Abstract
Cellular turnover represents a fundamental aspect of immunological homeostasis. While many factors appear to regulate leukocyte removal during inflammatory resolution, recent studies suggest that members of the galectin family play a unique role in orchestrating this process. Unlike cellular removal through apoptotic cell death, several members of the galectin family induce surface expression of phosphatidylserine (PS), a phagocytic marker on cells undergoing apoptosis, in the absence of cell death. However, similar to PS on cells undergoing apoptosis, galectin-induced PS exposure sensitizes cells to phagocytic removal. As galectins appear to prepare cells for phagocytic removal without actually inducing apoptotic cell death, this process has recently been coined preaparesis. Given the unique characteristics of galectin-induced PS exposure in the context of preaparesis, we will examine important considerations when evaluating the potential impact of different galectin family members on PS exposure and cell viability.
Keywords: Galectin, Phosphatidylserine (PS), Inflammatory resolution, Leukocyte turnover, Preaparesis
1 Introduction
Appropriate removal of leukocytes during inflammatory resolution represents a fundamental process in immunological homeostasis [1, 2]. Although many factors, including members of the TNF family [3], appear to regulate this process, recent studies suggest that several members of the galectin family also regulate leukocyte turnover [4]. While most immunoregulatory factors induce leukocyte removal by initiating apoptotic cell death, galectins possess a unique capacity to trigger phagocytic removal in the absence of apoptosis [5–7]. Galectin engagement of neutrophil ligands induces surface expression of phosphatidylserine (PS), similar to cells undergoing apoptotic cell death [8]. However, unlike apoptosis, galectin-induced PS exposure occurs in the conspicuous absence of cell death [5–7, 9, 10]. While galectins fail to induce apoptotic cell death in neutrophils, galectin-induced PS exposure maintains the ability to sensitize cells to phagocytic removal [7]. Thus, galectins possess a unique ability to prepare cells for phagocytic removal without inducing cell death, a process recently termed preaparesis [6].
During cellular turnover, apoptotic cell death often provides a distinct pathway for enabling cell removal without inducing significant inflammation [1, 2]. This largely reflects the ability of cells undergoing apoptosis to maintain membrane integrity prior to successful phagocytic removal. However, once an apoptotic program is engaged, cells only maintain membrane integrity for a limited time before membrane integrity becomes compromised and late apoptosis/necrosis occurs [1, 2]. In the setting of acute inflammation, not only are inflammatory signals already present, but the number of neutrophils often far exceeds the number of phagocytic cells needed for rapid removal during inflammatory resolution [11]. As a result, induction of apoptosis in neutrophils would be predicted to result in a significant number of late apoptotic events prior to efficient phagocytic removal, which may actually increase inflammatory stimuli [11, 12]. Furthermore, given the inflammatory environment in which neutrophils often reside, the likelihood of maintaining membrane integrity in this setting is also reduced [12, 13]. Consistent with this, previous studies suggested that neutrophils evolved a unique mechanism of turnover that occurs independent of apoptosis [11, 14, 15]. The ability of galectins to induce PS exposure in the absence of cell death, while retaining the ability to sensitize these cells for phagocytic removal, likely enables neutrophils to maintain membrane integrity until successfully phagocytosed.
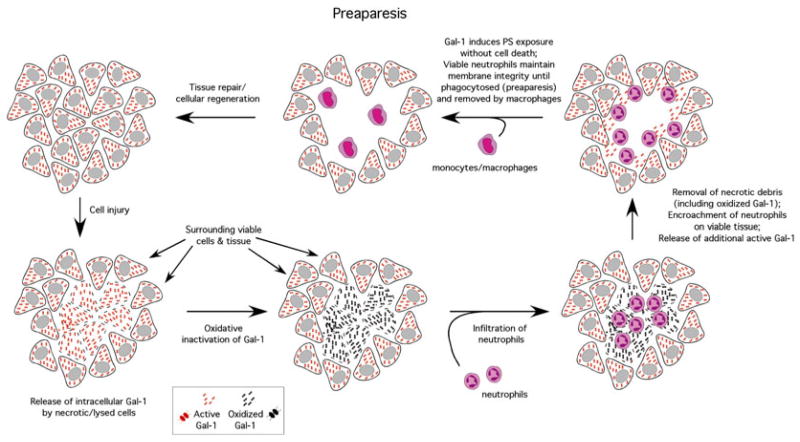
As neutrophils do not typically utilize the same level of directed immunological activity of T cells and other cells involved in adaptive immunity, the ability of galectins to induce preaparesis in neutrophils, while typically inducing apoptosis in T cells [6, 16], may in part reflect engagement of unique pathways not only important in turnover but also important in the spatial and temporal regulation of neutrophil function. Galectins possess a unique sensitivity to oxidative or proteolytic inactivation once outside the cell [7, 9, 17–19]. As a result, galectin released from damaged tissue at the time of an initial inflammatory insult likely undergoes inactivation prior to significant neutrophil accumulation. However, as neutrophils begin to encroach on viable tissue following the removal of necrotic debris, release of reduced, and therefore active, galectin likely results in galectin engagement and induction of preaparesis in neutrophils, thereby facilitating macrophage-mediated removal (Fig. 1) [9, 20, 21]. In contrast, as T cells often do not accumulate at the same rate or magnitude as neutrophils and also display a targeted approach to their activity [22, 23], apoptotic cell death is likely sufficient to induce cellular removal without causing deleterious consequences in the setting of inflammation.
Fig. 1.
Galectins may contribute to the spatial and temporal regulation of neutrophil turnover. Intracellular galectin remains reduced and active. However, following cellular injury, intracellular galectin becomes exposed to the extracellular oxidizing environment, oxidizes and becomes inactive. Following galectin inactivation, neutrophils infiltrate, which allows for neutralization of potential pathogens and removal of necrotic tissue. During this inflammatory episode, most of the galectin released during the primary injury likely becomes oxidized, preventing galectins from inhibiting a productive inflammatory response. Following removal of necrotic tissue and removal of pathogens, encroachment of neutrophils on surrounding viable tissue results in cellular damage and release of reduced and therefore active galectin. Galectin engages neutrophil receptors and induces PS exposure without altering cellular viability, a process termed preaparesis, which allows neutrophils to maintain membrane integrity until successfully phagocytosed
Given the unique sensitivity of galectins to oxidative inactivation coupled with their unprecedented ability to induce leukocyte turnover independent of cell death [7, 9, 17], examination of the potential impact of galectins on cellular viability requires careful analysis of galectin activity and cellular function. In this chapter, we will describe methods used to examine the potential impact of galectins on cellular viability and PS exposure, including potential pitfalls commonly associated with these approaches.
2 Materials
2.1 Neutrophil Isolation
Dextran (6 % dextran 70 in 0.9 % Sodium Chloride injection USP—Braun Medical Inc.).
0.2 % NaCl.
1.6 % NaCl.
Hanks’ Balanced Salts Solution (HBSS) (standard with Mg and Ca) (HyClone).
human serum albumin (HSA) (Fisher scientific).
Ficoll (GE Healthcare).
Butterfly needle (BD).
60 mL syringe (Cole-Parmer).
15 mL Falcon tube (BD).
50 mL Falcon tube (BD).
Wright-Giemsa stain (Sigma-Aldrich).
fMLP (Sigma-Aldrich).
2.2 Cell Culture
HL60 cells (ATCC CCL240).
RPMI (Gibco).
FBS (Gibco, Life Technologies).
Glutamine (Life Technologies).
Penicillin/streptomycin (Sigma-Aldrich).
Supplemented RPMI media: RPMI with 10 % FBS, 1 % glutamine, 1 % penicillin/streptomycin.
2.3 Galectin Preparation and Incubation with Cells
Recombinant galectin.
PBS standard pH 7.4 (HyClone).
β-Mercaptoethanol (βME) (Fisher).
PD-10 (or equivalent) small gel filtration column (GE Healthcare).
RPMI media (Gibco).
Lactose (Fisher).
Thiodigalactoside (TDG) (Carbosynth).
0.1 M lactose in HBSS.
Sterile 48-well tissue culture plate.
2.4 Annexin V Staining of Galectin-Treated Cells
-
1
HEPES/NaOH.
-
2
NaCl.
-
3
CaCl2.
-
4
Hanks’ Balanced Salts Solution (HBSS) (standard with Mg and Ca) (HyClone).
-
5
Annexin V, Alexa Fluor 488 (Life Technologies).
-
6
Propidium iodide (PI) (Life Technologies).
Prepared Buffers
3 Methods
3.1 Isolation of Human Neutrophils from Whole Blood
Using a 60 mL syringe, draw 300 μL of heparin (1,000 U/mL) (see Note 3).
Draw 30 mLs of whole blood into this 60 ml syringe using a butterfly needle by employing standard phlebotomy procedures (see Note 4).
Carefully rotate capped syringe in order to adequately mix heparin with blood to prevent clotting.
Draw 15 mLs of dextran into the whole blood-heparin mixture (see Note 5).
Carefully mix the dextran solution with the whole blood solution.
Affix the syringe with the opening facing upright against a wall or other support in a sterile hood. The dextran/whole blood solution will begin to separate. The top layer, which appears to become devoid of red blood cells (RBCs), is the layer that contains neutrophils.
At 30 min, 45 min, and 1 h after affixing the syringe in the upright position, remove this top layer (see Note 6).
Once the top layer of solution is completely removed, place this solution in a 50 mL sterile tube and centrifuge for 7 min at 600 × g at RT (see Note 7).
After centrifugation, discard the supernatant (see Note 8).
Resuspend the pellet in 25 mL of a 0.2 % NaCl solution, and incubate at RT for 20 s (see Note 9).
Immediately add 25 mL 1.6 % NaCl and mix well.
Place cells in a centrifuge for 7 min at 600 × g at RT.
Discard supernatant and resuspend pellet in 50 mL of HBSS with 0.5 % human serum albumin.
Place cells in a centrifuge for 7 min at 600 × g at RT.
Add 6 mL Ficoll to a sterile 15 mL Falcon tube.
Resuspend pellet isolated in step 14 in 6 mL of HBSS with 0.5 % human serum albumin.
Place the leukocyte solution on top of the Ficoll by tilting the tube at a 45° angle and carefully layer the leukocyte solution on top of the Ficoll (see Note 10).
Centrifuge the layered leukocyte/Ficoll solution for 30 min at 600×g at RT (see Note 11).
Remove the middle band (this layer primarily consists of lymphocytes).
Remove the pellet (this primarily consists of neutrophils) and resuspend in 12 mL HBSS with 0.5 % human serum albumin.
Centrifuge cells for 7 min at 600 × g at RT.
Wash two more times in 12 mL HBSS with 0.5 % human serum albumin by centrifugation for 7 min at 600 × g at RT.
Examine the cells for neutrophil content by staining with Wright-Giemsa stain per the manufacturer’s protocol, followed by morphological analysis of neutrophils (see Note 12).
Following the final wash, cells can be resuspended in supplemented RPMI.
Count the number of cells per mL using a standard hemocytometer.
For activation, resuspend the cells in RPMI at 1 × 106/mL, and add 1 μg of fMLP for every mL. Incubate at 37 °C for 15 min. Following incubation, wash cells in supplemented RPMI by centrifuging for 7 min at 600 × g at RT twice.
3.2 Maintaining Cell Culture of Non-adherent HL60 Cells
3.3 Galectin Preparation and Incubation with Cells
Thaw frozen vial of recombinant galectin by placing on ice (4 °C) (see Note 15).
Re-equilibrate a PD-10 column with 5 column volumes of cold PBS.
To remove lactose and βME from galectin, add 1 mL of recombinant galectin solution to the re-equilibrated PD-10 column (see Note 16).
Collect 0.5 mL fractions from the PD-10 column following the addition of recombinant galectin.
Once the recombinant galectin solution has completely penetrated the column, add 2 mL of cold PBS.
Continue collecting 0.5 mL fractions from the PD-10 column following the addition of cold PBS, adding additional PBS as needed to elute all galectin from the column and to prevent the column from drying.
Examine protein content of each fraction by measuring the OD 280 nm. This is typically done by diluting each fraction tenfold (i.e., 10 μL of each fraction in 90 μL PBS) followed by examination of the OD 280 nm on a standard spectrophotometer (see Note 17).
Place pooled recombinant galectin on ice until ready to use.
Prepare galectins in PBS at five times the desired final concentration.
Pipet 200 μL of cells in supplemented RPMI media at a concentration of 1×106 cells/mL into each well of a 48-well plate (see Note 18).
Pre-warm galectin to 37 °C and add 50 μL of recombinant galectin solution or PBS control to each well (see Note 19).
Incubate at 37 °C for the indicated time (see Note 20).
3.4 Staining Cells with Annexin V
At the end of the incubation time point, add 50 μL of 120 mM lactose in RPMI to remove bound galectin and disengage cells (see Note 21).
Incubate for 5 min at 37 °C.
Examine cells for agglutination following incubation (see Note 22).
Once cells appear to be sufficiently disengaged, wash cells three times in HBSS by centrifuging cells at 600 × g for 7 min.
Place cells on ice.
Resuspend cells in 100 μL Annexin V and PI staining solution precooled to 4 °C.
Incubate at 4 °C for 15 min in the dark.
Add 400 μL of Annexin V staining buffer precooled at 4 °C to each sample.
Fig. 2.
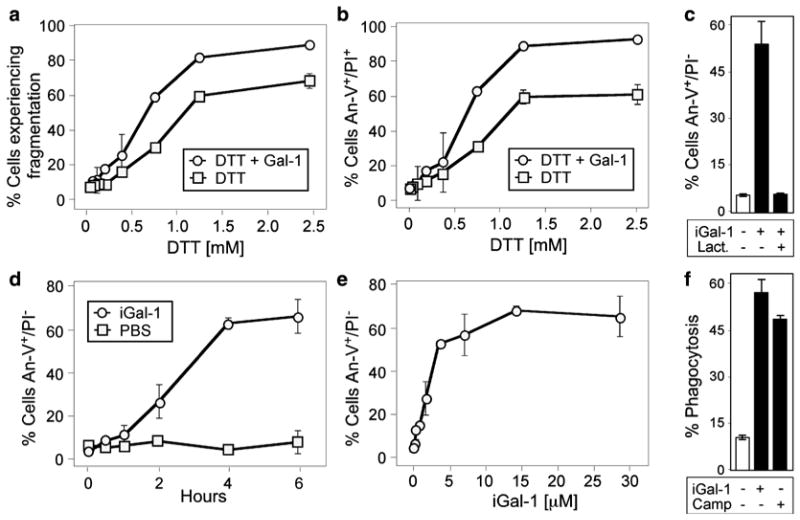
DTT sensitizes cells to Gal-1-induced apoptosis. (a) Promyelocytic HL60 cells (cells) were incubated with PBS, 10 μM Gal-1, or various concentrations of DTT with or without 10 μM Gal-1 as indicated for 9 h followed by detection for cellular fragmentation as indicated by changes in forward (FSC) and side scatter (SSC) profiles of cells. (b) Cells were incubated with PBS, 10 μM Gal-1, or various concentrations of DTT with or without 10 μM Gal-1 as indicated for 9 h followed by detection for cell death by PS exposure and membrane integrity loss by An-V-FITC and PI staining. (c) Cells were incubated with PBS, 10 μM iodoacetamide-treated Gal-1 (iGal-1), or 10 μM iGal-1 with 20 mM lactose followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (d) Cells were incubated with PBS or 10 μM iGal-1 for the indicated time followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (e) Cells were incubated with PBS or the indicated concentration of iGal-1 for 8 h followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (f) Cells were incubated with PBS, 10 μM iGal-1, or 10 μM Camp for 8 h followed by incubation of peritoneal macrophages for 1 h and microscopic examination of phagocytosis. This research was originally published in Molecular Biology of the Cell. Stowell SR, Karmakar S, Arthur CM, Ju T, Rodrigues LC, Riul TB, Dias-Baruffi M, Miner J, McEver RP, Cummings RD. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. 2009 Mar;20(5):1408–18
Fig. 3.
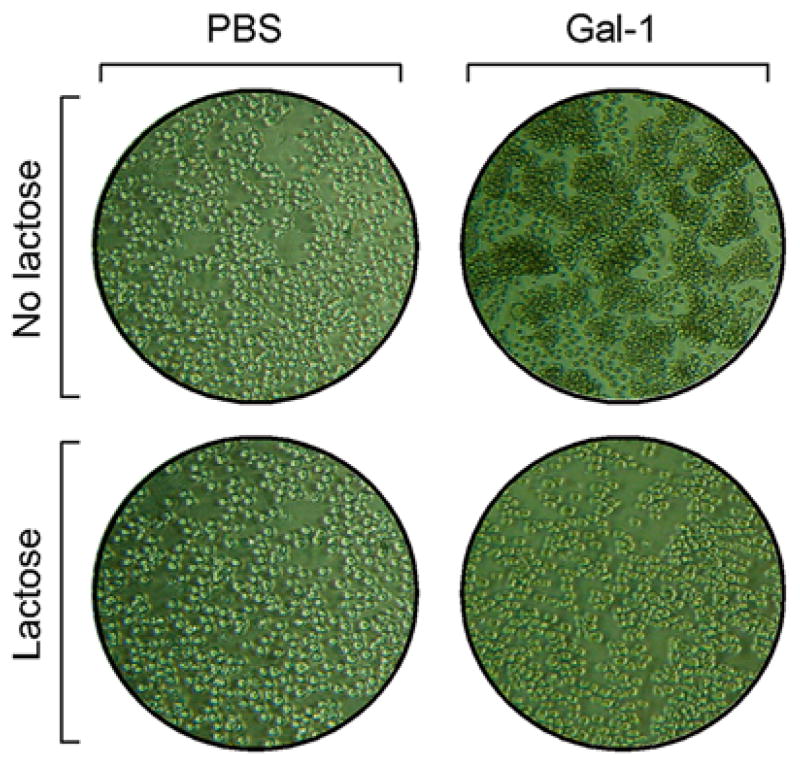
Incubation of leukocytes with galectins induces agglutination. Promyelocytic HL60 cells were incubated with PBS or 10 μM galectin-1 (Gal-1) for 4 h followed by microscopic evaluation for agglutination (no lactose). Following incubation with PBS or Gal-1, lactose was added at a final concentration of 20 mM lactose for 15 min at 37 °C followed by microscopic evaluation as indicated
Fig. 4.
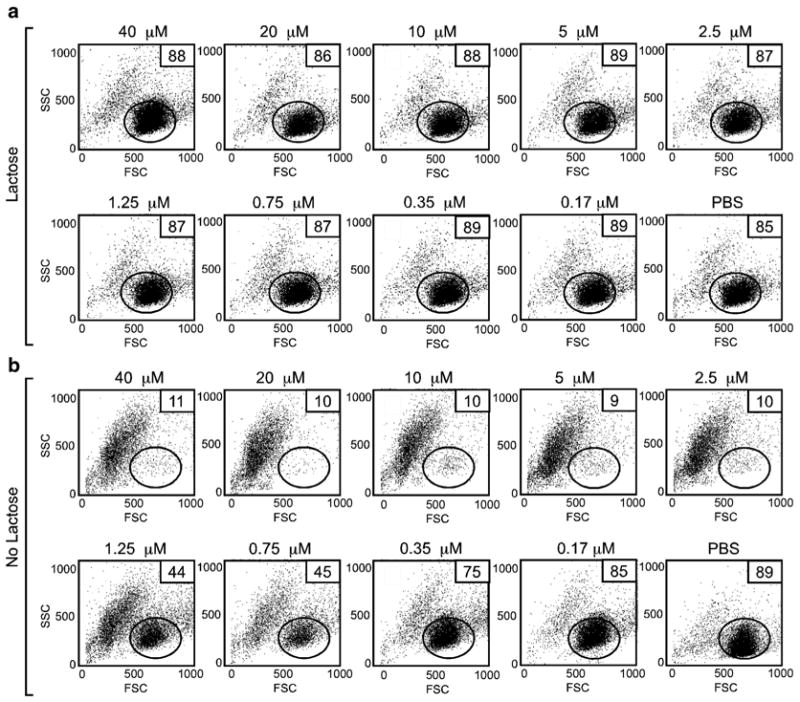
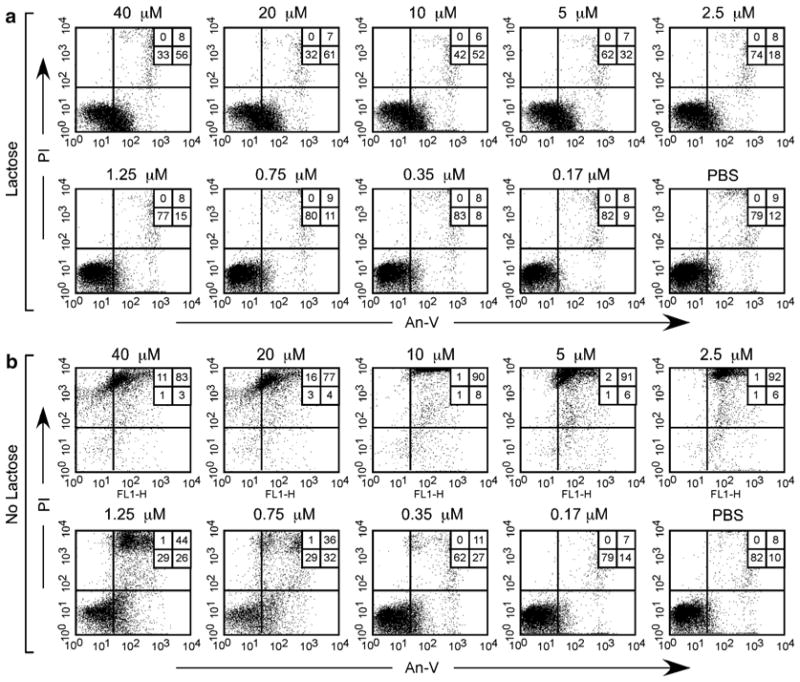
Failure to disengage cells can result in significant changes in the forward and side scatter profiles following flow cytometric analysis. Promyelocytic HL60 cells were incubated at the indicated concentrations of galectin-1 (Gal-1) for 4 h followed by addition of PBS (no lactose) or lactose at a final concentration of 20 mM (lactose) and incubated for an additional 15 min. Following incubation, cells were washed and analyzed for potential alterations in the forward and side scatter profiles as indicated. Gate = % of cells experiencing no fragmentation. The percent of cells in the gate for each condition is shown
Fig. 5.
Failure to disengage cells can result in significant Annexin V and propidium iodide double-positive staining. Promyelocytic HL60 cells were incubated at the indicated concentrations of galectin-1 (Gal-1) for 4 h followed by addition of PBS (no lactose) or lactose at a final concentration of 20 mM (lactose) and incubated for an additional 15 min. Following incubation, cells were washed and stained with Annexin V (An-V) and propidium iodide (PI) as outlined. The percent of cells in each quadrant is shown
Fig. 6.
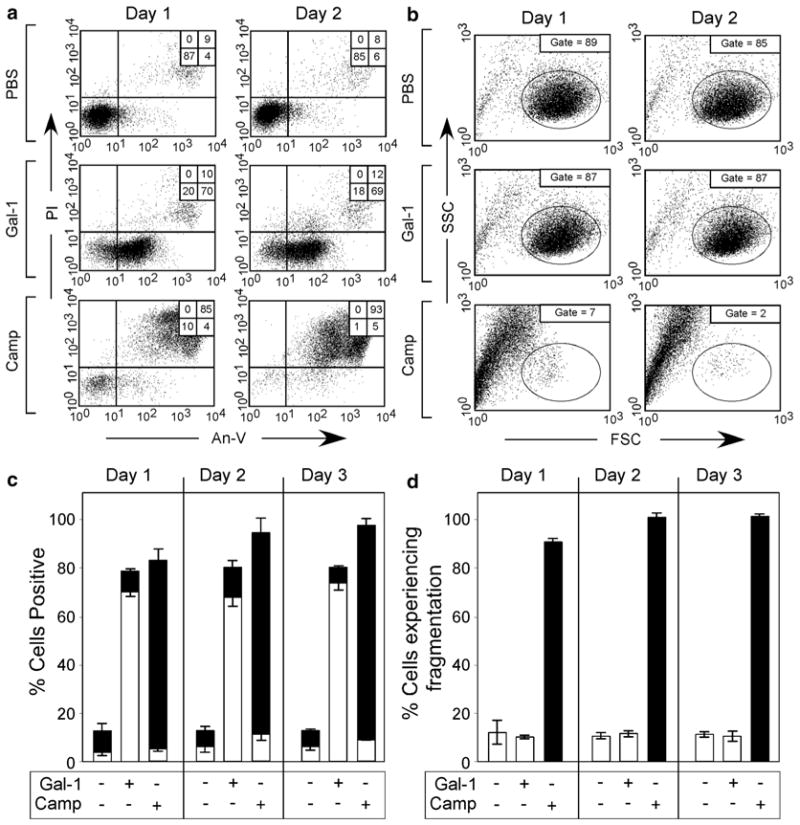
Gal-1 induces continuous PS exposure in the absence of cellular fragmentation. (a) Cells were incubated with PBS, 10 μM iodoacetamide-alkylated Gal-1 (iGal-1), or 10 μM Camp for 1 or 2 d as indicated, followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (b) Cells were incubated with PBS, 10 μM iGal-1, or 10 μM Camp for 1 or 2 d as indicated, followed by examination for cellular fragmentation as indicated by changes in forward (FSC) and side scatter (SSC) profiles of cells. Gate = % of cells experiencing no fragmentation. (c ) Quantification of cells treated in (a). White bars = % An-V+, PI−; black bars = % An-V+, PI+. (d) Quantification of cells treated in (b). This research was originally published in Molecular Biology of the Cell. Stowell SR, Karmakar S, Arthur CM, Ju T, Rodrigues LC, Riul TB, Dias-Baruffi M, Miner J, McEver RP, Cummings RD. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. 2009 Mar;20(5):1408–18
Acknowledgments
This work was supported in part by grants from the National Blood Foundation, American Society of Hematology and Hemophilia of Georgia to S.R.S.
Footnotes
Following buffer preparation, cool buffers to 4 °C prior to staining the cells.
The amount of Annexin V used may vary based on the manufacturer of the product.
Typically 100 μL of heparin stock solution (1,000 U/mL) is used for every 10 mL of whole blood drawn.
Institutional review board (IRB) or equivalent approval must be obtained prior to drawing blood from healthy human volunteers.
The total amount of dextran added typically equals half the total starting volume of whole blood. This is often done by drawing the dextran solution into a separate container followed by injecting the dextran solution into the whole blood-heparin mixture to prevent contamination of the dextran stock.
The 30 and 45 min interval removal of the top layer appears to facilitate additional separation. However, these steps are not absolutely necessary.
Although protocols can differ, we found that placing neutrophils on ice, followed by rewarming, can result in significant neutrophil activation. As a result, a concerted effort is made to avoid cold temperatures during neutrophil isolation. All procedures should be done under a sterile laminar flow hood.
The pellet will look red due to significant red blood cell contamination.
This should lyse the red blood cells. As this is a hypotonic RBC lysis method, care should be taken to not over-incubate the cells.
Care should be taken to not allow the leukocyte HBSS solution to penetrate the Ficoll solution.
Do not use the brakes on the centrifuge as this can disrupt the desired interfaces generated during centrifugation.
Using this protocol typically >90 % of the isolated cells are neutrophils.
As HL60 cells will differentiate in the presence of dimethyl sulfoxide (DMSO) and DMSO is a commonly employed cryo-preservant, care should be taken to remove DMSO as soon as possible following initiation of the HL60 culture.
Care should be taken to not allow cells to exceed 1 × 106 cells/mL. Cells can be counted using a standard hemocytometer. Equally important, the media should be regularly changed to allow cells to remain in a physiologic pH. Using this approach, typically >90 % of the cells remain viable at baseline. When cells are allowed to become overcrowded and/or the media is changed infrequently, cellular viability suffers and the overall outcome of galectin-HL60 cell interactions can be altered.
All galectin preparation should be done under a sterile laminar flow hood. Potential lipopolysaccharide (LPS) contamination should be assessed prior to preparing galectin for incubation with cells. This can be done using the commercially available limulus amebocyte lysate (LAL) assay (Pierce). If significant LPS contamination is noted, LPS removal can be achieved by passing the endotoxin-contaminated recombinant galectin sample over a polymyxin B column (Sigma-Aldrich) according to the manufacturer’s protocol. Repeat removal should be employed until LPS is undetectable.
Several galectins display unique sensitivity to oxidative inactivation [5, 9]. This should be considered when assessing the potential ability of an individual galectin to induce PS exposure when incubated with a given cell. Methods to alkylate galectin-1 and protect it from oxidative inactivation have been developed [9]. However, it should be noted that for most galectins, oxidative inactivation is a gradual process and that biological activity of these proteins can be assessed in the absence of reducing conditions or other chemical modifications if care is taken to use them immediately following removal of βME (or other reducing agent) and ligand (see Fig. 2 for impact of DTT on cellular viability and sensitivity to Gal-1-induced PS exposure).
To extrapolate the protein concentration from the OD 280 nm values, we use the extinction coefficient for the particular galectin being examined to calculate actual concentration in mg/mL. The following Web sites http://www.basic.northwestern.edu/biotools/proteincalc.html and http://web.expasy.org/protparam/protparam-doc.html offer explanation and assistance in calculating the extinction coefficient and using this calculation to determine the actual concentration of a given protein in mg/mL, including caveats as to how these numbers may differ from the actual number. As methods of calculating the extinction coefficient only provide estimates, alternative approaches can be used to empirically determine these values. These include using a Bradford assay to calculate protein concentration or simply re-equilibrating the recombinant protein directly into water, lyophilizing, weighing, and then resuspending in the buffer of choice followed by empirically determining the extinction coefficients for a particular galectin family member.
When counting cells, use trypan blue (Sigma-Aldrich) to determine the percent of viable and nonviable cells. If the nonviable cell count is higher than 10 %, these cells are not sufficiently healthy to use in this assay.
Control wells containing galectin along with a final concentration of 20 mM lactose or 20 mM TDG (both hapten inhibitors of galectin-carbohydrate binding) should be included as controls to determine whether positive signaling events are likely due to galectin-carbohydrate interactions. Additionally, it is critical to incorporate an apoptosis-positive control. Typically Fas for neutrophils and camptothecin for HL60 cells can be used as an apoptosis-positive control, although a variety of proapoptotic agents can be employed.
PS exposure typically peaks between 2 and 4 h. A range of galectin concentrations and times should be employed to determine the optimal time for PS exposure to be realized. To determine whether PS exposure occurs in the absence of cell death, later time points, including 8, 12, and even 24 h, are recommended to determine whether PS exposure continues to occur in the absence of apoptosis using additional methods in parallel that are capable of detecting more definitive markers of apoptotic cell death (examination of DNA fragmentation, changes in mitochondrial potential, cellular fragmentation, etc.).
Unlike the use of antibodies for staining cell surface CD markers, galectins typically agglutinate cells in suspension. Agglutinated cells not only possess the ability to interfere with and potentially occlude appropriate fluid flow during flow cytometric analysis, but cellular fragmentation of agglutinated cells during flow cytometric analysis can result in false-positive results (see Figs. 3, 4, and 5). Although most antibodies used for flow cytometric examination of antigen reactivity at saturating concentrations do not induce agglutination [24–26], antibodies capable of inducing agglutination can induce similar fragmentation and PS positivity [27].
Each galectin family member has different affinity for lactose and leukocyte ligands. As a result, the concentration of lactose and the duration of incubation needs to be empirically determined for each galectin and cell population being analyzed. Thiodigalactoside (TDG) can be a more efficient inhibitor of galectins and therefore can occasionally be used when lactose appears to be insufficient to induce galectin release and disengagement of galectin-induced agglutinated aggregates.
When analyzing cells undergoing apoptosis, it is important to consider potential changes in the forward and side scatter profiles that may occur. The changes can be diagnostic of cells undergoing apoptosis, but may be inadvertently gated out during data acquisition and analysis. As a result, care should be taken when analyzing cells by flow cytometry to ensure that apoptosis-/late apoptosis-positive events are not gated out during data acquisition or analysis (Fig. 6).
Keep cells on ice and analyze as soon as possible (typically <1 h following completion of staining to avoid potential changes in viability associated simply with prolonged incubation in Annexin V staining buffer.) Fixation of cells can result in artificial exposure of PS and should be avoided.
References
- 1.Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84(4):1201–1208. [PubMed] [Google Scholar]
- 4.Cerliani JP, Stowell SR, Mascanfroni ID, Arthur CM, Cummings RD, Rabinovich GA. Expanding the universe of cytokines and pattern recognition receptors: galectins and glycans in innate immunity. J Clin Immunol. 2011;31(1):10–21. doi: 10.1007/s10875-010-9494-2. [DOI] [PubMed] [Google Scholar]
- 5.Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109(1):219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180(5):3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 7.Stowell SR, Karmakar S, Arthur CM, Ju T, Rodrigues LC, Riul TB, Dias-Baruffi M, Miner J, McEver RP, Cummings RD. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell. 2009;20(5):1408–1418. doi: 10.1091/mbc.E08-07-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405(6782):85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 9.Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, Karmakar S, Mehta P, Dias-Baruffi M, McEver RP, Cummings RD. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem. 2009;284(8):4989–4999. doi: 10.1074/jbc.M808925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karmakar S, Stowell SR, Cummings RD, McEver RP. Galectin-1 signaling in leukocytes requires expression of complex-type N-glycans. Glycobiology. 2008;18(10):770–778. doi: 10.1093/glycob/cwn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 12.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. AnnuRevPathol. 2013 doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179(3):1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98(4):1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 16.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378(6558):736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 17.Levi G, Teichberg VI. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981;256(11):5735–5740. [PubMed] [Google Scholar]
- 18.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J Biol Chem. 1995;270(10):5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- 19.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem. 1995;270(10):5207–5212. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- 20.Dias-Baruffi M, Stowell SR, Song SC, Arthur CM, Cho M, Rodrigues LC, Montes MA, Rossi MA, James JA, McEver RP, Cummings RD. Differential expression of immuno-modulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle. Glycobiology. 2010;20(5):507–520. doi: 10.1093/glycob/cwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerri DG, Rodrigues LC, Stowell SR, Araujo DD, Coelho MC, Oliveira SR, Bizario JC, Cummings RD, Dias-Baruffi M, Costa MC. Degeneration of dystrophic or injured skeletal muscles induces high expression of Galectin-1. Glycobiology. 2008;18(11):842–850. doi: 10.1093/glycob/cwn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248(450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 23.Zinkernagel RM, Doherty PC. The discovery of MHC restriction. Immunol Today. 1997;18(1):14–17. doi: 10.1016/s0167-5699(97)80008-4. [DOI] [PubMed] [Google Scholar]
- 24.Stowell SR, Liepkalns JS, Hendrickson JE, Girard-Pierce KR, Smith NH, Arthur CM, Zimring JC. Antigen modulation confers protection to red blood cells from antibody through fcgamma receptor ligation. J Immunol. 2013;191(10):5013–5025. doi: 10.4049/jimmunol.1300885. [DOI] [PubMed] [Google Scholar]
- 25.Girard-Pierce KR, Stowell SR, Smith NH, Arthur CM, Sullivan HC, Hendrickson JE, Zimring JC. A novel role for C3 in antibody-induced red blood cell clearance and antigen modulation. Blood. 2013;122(10):1793–1801. doi: 10.1182/blood-2013-06-508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stowell SR, Henry KL, Smith NH, Hudson KE, Halverson GR, Park JC, Bennett AM, Girard-Pierce KR, Arthur CM, Bunting ST, Zimring JC, Hendrickson JE. Alloantibodies to a paternally derived RBC KEL antigen lead to hemolytic disease of the fetus/newborn in a murine model. Blood. 2013;122(8):1494–1504. doi: 10.1182/blood-2013-03-488874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liepkalns JS, Hod EA, Stowell SR, Cadwell CM, Spitalnik SL, Zimring JC. Biphasic clearance of incompatible red blood cells through a novel mechanism requiring neither complement nor Fcgamma receptors in a murine model. Transfusion. 2012;52(12):2631–2645. doi: 10.1111/j.1537-2995.2012.03647.x. [DOI] [PubMed] [Google Scholar]