a Cochrane Review of 3 randomized controlled studies related to HZ vaccination efficacy found a risk ratio of having HZ to be 0.49 in all vaccinated patients versus unvaccinated patients.13 Furthermore, the 4 year follow up to the SPS showed continued efficacy, but reductions in burden of illness decreased to 50.1% from 61%.14 Therefore, improving vaccination rates among eligible patients is an important clinical priority.
We hypothesized that by introducing interventions to influence GIM physicians’ knowledge and attitudes related to the HZ vaccine, there would be a change in provider practices to increase the use of the HZ vaccine among eligible patients. Overall, average monthly HZ vaccine usage in the NYU pharmacy increased in the 10 months between surveys by 156% compared with the 3 months prior to the baseline survey before interventions. Among physicians that participated in both the baseline and follow up surveys, they were statistically more likely to know that there are more than 1,000,000 cases of HZ annually in the U.S. (30% baseline; 70% follow up; p=0.02), and more likely to report having education for physicians about the severity of HZ (7% baseline; 27% follow up; p=0.003).
Furthermore, consistent with Cabana et al.,23 worse prioritization of the HZ vaccine suggested worse adherence to national recommendations. 83% (30/36) at baseline but only 69% (25/36) on follow-up responded that HZ vaccination was an important clinical priority. Comparatively, physicians indicated that their clinical priorities regarding the importance of pneumococcal and influenza vaccination were above 90% for both surveys. Correspondingly, it was reported that less than 10% of their patients received the HZ vaccines by 57% (17/30) physicians at baseline compared to 40% (12/30) upon follow up. Greater than 50% of repondents reported that over 75% of their patients received the pneumococcal and influenza vaccines during both surveys. However, there was no statistical difference between baseline and follow up vaccination proportions among the 55 physicians who answered both surveys.
As in the baseline survey, significant differences in physician attitudes and practices remained among the 4 different provider sites in the follow up survey.22 BG and Vol were more likely to report cost as a barrier to vaccination, and BG, without the vaccine on formulary, expressed greater difficulties with ordering and administering the vaccine. As a result, BG physicians were more likely to respond that less than 10% of their patients received the HZ vaccine.
There are several reasons why we may not have seen major changes in many questions. First, the survey responders were potentially less likely to benefit from the interventions than the non-responders. The EMR reminders were available only on EPIC, which was only used by 13% of responders on the baseline and follow up survey. The NYU pharmacy showed a large increase in HZ prescriptions and physicians writing prescriptions following the interventions, but the percent of patients who used that pharmacy per physician was low in both the baseline and follow up surveys. This intervention would be more likely to affect the FGP and Vol, which used the NYU pharmacy more than BG and VA. However, the increase in educational programs in physician practices and usage at pharmacy suggest interventions may have had an impact not detected by survey.
Second, lack of adherence to guidelines is undermined by system and environmental factors.23 For example, patients on Medicaid or uninsured have overall lower HZ coverage and difficulty accessing adult vaccines, despite being covered by Medicaid.1,24 45% of responders in the baseline survey and 52% in the follow up survey were BG physicians where the HZ vaccine is not on formulary. Changes in attitudes and practices are unlikely to be seen in the survey results when nearly half of the sample size still cannot offer their patients the HZ vaccine at their practice site.
Third, one of the top “helpful” interventions identified in the baseline survey was nurse initiated prompting about vaccination with the patients, which was not included in the interventions. The interventions used were predefined at the start of the study based on successful interventions in the literature, particularly for EMR reminders. Chaudhry et al. evaluated clinical decision support software as part of EMR at the Mayo Clinic and found an increase in vaccine utilization of 43% and 54% at two clinics.25 However, there is evidence the physician reminders are less effective than patient reminders with regard to increasing influenza vaccination rates,26 and nurse initiated reminders with EMR reminders is more effective than EMR reminders alone.27 Therefore, additional practice interventions like nurse initiated prompting or patient reminder and recall systems could potentially show a greater change in physician practices.28–30 A continued expanded role for nurses in promoting vaccinations and other recommended preventive care should be considered.
Lastly, diffusion of a novel vaccine into standard clinical practice may take more time than the 1 year follow up to assess change. For example, in 2011 only 62.3% of eligible patients in the U.S. received the pneumococcal vaccine.21 Interventions to increase use of that vaccine started in 1981 with Medicare covering it, but by 1989 only 14.1% of eligible adults 65 and older were vaccinated.31 The HZ vaccine was only approved for immunocompetent patients 60 and older in 2006. Healthcare system-wide changes were only beginning during this study. For example, New York State began to allow certified pharmacists to administer the HZ vaccine with a prescription in October 2012 and Merck began a marketing campaign about shingles that year as well. More time may be needed to measure changes in patient and provider behaviors.
This study has additional limitations. The response rate was 29.8% for the baseline survey and only 25.4% for the follow up. The lower response rate during the follow up may be in part due to Hurricane Sandy, which closed much of the medical center, NYU pharmacy, and affected many voluntary practices during the follow up survey. In addition, the study relied on self-reported practices, which may not reflect actual practices that could be obtained from administrative or medical records. Finally, these results present the responses of physicians at one large, diverse major urban academic medical center, which may not be generalizable to other settings.
From this study we were able to add to the growing literature that there continues to be underutilization of the HZ vaccine, despite ongoing support for its safety and efficacy. Overall, GIM physicians know the severity of HZ and have improved knowledge related to the epidemiology of the disease. They do not, however, universally know about the ACIP recommendations, and that combined with the barriers of cost and difficulties administering the vaccine limit their positive attitudes towards the vaccine. Simple interventions such as having a nurse present in the pharmacy to administer the vaccine greatly increased usage. Additional practice-specific interventions like nurse initiated prompting may further increase use of the HZ vaccine and strengthen physician recommendations.
Acknowledgments
The authors would like to thank the Division of General Internal Medicine Physicians, Dr. Marc Gourevitch for providing DGIM NYU faculty email addresses, and Thomas O’Brien at the NYU Pharmacy. This study was supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. Also supported in part by grant UL1 TR000038 from the National Center for the Advancement of Translational Science (NCATS), National Institutes of Health.
Footnotes
Conflicts of Interest: No financial disclosures were reported by the authors of this paper.
References
- 1.Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older, in the U.S., 2008. Am J Prev Med. 2011 Feb;40(2):e1–6. doi: 10.1016/j.amepre.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Rimland D, Moanna A. Increasing incidence of herpes zoster among Veterans. Clin Infect Dis. 2010 Apr 1;50(7):1000–1005. doi: 10.1086/651078. [DOI] [PubMed] [Google Scholar]
- 3.Tseng HF, Smith N, Harpaz R, et al. Herpes Zoster Vaccine in Older Adults and the Risk of Subsequent Herpes Zoster Disease. JAMA: The Journal of the American Medical Association. 2011 Jan 12;305(2):160–166. doi: 10.1001/jama.2010.1983. 2011. [DOI] [PubMed] [Google Scholar]
- 4.Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007 Nov;82(11):1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 5.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005 Aug;20(8):748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghaznawi N, Virdi A, Dayan A, et al. Herpes zoster ophthalmicus: comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology. 2011 Nov;118(11):2242–2250. doi: 10.1016/j.ophtha.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011 Feb;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005 Jun 2;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 9.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008 Jun 6;57(RR-5):1–30. quiz CE32–34. [PubMed] [Google Scholar]
- 10.Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011 Nov 11;60:1528. [PubMed] [Google Scholar]
- 11.Schmader KE, Levin MJ, Gnann JW, et al. Efficacy, Safety, and Tolerability of Herpes Zoster Vaccine in Persons Aged 50,Äì59 Years. Clinical Infectious Diseases. 2012 Apr 1;54(7):922–928. doi: 10.1093/cid/cir970. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter R, Tran TN, Hansen J, et al. Safety of Zostavax--a cohort study in a managed care organization. Vaccine. 2012 Oct 19;30(47):6636–6641. doi: 10.1016/j.vaccine.2012.08.070. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi AM, Gomes Silva BN, Torloni MR, et al. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD008858.pub2. CD008858. [DOI] [PubMed] [Google Scholar]
- 14.Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012 Nov 15;55(10):1320–1328. doi: 10.1093/cid/cis638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng HF, Chi M, Smith N, et al. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis. 2012 Jul 15;206(2):190–196. doi: 10.1093/infdis/jis334. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. Jama. 2012 Jul 4;308(1):43–49. doi: 10.1001/jama.2012.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freed GL, Clark SJ, Cowan AE, et al. Primary care physician perspectives on providing adult vaccines. Vaccine. 2011 Feb 17;29(9):1850–1854. doi: 10.1016/j.vaccine.2010.12.097. [DOI] [PubMed] [Google Scholar]
- 18.Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med. 2010 May 4;152(9):555–560. doi: 10.7326/0003-4819-152-9-201005040-00005. [DOI] [PubMed] [Google Scholar]
- 19.Opstelten W, van Essen GA, Hak E. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine. 2009 Jan 7;27(2):192–196. doi: 10.1016/j.vaccine.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 20.Adult vaccination coverage - United States, 2010. MMWR. Morbidity and mortality weekly report. 2012 Feb 3;61:66–72. [PubMed] [Google Scholar]
- 21.Noninfluenza vaccination coverage among adults - United States, 2011. MMWR. Morbidity and mortality weekly report. 2013 Feb 1;62:66–72. [PMC free article] [PubMed] [Google Scholar]
- 22.Elkin Z, Cohen E, Goldberg J, et al. Studying Physician Knowledge, Attitudes, and Practices Regarding the Herpes Zoster Vaccine to Address Perceived Barriers to Vaccination. Cornea. 2013 doi: 10.1097/ICO.0b013e318283453a. (In Press) [DOI] [PubMed] [Google Scholar]
- 23.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. Jama. 1999 Oct 20;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 24.Orenstein WA, Mootrey GT, Pazol K, et al. Financing immunization of adults in the United States. Clin Pharmacol Ther. 2007 Dec;82(6):764–768. doi: 10.1038/sj.clpt.6100401. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry R, Schietel SM, North F, et al. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract. 2012 Feb 5; doi: 10.1111/j.1365-2753.2011.01814.x. [DOI] [PubMed] [Google Scholar]
- 26.Thomas RE, Russell M, Lorenzetti D. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2010;(9) doi: 10.1002/14651858.CD005188.pub2. CD005188. [DOI] [PubMed] [Google Scholar]
- 27.Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gen Intern Med. 1999 Jun;14(6):351–356. doi: 10.1046/j.1525-1497.1999.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3) doi: 10.1002/14651858.CD003941.pub2. CD003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: A review. Jama. 2000 Oct 11;284(14):1820–1827. doi: 10.1001/jama.284.14.1820. [DOI] [PubMed] [Google Scholar]
- 30.Wright A, Poon EG, Wald J, et al. Randomized controlled trial of health maintenance reminders provided directly to patients through an electronic PHR. J Gen Intern Med. 2012 Jan;27(1):85–92. doi: 10.1007/s11606-011-1859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch JA. Strategies to overcome barriers to pneumococcal vaccination in older adults: an integrative review. J Gerontol Nurs. 2012 Feb;38(2):31–39. doi: 10.3928/00989134-20110831-03. [DOI] [PubMed] [Google Scholar]


