Abstract
Background
Opioid use disorder is often treated with short term hospitalization and medically supervised withdrawal from opioids followed by counseling alone without medication assisted treatment (MAT). More evidence is needed to confirm the expectation that the rate of relapse would be high after short term inpatient treatment and withdrawal from opioids without followup MAT.
Objective/Methods
To examine relapse to opioid use disorder in a randomized, multi-site effectiveness trial of extended-release injection naltrexone (XR-NTX) vs community-based treatment as usual (TAU) without medication, as a function of the type of clinical service where treatment was initiated–short-term inpatient (N = 59), long-term inpatient (N = 48), or outpatient (N = 201). Inpatients typically were admitted to treatment actively using opioids and had completed withdrawal from opioids before study entry. Outpatients typically presented already abstinent for varying periods of time.
Results
One month after randomization, relapse rates on TAU by setting were: short-term inpatient: 63%; long term inpatient: 14%; outpatient: 28%. On XR-NTX relapse rates after one month were low (< 12%) across all three settings. At the end of the 6 month trial, relapse rates on TAU were high across all treatment-initiation settings (short term inpatient 77%; long term inpatient 59%; outpatient 61%), while XR-NTX exerted a modest protective effect against relapse across settings (short term inpatient: 59%; long term inpatient 46%; outpatient 38%).
Conclusions
Short term inpatient treatment is associated with a high rate of relapse among patients with opioid use disorder. These findings support the recommendation that medically supervised withdrawal from opioids should be followed by medication assisted treatment.
Keywords: opioid use disorder, injection naltrexone, medication assisted treatment, detoxification, medically supervised withdrawal, inpatient, residential, relapse
Introduction
Inpatient or residential treatment is a time-honored intervention for substance use disorders. Beneficial features of this approach include medically supervised withdrawal from substances of abuse, removal of the individual from the natural environment in which substance use was taking place, and initiating and setting the stage for ongoing psychosocial treatment and self-help group participation on an outpatient basis after discharge.
Until recently, many if not most inpatient and residential treatment programs did not routinely offer initiation of medications for maintenance of abstinence and prevention of relapse. However, this approach is problematic for opioid use disorder. Medications for opioid use disorder—methadone (an opioid agonist), buprenorphine (an opioid partial agonist), and injection naltrexone (an opioid antagonist)–are highly effective at maintaining abstinence and preventing relapse (Hser et al., 2014; Krupitsky et al., 2011; Mattick et al., 2014). The risk of relapse to opioid use disorder is uniquely high because of death from overdose. This risk is in theory especially high after a period of abstinence, such as after inpatient or residential treatment, because of loss of tolerance. Correspondingly, large observational studies have shown a spike in opioid overdose deaths after release from controlled settings such as prison or inpatient treatmen (Binswanger et al., 2013; Bird and Hutchinson 2003; Ravndal and Amundsen 2010; Seaman, Brettle, and Gore 1998).
Despite these concerns, the evidence-base on the outcome of opioid use disorder after an episode of inpatient or residential treatment remains limited. A handful of studies of clinical course of opioid use disorder after inpatient treatment are available, showing high rates of relapse, but also that a proportion of patients reduce their drug use or sustain abstinence (Broers et al., 2000; Chutuape et al., 2001; Gossop et al., 1989). Most controlled trials of medication treatments for opioid use disorder have been based in outpatient settings (Hser et al., 2014; Mattick et al., 2014; Weiss et al., 2011). The pivotal trial demonstrating the effectiveness of injection naltrexone (XR-NTX) for opioid use disorder, conducted in Russia, initiated active or placebo injections among inpatients, and then followed them as outpatients for 6 months. XR-NTX produced significantly more abstinence and retention in treatment over 6 months, compared to placebo (Krupitsky et al., 2011). Still, on placebo about 30% of patients were retained in treatment and predominantly abstinent (Nunes et al., 2015). To initiate naltrexone, a patient must be fully withdrawn from opioids, in order to avoid precipitated withdrawal. Thus, inpatient units, as utilized in the Russian trial, are an ideal setting for initiating naltrexone, because medically supervised withdrawal from opioids can be accomplished in a protected setting. An outpatient initiating naltrexone must have already achieved and sustained abstinence for at least a week or more, which might suggest a greater level of control and lower risk of relapse over the long term.
A recently completed, U.S.-based trial demonstrating the effectiveness of injection naltrexone for treatment of opioid use disorder (Lee et al., 2016) offered the opportunity to further examine relapse after inpatient treatment for opioid use disorder, since some of the patients initiated the trial during short-term inpatient stays, some during long-term residential treatment, and some initiated the trial as outpatients having already achieved abstinence. Participants were randomly assigned to receive either community-based treatment as usual (TAU) consisting mainly of psychosocial treatment without medication, or TAU + monthly injection naltrexone (XR-NTX) for 6 months. We hypothesized that the rate of relapse in the TAU condition would be highest, and the protective effect of XR-NTX (i.e. the difference in relapse between the XR-NTX + TAU versus TAU conditions) greatest among those initiating treatment as inpatients.
Methods
Overview
This report is secondary analysis of a 6-month, multi-site, randomized, controlled effectiveness trial of monthly injection naltrexone (XR-NTX) (brand name: Vivitrol) for prevention of relapse among patients with a history of opioid use disorder as well as recent criminal justice involvement. Details of the methods (Lee et al., 2015) and primary outcome analyses (Lee et al., NEJM 2016) have been reported previously. The study was reviewed and approved by the Institutional Review Boards at each of the participating sites, and all participating patients gave written informed consent. The present analysis focuses on comparing outcomes between patients initiating the trial on short term inpatient/residential units, versus long-term inpatient/residential units, versus outpatient settings.
Participants and Settings
Participants had a lifetime history of opioid use disorder (heroin or prescription opioids), and criminal justice involvement, but were not currently prisoners. Rather, they were either under community supervision (parole or probation) or had some other form of criminal justice involvement, such as an arrest, within the last 12 months. Though having a lifetime history of active opioid use disorder and thus at risk for relapse, participants had to have a stated goal of opiate-free treatment (i.e. not seeking agonist maintenance treatment with buprenorphine or methadone), and be currently abstinent and able to pass a challenge test with the short acting opioid antagonist naloxone, confirming their readiness to start naltrexone.
Participants were recruited from 5 sites across the eastern U.S., in Philadelphia, PA, Baltimore, MD, Providence, RI, and New York, City (two sites, one at New York University Medical Center, and one at Columbia University Medical Center). Two of those sites (New York University Medical Center, and Providence) recruited exclusively outpatients who had already achieved abstinence from opioids at some point in the last 6 months. One site (Baltimore, MD) recruited mainly inpatients who had been admitted to either short term (up to 4 weeks) or long term (up to 6 months) inpatient/residential treatment programs. Two sites (Pennsylvania and Columbia University Medical Center) recruited a mixture of outpatients and inpatients. For the purposes of the present analysis, participants were classified according to whether they entered the trial as either: 1) outpatient; 2) short term inpatient-residential, defined as an expected residential stay of less than 4 weeks; 3) long-term inpatient-residential, defined as a longer expected stay, typically 3 to 6 months.
Procedures
Prospective participants first underwent a psychiatric and medical evaluation to confirm eligibility, and had to be abstinent from all opioids, with an opioid negative urine toxicology and able to pass a naloxone challenge test, in order to be randomized. Participants were randomly assigned to either treatment as usual (TAU) in the community, typically consisting of some form of counseling without study medication, or TAU plus monthly injections of injection naltrexone (XR-NTX) (Vivitrol), for a 6 month trial. All participants, across both conditions, received regular follow-up visits with a medical clinician (weekly, then biweekly) throughout the trial. At each follow-up visit, participants provided a urine sample that was tested for opioids and other substances, and self-reported substance use with the time-line follow-back calendar method.
Data Analysis
The primary outcome measure was Relapse (time to relapse) to regular opioid use, operationalized as either two consecutive opioid positive urines or at least seven consecutive days of self-reported opioid use with missing urine tests imputed as positive. Relapse is considered a clinically meaningful outcome among patients with opioid use disorder in abstinence-based treatment, because once regular opioid use is resumed it is typically sustained and requires either another medically supervised withdrawal, or medication treatment (such as methadone or buprenorphine maintenance) to get it back under control. Patients on naltrexone treatment typically do not take opioids regularly while the medication is present at adequate blood levels (except for occasional ‘testing of the blockade’), and the typical failure mode is to stop taking the medication (miss a scheduled dose) after which the blockade wears off. Relapse to regular opioid use at that point requires another withdrawal from opioids to re-establish abstinence before naltrexone can be re-initiated.
The data analysis evaluated time to Relapse in an 2 treatment condition (TAU vs TAU + XR-NTX) by 3 setting type (outpatient, vs short term inpatient, vs long term inpatient) design. The original plan was to use survival analysis with Cox models. However, with the 2 by 3 design, the proportional hazards assumption was not confirmed. We therefore fit separate logistic regressions for the binary outcomes of Relapse (yes/no) at 5 weeks after randomization to capture rapid relapse, and Relapse (yes/no) by 6 months after randomization to capture cumulative relapse across the entire trial. Five weeks was chosen to capture rapid relapse, which was of particular interest given the likely high risk of relapse and overdose in the immediate weeks after discharge from controlled settings (Binswanger et al., 2013; Bird and Hutchinson 2003; Ravndal and Amundsen 2010; Seaman, Brettle, and Gore 1998). Also, 5 weeks is the outside limit of blockade by XR-NTX (there being at least partial blockade through the fifth week (Comer et al., 2002). Survival curves are displayed for descriptive purposes. Because even a single episode of opiate use, particularly after a detoxification or period of abstinence, carries a risk of overdose, we also examined the binary outcomes of any opioid use (versus none) in the first 5 weeks of the trial, and any opioid use (versus none) over all 26 weeks. All tests were two-tailed at alpha level 0.05.
Results
Participants
The overall sample consisted of 308 patients with a history of opioid use disorder, randomly assigned to treatment as usual (TAU) (N = 155) or TAU + injection naltrexone (XR-NTX) (N = 153). Overall, this was a predominantly male (85%), minority (African American: 50%; Hispanic: 27%) sample with mean age of 44 years, low educational attainment (mean of 11.5 years of education) and high unemployment (82%). Most (89%) had a lifetime history of heroin use (as opposed to prescription opioids only), and 41% had a lifetime history of injection drug use. Most (80%) were under parole, probation, or some other form of criminal justice supervision.
Table 1 shows demographic and clinical features of the sample divided into those who entered the trial in outpatient, or short-term inpatient, or long-term inpatient settings. As can be seen, the largest fraction of the sample entered the trial as outpatients (N = 201) having already achieved abstinence from opioids, with smaller numbers entering from short term (N = 59) or long-term (N = 48) inpatient settings. The patients differed across the settings on several features. Inpatients (short or long term), compared to outpatients, were more likely unemployed and under criminal justice supervision, and more likely to have a lifetime history of heroin use and of intravenous drug use, differences consistent with greater overall severity and impairment among the inpatients. As expected, few long term inpatients had evidence of substance use in the 30 days before randomization. Among outpatients, 37% reported some opioid use in the last 30 days, indicating they achieved abstinence and readiness to initiate naltrexone in the month before randomization. That only 44% of short term inpatients reported opioid use in the 30 days before randomization suggests that over half of the short term inpatients initiated the study at the end of 30 day inpatient stays.
Table 1.
Baseline characteristics of participants according to clinical settings under which they entered the trial
| Characteristic | Short-term Inpatient (N=59) | Long-term Inpatient (N=48) | Outpatient (N=201) | F or Chi-square, p-value |
|---|---|---|---|---|
| Mean ± SD or N (%) | ||||
| Age | 42.6±10.2 | 46.5±8.9 | 43.5±9.1 | F(2, 304)=2.6, p=0.08 |
| Male | 43 (72.9) | 41 (85.4) | 177 (88.1) | X2(2)=8.1, p=0.02 |
| Race/Ethnicity | ||||
| White | 17 (28.9) | 6 (12.5) | 38 (18.9) | X2(6)=21.5, p=0.002 |
| Black | 31 (52.5) | 34 (70.8) | 90 (44.8) | |
| Hispanic | 9 (15.3) | 6 (12.5) | 67 (33.3) | |
| Other | 2 (3.3) | 2 (4.2) | 2 (1.0) | |
| Years of education | 11.5±2.1 | 11.1±2.3 | 11.6±1.8 | F(2, 301)=1.0, p=0.35 |
| Current employment | 7 (11.9) | 1 (2.1) | 47 (23.4) | X2(2)=13.8, p=0.001 |
| Status with respect to supervision by criminal justice system | ||||
| Current supervision | 45 (76.3) | 35 (72.9) | 165 (82.1) | X2(2)=2.5, p=0.29 |
| Probation | 33 (55.9) | 22 (45.8) | 62 (30.9) | X2(4)=43.9, p<0.0001 |
| Parole | 11 (18.6) | 5 (10.4) | 95 (47.2) | |
| Other | 1 (1.7) | 8 (16.7) | 8 (4.0) | |
| No supervision | 14 (23.7) | 13 (27.1) | 36 (17.9) | |
| Heath insurance | ||||
| Any | 42 (71.2) | 40 (83.3) | 138 (68.7) | X2(2)=4.1, p=0.13 |
| Medicaid | 11 (18.6) | 11 (22.9) | 113 (56.2) | X2(2)=65.8, p<0.0001 |
| Opioid use during lifetime | ||||
| Opioid use disorder | 59 (100) | 48 (100) | 201 (100) | --- |
| Heroin use | 58 (98.3) | 48 (100) | 166 (82.6) | X2(2)=16.2, p<0.001 |
| Other, non-heroin, opioid use | 24 (40.7) | 18 (37.5) | 109 (54.2) | X2(2)=7.52, p=0.02 |
| Injection-drug use | 35 (59.3) | 25 (52.1) | 66 (32.8) | X2(2)=16.2, p<0.001 |
| Opioid use in past 30 days | ||||
| Heroin use | 25 (42.4) | 4 (8.3) | 46 (22.9) | X2(2)=17.1, p<0.001 |
| Other, non-heroin, opioid use | 8 (13.6) | 2 (4.2) | 47 (23.4) | X2(2)=11.2, p=0.004 |
| Any opioid use | 26 (44.1) | 5 (10.4) | 75 (37.3) | X2(2)=15.6, p<0.001 |
| Cocaine use in past 30 days | 13 (22.0) | 0 (0) | 46 (22.9) | X2(2)=13.8, p=0.001 |
| Heavy alcohol use in past 30 days | 4 (6.8) | 0 (0) | 33 (16.4) | X2(2)=12.1, p=0.002 |
Relapse across Treatment Conditions and Clinical Settings
Figure 1 shows the survival curves comparing time to Relapse between the treatment conditions (TAU vs TAU + XR-NTX) across each of the three clinical settings in which participants initiated the study—short-term inpatient, long-term inpatient, and outpatient. Table 2 shows the cumulative rates of relapse at 5 weeks after randomization (upper panel) and at end of study (week 26) (lower panel), across the two treatment conditions and three clinical settings.
Figure 1.
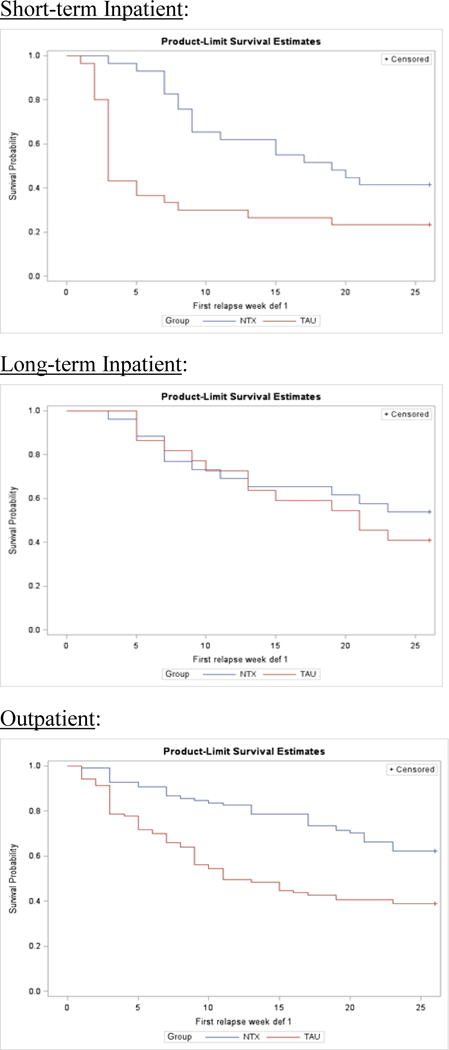
Survival curves for time to relapse comparing treatment as usual (TAU) to injection naltrexone (XR-NTX + TAU) across three settings in which patients initiated treatment: short-term inpatient (top panel), long-term inpatient (middle panel), outpatient (bottom panel).
Table 2.
Cumulative relapse rates at 5 weeks after randomization (upper panel) and at 26 weeks/end of study (lower panel), by treatment condition (TAU vs XR-NTX + TAU) and by setting in which the patient initiated the study–short-term inpatient (n = 59), versus long-term inpatient (n = 48), versus outpatient (n = 201). Values in the Table are percent relapsed (N relapsed/N total), and odds ratios for contrasts between treatment groups
| TAU | XR-NTX + TAU | Odds Ratio (95% Confidence Interval) | |
|---|---|---|---|
| Cumulative Relapse at 5 Weeks | |||
| Short-term inpatient (n = 59) | 63 (19/30) | 7 (2/29) | 0.043 (0.009–0.216)* |
| Long-term inpatient (n = 48) | 14 (3/22) | 12 (3/26) | 0.826 (0.149–4.576)* |
| Outpatient (n = 201) | 28 (29/103) | 9 (9/98) | 0.258 (0.115–0.579)* |
| Cumulative Relapse at End of Study/26 weeks | |||
| Short-term inpatient (n = 59) | 77 (23/30) | 59 (17/29) | ** |
| Long-term inpatient (n = 48) | 59 (3/22) | 46 (12/26) | ** |
| Outpatient (n = 201) | 61 (63/103) | 38 (37/98) | ** |
For cumulative Relapse at 5 weeks, the treatment condition by setting interaction is significant (X2(2) = 6.36, p = 0.042).
For cumulative Relapse at end of study/26 weeks, the treatment condition by setting interaction was not significant. Main effect of treatment was significant (X2(2) = 13.36, p = .0003). Main effect of setting also significant, with relapse in the outpatient group significantly lower than in the short-term inpatient group (OR = 0.453; 95% CI = 0.242 to 0.849).
5-Week Cumulative Relapse
The logistic regression model with 5-week cumulative relapse as the dependent variable, and treatment condition and setting as the independent variables, yielded a significant interaction between treatment condition and setting (chi-square = 6.36, p = 0.042), indicating that the differences in relapse rates between treatment conditions vary significantly across clinical settings. Among patients initiating the study on short term inpatient units (Figure 1, upper panel, Table 2 upper panel), over the first 5 weeks after randomization there is little relapse among patients treated with XR-NTX + TAU (7%), but a high rate of relapse among patients assigned to TAU (63%; odds ratio = 0.043, 95% confidence interval: 0.009 to 0.216). Among patients initiating the study on long-term inpatient units (Figure 1, middle panel), over the first 5 weeks after randomization there is little relapse on either XR-NTX + TAU (12%) or TAU (14%; odds ratio = 0.826; 95% confidence interval: 0.149 to 4.576). Among patients initiating the study as outpatients (Figure 1, bottom panel), over the first 5 weeks after randomization, there is again little relapse among those treated with XR-NTX + TAU (9%), and a moderately higher rate of relapse among those on TAU (28%; odds ratio = 0.258; 95% confidence interval: 0.115 to 0.579).
Cumulative Relapse by End of Study—26 Weeks
Inspection of the relapse curves in Figure 1 shows continued relapse across treatment conditions and settings over the 26 weeks of the trial. The logistic regression model with cumulative relapse at end of study (26 weeks) as dependent variable, and treatment condition and setting as independent variables, did not yield a significant interaction of treatment by setting (X2(2)=0.44, p=0.803). The main effect of treatment was significant (X2(2) = 13.36, p < .0003), indicating relapse rates were lower on the XR-NTX + TAU condition across treatment settings. The main effect of setting was also significant (X2(2) = 6.15, p =0.046). In pair-wise comparisons between settings, relapse rates were lower overall among patients who entered the study as outpatients, compared to those entering as short term inpatients (odds ratio = 0.453; 95% confidence interval: 0.242 to 0.849). The relapse rate among patients entering the study as long-term inpatients fell in between the outpatients and the short-term inpatients. In pairwise comparison the trend was in the direction of lower relapse among long term inpatients compared to short term inpatients, but not statistically significant (odds ratio = 0.524; 95% confidence interval: 0.235 to 1.171).
Any Opioid Use by 5 Weeks and 26 Weeks
Table 3 shows the cumulative rates of any episode of opioid use by 5 weeks after randomization (upper panel) and by end of study (week 26) (lower panel), across the two treatment conditions and three clinical settings. For both the 5 week and 26 week any use outcomes, the logistic regression models yield main effects of treatment (5-week: X2(1) = 20.27, p = .0001; 26-week: X2(1) = 6.15, p = .013) and of setting (5-week: X2(2) = 13.83 (p = .001); 26-week: X2(2) = 14.28 (p = .0008), with no significant treatment by setting interactions. As can be seen in Table 3, there is a protective effect of XR-NTX treatment against any opioid use that is particularly evident over the first 5 weeks in the short term inpatient and outpatient settings. Inspecting the effects of setting in the table, rates of any opioid use are highest among those who started the study as short-term inpatients, and by week 26 almost all the short-term inpatients (93% across both treatment conditions) had used opioids at least once. For those who started the study as long term inpatients, rates of any use are low across the first 5 weeks (18% on TAU, 19% on XR-NTX), consistent with a protective effect of the controlled residential setting. However, by 26 weeks (at which point most would have transitioned out of the long term residential settings) rates of any use are high (86% on TAU, 69% on XR-NTX). Those starting the study as outpatients also have substantial rates of any use by week 26 (74% on TAU, 59% on XR-NTX), although lower than the two inpatient groups.
Table 3.
Cumulative rates of any opioid use at 5 weeks after randomization (upper panel) and at 26 weeks/end of study (lower panel), by treatment condition (TAU vs XR-NTX + TAU) and by setting in which the patient initiated the study–short-term inpatient (n = 59), versus long-term inpatient (n = 48), versus outpatient (n = 201). Values in the Table are percent using any opioids (N used/N total).
| TAU | XR-NTX + TAU | |
|---|---|---|
| Cumulative Any Opioid Use at 5 Weeks* | ||
| Short-term inpatient (n = 59) |
70 (21/30) | 31 (9/29) |
| Long-term inpatient (n = 48) |
18 (4/22) | 19 (5/26) |
| Outpatient (n = 201) |
42 (43/103) | 16 (16/98) |
| Cumulative Any Opioid Use at End of Study/26 weeks** | ||
| Short-term inpatient (n = 59) |
93 (28/30) | 93 (27/29) |
| Long-term inpatient (n = 48) |
86 (19/22) | 69 (18/26) |
| Outpatient (n = 201) |
74 (76/103) | 59 (58/98) |
For any use by 5 weeks, main effect of treatment (X2(1) = 20.27, p = .0001) and setting are significant X2(2) = 13.83 (p = .001), with any use in the short term inpatient group significantly higher than the long term inpatient (OR = 10.50; 95% CI: 2.76 – 39.92) and outpatient settings (OR = 3.26; 95% CI: 1.36 – 7.80). The treatment by setting interaction was not significant.
For any use by End of Study/26 weeks, main effects of treatment (X2(1) = 6.15, p = .013) and setting (X2(2) = 14.28 (p = .0008) are significant, with any use in the short term inpatient group significantly higher than the long term inpatient (OR = 4.024, 95% CI: 1.183 to 13.687) and outpatient settings (OR = 7.100, 95% CI: 2.455 to 20.532). The treatment by setting interaction was not significant.
Relapse in Relation to Last Dose of XR-NTX
To examine the relationship between non-adherence or early discontinuation of medication and relapse, we compared, within the injection naltrexone + TAU group, the week when the last dose of XR-NTX was taken to the week of relapse. Of the 66 relapses that occurred among patients treated with injection naltrexone (collapsing across the three treatment initiation settings), most (55, 83%) occurred after the patient’s last dose of medication.
Discussion
In a multi-site effectiveness trial of injection naltrexone (XR-NTX) for prevention of relapse to opioid use disorder among patients not seeking opiate substitution treatment, we examined rates of relapse to opioid use as a function of the type of setting in which patients initiated the trial. Many patients initiated the trial as outpatients, having already achieved and sustained abstinence on an outpatient basis. Others had been admitted to either short-term inpatient or long term inpatient programs and initiated the trial in those settings. As hypothesized, among patients initiating the trial on short term inpatient units, there was a high rate of relapse to regular opioid use in the early weeks after discharge–a cumulative relapse rate of 63% by week 5–among patients assigned to treatment as usual (TAU) without study medication. Whereas, those assigned to XR-NTX had little relapse in the first 5 weeks (7%). Among the short term inpatients, XR-NTX also reduced the rate of any opioid use in the first 5 weeks (31%), compared to TAU (70%). The high rate of relapse, and of any opioid use, in the TAU condition replicates the findings of other studies that examined outcome after short-term inpatient or residential treatment (Broers et al., 2000; Chutuape et al., 2001; Gossop et al., 1989), and confirms clinical concerns about the risk of discharging patients with opioid use disorder without an effective medication treatment. The low rate of early relapse in the XR-NTX condition, and the fact that not all patients on XR-NTX who used opioids relapsed, supports the potential protective effect of an evidence-based medication like XR-NTX as part of the discharge plan for patients with opioid use disorder undergoing medically supervised withdrawal during short term inpatient or residential treatment.
Other aspects of the findings were surprising and somewhat contrary to our hypotheses. Disappointingly, among patients from short-term inpatient units treated with XR-NTX, there was considerable relapse after week 5 through the end of the 6-month study, such that the protective effect of XR-NTX, compared to TAU, while still present, was diminished. Indeed, there was substantial relapse over the 6 month trial on XR-NTX across all three treatment initiation settings. This result likely relates to patients not returning for second or subsequent monthly injections of XR-NTX, and indeed most (83%) of the relapses in the XR-NTX condition occurred after the patient’s last recorded dose of medication. The relapse rate on XR-NTX would likely have been much lower had more patients adhered to the full 6 months of doses. The trial offered no specific interventions designed to boost medication adherence other than standard clinical support. Behavioral methods such as contingency management, cognitive behavioral therapies, and involvement of significant others have been shown to improve adherence to medication assisted treatment with oral naltrexone (Carroll et al., 2001; Carroll et al., 2002; Fals-Stewart and O’Farrell 2003; Nunes et al., 2006; Preston et al., 1999) and buprenorphine (Christensen et al., 2014), and future studies should consider examining such behavioral methods for improving adherence to injection naltrexone.
Among the outpatients, many of whom had already sustained abstinence for weeks or months prior to entering the study, the amount of relapse observed was also high. Among outpatients in the TAU condition, relapse occurred steadily over the course of the trial, and the cumulative rate of relapse at 6 months was 61%, while 74% had used an opioid at least once. This suggests the disquieting clinical implication that even among patients with opioid use disorder who have been abstinent and doing well for a period of time as outpatients, relapse risk is still high. This finding is consistent with long term follow up studies of samples of patients with opioid use disorder treated initially with buprenorphine or methadone, showing that the risk of return to opioid use is high, and the best predictor of abstinence is staying on medication (Hser et al., 2016; Weiss et al., 2015). In the present study XR-NTX exerted a protective effect among the outpatients, reducing the relapse rate to 38%. The clinical implication is that patients with opioid use disorder, even if they have achieved abstinence for a period of time, should consider receiving prophylaxis with an effective medication. XR-NTX is well suited for this purpose, since the other effective medications, buprenorphine or methadone, would re-expose an abstinent individual to opioids and re-initiate physiological dependence.
Patients initiating the study during long term inpatient treatment experienced low rates of early relapse and early use of any opioid (over the first five weeks) in both TAU and XR-NTX conditions. This finding suggests a protective effect of long-term residential treatment, removing and protecting patients from exposure to the natural environments where drug use is likely. Again, as with patients initiating the study as outpatients, relapse gradually recurred over the 6 month trial, as patients leave the residential setting and return to their natural environments.
Although the high rates of relapse have been emphasized, 23% (short-term inpatient group), 39% (outpatient group), and 41% (long term inpatient group) on the TAU condition did not relapse across the 6 month trial. Thus, a minority of patients with opioid use disorder appear to do well over a six month follow-up without a maintenance medication. Caution is warranted in that most patients on TAU had used opioids at least once by the 6 month point (short term inpatient: 93%; long term inpatient: 86%; outpatient: 74%) and use carries the risk of relapse over time. Further, there is the problem from a clinical perspective is that we do not have a good way to identify, prospectively, which patients with opioid use disorder may fall into that minority who are not going to use opioids and thus would not need maintenance medication to help prevent relapse. More work is needed to explore clinical features that might predict the need for long term treatment with medication.
The main effects of setting on rates of relapse over 26 weeks (Table 2), and on rates of any use of opioids (Table 3), indicate that those patients who initiated treatment on short term inpatient units had the highest overall risk of opioid use and of relapse, while those who initiated treatment as outpatients had the lowest risks. This makes sense on the face of it, in that patients with opioid use disorder who are able to sustain abstinence as outpatients (as required to gain entry into the trial) likely have a greater level of control over their opioid use. Differences in clinical and demographic features between settings shown in Table 1 (among inpatients: more unemployment, criminal justice supervision, and more lifetime heroin and injection drug use) suggest that the case-mix of patients on short-term and long term inpatient settings was more severely ill than that of outpatients. Thus, differences in overall outcome and treatment effects (XR-NTX vs TAU) across settings may be due to differences in patients’ clinical severity, rather than direct effects of the settings in which they initiated treatment. A companion analysis of baseline variables as moderators of the effect of treatment in this trial (Friedmann et al., in press) found only one significant moderator—heavy alcohol use in the month prior to admission associated with poor outcome on XR-NTX.
Strengths of this analysis include the large, randomized effectiveness trial, a community-based sample likely to be representative of the important population of patients with opioid use disorder who have had criminal justice involvement, and the clinically meaningful primary outcome measure of relapse.
Limitations include that this was a secondary analysis: clinical setting when a patient initiated the trial was naturalistic, was not part of the a priori design, and was not randomly assigned. Clinical settings were not evenly distributed across the five sites that participated in the trial, such that setting and site are confounded. The study design did not control features of the clinical settings, which varied naturalistically. Only a handful of inpatient or residential settings were involved and they may not be representative of the full range of such treatment programs.
In summary, patients with opioid use disorder admitted to short-term inpatient treatment experienced high rates of early relapse to opioid use when assigned to usual treatment without a maintenance medication, while extended-release injection naltrexone (XR-NTX) reduced early relapse. This result suggests a high risk of relapse for patients with opioid use disorder admitted to short term inpatient treatment with medically supervised withdrawal from opioids. The data supports the clinical recommendation that an effective medication should be part of the discharge and aftercare plans for inpatients with opioid use disorder. One can further argue that the system of “detoxification units” for opioid use disorder should be reengineered into “medication induction centers” in which effective medications are initiated in a controlled environment. Across the 6-month trial, a substantial rate of relapse was observed for all three clinical settings from which patients initiated the trial (short-term inpatient, long-term inpatient, outpatient). Even outpatients, who had been abstinent for a period of time before entering the trial, had a clinically important rate of relapse. While XR-NTX continued to exert a protective effect across the 6-month follow-up, substantial relapse among those assigned to XR-NTX suggests the need for approaches to improve adherence to medication assisted treatment and manage relapse risk over the long term. Finally, a minority of patients assigned to usual treatment (TAU) across all three treatment settings did not relapse during the 6 month trial. This finding suggests there is a subgroup of patients with opioid use disorder who may remain relapse-free over the long term without medication assisted treatment. However, there is at present no accurate way to predict who may do well without medication, and relapse to opioids carries the risk of overdose and death. Thus, patients and their clinicians face a risky choice if not opting for medication, and future work is needed in an effort to determine when patients with opioid use disorder may safely discontinue or forgo medication treatment.
Highlights.
Among patients with opioid use disorder, the risk of relapse was high over the month after discharge from short term inpatient treatment without medication assisted treatment.
Long-acting injectable naltrexone (XR-NTX) protected against relapse after discharge from short term inpatient treatment.
Short term inpatient “detoxification” units should be re-engineered into “induction units” for medication assisted treatments.
Even among outpatients with opioid use disorder who present already abstinent, or patients on long-term residential treatment, relapse over a 6 month follow-up was high and XR-NTX was protective.
More work is needed to improve long-term adherence to XR-NTX and other medication assisted treatments for opioid dependence.
Acknowledgments
Supported by the National Institute on Drug Abuse (NIDA) through a collaborative clinical trial mechanism, PAR-07-232 (R01DA024549, to Dr. Friedmann; R01DA024550, to Dr. Kinlock; R01DA024553, to Dr. O’Brien; R01DA024554, to Dr. Nunes; and R01DA024555, to Dr. Lee), and additional support (K24DA022412, to Dr. Nunes). Trial medication was provided in kind from an investigator-initiated grant from Alkermes. Funding from the Dana Foundation to Dr. O’Brien supported the conduct of a five-site pilot study. Dr. Lee reports receiving grant support and study medication from Alkermes and study medication from Indivior (formerly Reckitt Benckiser). Dr. Friedmann reports receiving fees for serving on an advisory board and travel support from Indivior and an honorarium for leading a roundtable discussion from Orexo. Dr. Kinlock reports receiving grant support and study medication from Alkermes. Dr. Nunes reports serving on an advisory board for Alkermes, receiving study medication from Reckitt Benckiser and Duramed Pharmaceuticals, being lead investigator for a NIDA-funded study of a computer-delivered behavioral intervention supplied by HealthSim, and being site principal investigator for a study funded by, and receiving travel support from, Brainsway. Dr. Gordon reports receiving grant support and study medication from Alkermes. Dr. Fishman reports receiving travel support from Alkermes. Dr. O’Brien reports receiving consulting fees from Alkermes. No other potential conflict of interest relevant to this article was reported. We thank the trial participants and combined research staff across all sites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison–a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–65. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996–99. Addiction. 2003;98(2):185–90. doi: 10.1046/j.1360-0443.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in Geneva: follow-up at 1 and 6 months. Drug Alcohol Depend. 2000;58(1–2):85–92. doi: 10.1016/s0376-8716(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan DA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58(8):755–61. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol. 2002;10(1):54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Christensen DR, Landes RD, Jackson L, Marsch LA, Mancino MJ, Chopra MP, Bickel WK. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964–72. doi: 10.1037/a0037496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three-, and six-month outcomes after brief inpatient opioid detoxification. Am J Drug Alcohol Abuse. 2001;27(1):19–44. doi: 10.1081/ada-100103117. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl) 2002 Feb;159(4):351–60. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ. Behavioral family counseling and naltrexone for male opioid-dependent patients. J Consult Clin Psychol. 2003;71(3):432–42. doi: 10.1037/0022-006x.71.3.432. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Wilson D, Nunes EV, Hoskinson R, Lee JD, Gordon M, Murphy SM, Bonnie RJ, Chen DT, Boney TY, O’Brien CP. Do patient characteristics moderate the effect of extended-release naltrexone (XR-NTX) for opioid use disorder? Journal of Substance Abuse Treatment. doi: 10.1016/j.jsat.2017.01.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Gossop M, Green L, Phillips G, Bradley B. Br J Psychiatry. 1989;154:348–53. doi: 10.1192/bjp.154.3.348. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Morris N, Arnold-Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66(10):1108–1115. doi: 10.1001/archgenpsychiatry.2009.130. [DOI] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, Ling W. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014 Jan;109(1):79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Woody G, Liu D, Wakim P, Matthews AG, Hatch-Maillette M, Jelstrom E, Wiest K, McLaughlin P, Ling W. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016;111(4):695–705. doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. The Lancet. 2011;377(9776):1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Wahlgren V, Tsoy-Podosenin M, Bushara N, Burakov A, Masalov D, Romanova T, Tyurina A, Palatkin V, Slavina T, Pecoraro A, Woody GE. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69(9):973–81. doi: 10.1001/archgenpsychiatry.2012.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Boney TY, Hoskinson RA, Jr, McDonald R, Gordon M, Fishman M, Chen DT, Bonnie RJ, Kinlock TW, Nunes EV, Cornish JW, O’Brien CP. Extended-release naltrexone to prevent relapse among opioid dependent, criminal justice system involved adults: rationale and design of a randomized controlled effectiveness trial. Contemp Clin Trials. 2015;41:110–7. doi: 10.1016/j.cct.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, Jr, Wilson D, McDonald R, Rotrosen J, Gourevitch MN, Gordon M, Fishman M, Chen DT, Bonnie RJ, Cornish JW, Murphy SM, O’Brien CP. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med. 2016;374(13):1232–42. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014 Feb 6;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: a ceiling on effectiveness? Am J Drug Alcohol Abuse. 2006;32(4):503–17. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Krupitsky E, Ling W, Zummo J, Memisoglu A, Silverman BL, Gastfriend DR. Treating Opioid Dependence With Injectable Extended-Release Naltrexone (XR-NTX): Who Will Respond? J Addict Med. 2015;9(3):238–43. doi: 10.1097/ADM.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54(2):127–35. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Ravndal E, Amundsen EJ. Mortality among drug users after discharge from inpatient treatment: an 8-year prospective study. Drug Alcohol Depend. 2010;108(1–2):65–9. doi: 10.1016/j.drugalcdep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Seaman SR, Brettle RP, Gore SM. Mortality from overdose among injecting drug users recently released from prison: database linkage study. BMJ. 1998;316(7129):426–8. doi: 10.1136/bmj.316.7129.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–46. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Griffin ML, Provost SE, Fitzmaurice GM, McDermott KA, Srisarajivakul EN, Dodd DR, Dreifuss JA, McHugh RK, Carroll KM. Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug Alcohol Depend. 2015;150:112–9. doi: 10.1016/j.drugalcdep.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


