Abstract
Learned safety, a learning process in which a cue becomes associated with the absence of threat, is disrupted in individuals with post-traumatic stress disorder (PTSD). A bi-directional relationship exists between smoking and PTSD and one potential explanation is that nicotine-associated changes in cognition facilitate PTSD emotional dysregulation by disrupting safety associations. Therefore, we investigated whether nicotine would disrupt learned safety by enhancing fear associated with a safety cue. In the present study, C57BL/6 mice were administered acute or chronic nicotine and trained over three days in a differential backward trace conditioning paradigm consisting of five trials of a forward conditioned stimulus (CS)+ (Light) coterminating with a footshock unconditioned stimulus followed by a backward CS− (Tone) presented 20 s after cessation of the unconditioned stimulus. Summation testing found that acute nicotine disrupted learned safety, but chronic nicotine had no effect. Another group of animals administered acute nicotine showed fear when presented with the backward CS (Light) alone, indicating the formation of a maladaptive fear association with the backward CS. Finally, we investigated the brain regions involved by administering nicotine directly into the dorsal hippocampus, ventral hippocampus, and prelimbic cortex. Infusion of nicotine into the dorsal hippocampus disrupted safety learning.
Keywords: Learned safety, nicotine, hippocampus, prelimbic cortex, backward conditioning
Introduction
The ability to modulate and control emotional processes is critical for normal functioning. However, in individuals with post-traumatic stress disorder (PTSD), emotional processing becomes dysregulated (Rauch et al., 2006), resulting in devastating symptoms, including re-experiencing trauma, avoidance of trauma associated cues, and increased arousal (Jovanovic et al., 2010; Rothbaum and Davis, 2003). Alterations in fear-related neurocognitive processes appears to be a critical component of PTSD (Shin et al., 2006). For example, individuals with PTSD present with altered anatomical and neural activation patterns in fore-brain regions important for emotional control and cognition, including the prefrontal cortex and hippocampus (Rauch et al., 2006; Shin et al., 2004, 2006). Similarly, behavioral work in human clinical populations suggests that PTSD is associated with deficits in fear inhibition (Christianson et al., 2012; Rauch et al., 2006). For example, individuals with PTSD fail to inhibit fear in the presence of cues and contexts that indicate the absence of danger (Blechert et al., 2007; Jovanovic et al., 2009, 2010, 2012). Failure to adaptively inhibit fear is proposed to be an important factor in pathological anxiety, such as that seen in PTSD (Davis et al., 2000; Kutlu et al., 2014; Lissek et al., 2005). Thus, disruptions in fear-related learning processes, which modulate fear inhibition, may be a critical feature of PTSD symptomology.
To investigate the role of fear and fear inhibition in PTSD, Pavlovian fear conditioning, in which a neutral conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US), has been used (VanElzakker et al., 2014). For example, individuals with PTSD have deficits in fear extinction, where after fear conditioning, repeated unreinforced CS presentations result in the formation of a new “safe” association with the CS, which is expressed as a decrease in fear responding to the CS (Milad et al., 2009). However, PTSD-associated deficits in fear inhibition are not limited to previously conditioned danger cues, but are also observed in the formation of safety associations with neutral cues, that is, learned safety. Learned safety is a form of associative learning related to, but dissociable from, conditioned fear. Similar to conditioning of fear, learned safety involves presentation of an aversive US; however, the CS is presented such that it is predictive of the absence of the US. Subsequently, learned safety cues are able to elicit decreased fear in the presence of previously conditioned fear cues. Thus, learned safety has been studied as a type of conditioned inhibition (Christianson et al., 2012; Pollak et al., 2010), and learning of safety may be assessed experimentally using summation testing (Hammond, 2013). During summation testing, a fear cue (CS+) is presented in compound with a safety cue (CS−), which elicits a reduced fear response in comparison with presentation of the CS+ alone (Christianson et al., 2012; Rescorla, 1969; Williams et al., 1992). Indeed, the ability of learned safety cues to decrease fear is thought to be an adaptive learning process recruited for modulation of emotional processes (Kong et al., 2014).
A number of studies have found that PTSD is associated with deficits in learned safety (Grillon and Morgan, 1999; Jovanovic et al., 2009, 2010, 2012). For example, individuals with PTSD showed deficits in a danger/safe discrimination paradigm (Jovanovic et al., 2009, 2010), with similar fear-potentiated startle to safety and danger cues. Similarly, safety cues were found to be less effective at reducing distress in individuals with high trait anxiety (Gazendam et al., 2013). Thus, the inability to learn safety-predicting cues could play an important role in development or maintenance of PTSD symptoms.
There is a robust bidirectional association between smoking and stress and anxiety-related disorders, including PTSD (Feldner et al., 2007). Individuals with PTSD smoke more heavily and at higher rates (45% vs. 23%) compared with non-PTSD individuals (Cougle et al., 2010; Lasser et al., 2000). Conversely, prior smoking has been associated with a greater risk of developing PTSD (Koenen et al., 2005), and individuals that reported smoking 18 months after trauma showed higher likelihood of subsequent development of anxiety symptoms and PTSD 27 months later (der Velden et al., 2007). Therefore, while it is not known how smoking facilitates or exacerbates PTSD symptoms, it appears to be a potent risk factor for development of PTSD. One possibility is that smoking alters fear learning processes that underlie PTSD anxiety-related symptoms (Kutlu and Gould, 2015). Nicotine, the psychoactive ingredient in tobacco, has strong effects on cognition in both clinical populations and pre-clinical models (Amitai and Markou, 2009; Gould et al., 2012; Kenney et al., 2012b; Levin et al., 1990; Rezvani and Levin, 2001). Importantly, acute nicotine enhanced hippocampus-dependent forms of fear learning, including trace and contextual fear conditioning (Gould et al., 2004; Gould and Wehner, 1999). In contrast, chronic nicotine does not change fear learning, suggesting the development of tolerance (Davis et al., 2005; Raybuck and Gould, 2009). Therefore, acute nicotine consumption, such as during smoking initiation, may facilitate or exacerbate PTSD symptoms by modulating hippocampus-dependent fear learning processes.
The cholinergic system is implicated in fear learning and fear inhibition (Kutlu and Gould, 2015; Wilson and Fadel, 2016), and nicotine acts directly on nicotinic acetylcholine receptors (nAChRs), ligand gated ion channels located throughout the central nervous system (Lindstrom, 1997). For example, nAChR pharmacological blockade prevents enhancement of contextual fear learning by acute nicotine (Davis and Gould, 2006). Further, activation of α4β2 nAChRs elicits enhanced contextual fear learning (Yildirim et al., 2015). Similarly, pharmacological and genetic approaches have demonstrated that high-affinity α4 and β2 containing nAChRs are important for nicotine’s effects (Davis et al., 2007; Kutlu et al., 2016a). Animals lacking α4 or β2 nAChR subunits show normal fear extinction when administered acute nicotine, while wildtype animals show disruptions in fear extinction (Kutlu et al., 2016a). Importantly, little is known regarding the nAChR system and danger/safe cue discrimination. One study found that acute nicotine disrupted context discrimination in a time-dependent manner, suggesting that nicotine results in discrimination deficits (Kutlu et al., 2014). In contrast, work using appetitive reinforcement found that nAChR activation enhanced cue discrimination and behavioral inhibition (MacLeod et al., 2006, 2010).
It is currently an outstanding question whether nicotine can alter learning a discrete safety cue. For example, acute nicotine has no effect on cued associative learning when the CS and US temporally overlap (Gould and Wehner, 1999), which suggests that nicotine does not intrinsically alter learning all discrete cue associations. However, acute nicotine has been shown to potentiate trace fear learning, a form of cued associative learning in which the CS and US do not temporally overlap (Gould et al., 2004). Therefore, in this study we sought to investigate the effects of acute and chronic nicotine on safety learning when the putative safety CS was presented in temporal proximity to the US. Additionally, the behavioral design of this study, in which the US and putative safety cue were separated by a temporal interval, has particular translational relevance as real-life traumatic events may occur under ambiguous conditions (Lissek et al., 2006). Moreover, the formation of inappropriate danger associations may be a critical aspect of PTSD (Grillon and Morgan, 1999).
Previous work suggests involvement of the hippocampus and medial prefrontal cortex (mPFC) in nicotine’s effects on cognition and fear learning (Kenney, et al., 2012b; Raybuck and Gould, 2010). Furthermore, manipulations of the mPFC have been found to disrupt some forms of cue discrimination (Meyer and Bucci, 2014; Sangha et al., 2014) and conditioned inhibition (Rhodes and Killcross, 2007), and individuals with PTSD have altered prefrontal cortical and hippocampal activity (Rauch et al., 2006; Shin et al., 2004, 2006). Finally, learning trace associations is dependent on the mPFC and hippocampus (Connor and Gould, 2016; Gilmartin and Helmstetter, 2010; Gilmartin et al., 2013b; Misane et al., 2005; Raybuck and Gould, 2010). Therefore, we investigated the effect of nicotine administered directly into the dorsal hippocampus, ventral hippocampus, and prelimbic cortex on backward trace conditioned safety.
Methods
Subjects
For all experiments, male C57BL/6 mice, 8–12 weeks old (Jackson Laboratory, Bar Harbor, ME, USA) were used. Mice were housed in groups of four and maintained on a 12 h light/ dark cycle, food and water access was ad libitum. All training and testing occurred between the hours of 09:00 and 19:00. Housing and behavioral procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Backward trace conditioning was performed in four identical chambers contained within sound attenuating boxes (MED Associates, St. Albans, VT, USA). The sound attenuating boxes housed ventilation fans producing 69 dB background noise. The auditory CS was produced using a speaker located on the wall of the conditioning chamber. A 100 mA houselight was similarly mounted at the top of a chamber wall. The front, back and ceiling of the chamber was made of clear Plexiglas and the floor consisted of a metal grid connected to a shock generator. The shock generator produced a 2-s, 0.57 mA scrambled footshock. Testing occurred in a different room using four chambers housed within sound attenuating boxes (MED Associates, St. Albans, VT, USA). In contrast to the training chambers, the testing chambers had flat plastic floors and one wall housed an inactive nosepoke apparatus. Similar to training chambers, testing chambers housed ventilation fans that provided white noise (69 dB). For both training and testing a 6 kHz tone (85 dB) CS− was produced by a programmable audio generator (MED Associates, St. Albans, VT, USA) via speakers housed within both the training and testing chambers. Additionally, the house light was used as a CS+ cue at 65 lux. Both training and testing occurred under red light conditions so as to increase salience of the light CS+. The presentation of all stimuli was controlled by Med-PC software.
Drugs
Systemic administration
The effects of acute and chronic nicotine on learned safety were examined. For all systemic studies, nicotine hydrogen tartrate (Sigma, St. Louis, MO, USA), reported as freebase weight, was dissolved in 0.9% sterile saline. To investigate the effects of acute nicotine on backward trace conditioned safety a dose of 0.09 mg/kg was selected as it has previously been shown to enhance forward trace fear conditioning in C57BL/6J mice (Gould et al., 2004; Raybuck and Gould, 2009), and results in nicotine plasma levels similar to those found in smokers (Davis et al., 2005). Nicotine was administered intra-peritoneally, 2 min prior to training and testing. Administration prior to training and testing was based on prior work showing that acute systemic nicotine enhances trace fear conditioning when administered at training and testing (Davis and Gould, 2007).
For the chronic nicotine experiment, nicotine was delivered at 12.6 mg/kg per day for 14 days based on previous findings in mice that indicate that this dose of chronic nicotine lies within a range of doses (6.3–18 mg/kg per day) that result in plasma nicotine and cotinine levels similar to those of active smokers (Benowitz et al., 1988; Benowitz, 1996; Cole et al., 2014; Davis et al., 2005; Portugal et al., 2012b). In addition, this treatment schedule has previously been shown to model neural adaptations associated with chronic smoking, including upregulation of nicotinic receptors (Gould et al., 2012, 2014a).
Local administration
For local administration experiments, two doses of nicotine hydrogen tartrate were selected, 0.09 μg/ side and 0.18 μg/side (all doses reported in freebase). Nicotine hydrogen tartrate was dissolved in sterile saline, which was used as vehicle for control mice. Doses and administration parameters were based on prior work demonstrating that both are sufficient to enhance forward trace fear conditioning when infused into the dorsal hippocampus and prelimbic cortex prior to training and testing (Raybuck and Gould, 2010). Intracranial drug infusion procedures were similar to those previously described (Kenney et al., 2012b; Raybuck and Gould, 2010). Briefly, mice were restrained and dummy cannula removed. Drug was directly infused using 22 gauge internal cannula (dorsal hippocampus and ventral hippocampus) and 33 gauge internal cannula (prelimbic cortex) (Plastics One, Roanoke) attached to PE50 polyethylene tubing. Drug infusion rate for all three experiments was 0.5 μl/ min with a dosing volume of 0.5 μl per side. Infusions were controlled by microinfusion pump (KD Scientific, New Hope, PA, USA) with 10 μl Hamilton syringe (Reno, NV, USA). Internal cannulae remained in place for 1 min following infusion to allow for diffusion of drug. Mice were trained or tested immediately after infusion was complete.
Behavioral procedures
The dependent measure for all experiments, freezing, was defined as the absence of movement except for respiration (Blanchard and Blanchard, 1969). Freezing was measured using a time-sampling method, in which subjects were observed for 1 s every 10s. During each 1 s sample, mice were judged as freezing or active (Gould and Wehner, 1999). Freezing data were converted to and analyzed as % freezing. Finally, for all experiments, freezing was observed by experimenters blind to drug conditions.
For all studies, backward trace conditioning consisted of three training sessions occurring over the same number of days. The procedure was a CS+/CS− discrimination paradigm in which a signaled footshock is followed by an un-reinforced tone presentation (Figure 1). Within each session, five signaled US foot-shocks (2 s, 0.57 mA) were presented, followed by a 20 s trace interval. The footshock US was signaled by a CS+ (houselight, 30 s) that co-terminated with the US. Following the trace interval, a CS− (Tone, 6kHz, 85 dB) was presented. Each training session began with a 60 s baseline period prior to the first trial. The intertrial interval, range 90–120 s, was pseudorandomly assigned.
Figure 1.
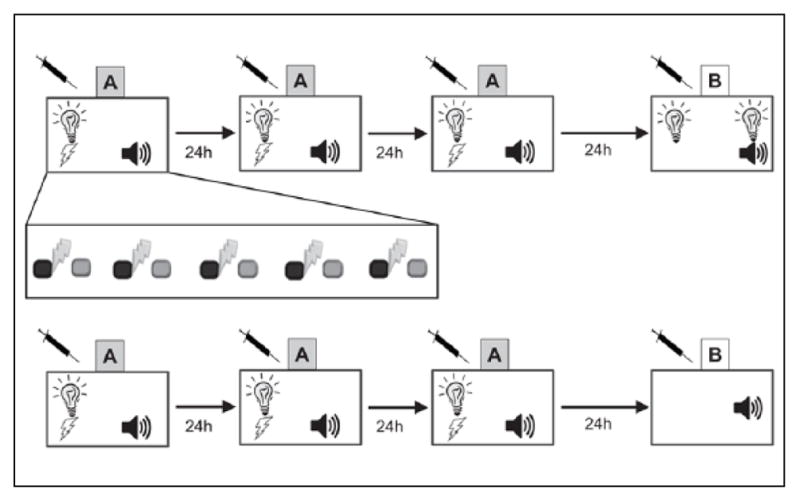
Design schematics for training and testing of backwards trace conditioned safety and fear. (a) Mice were trained over three days in context A with five trials. Exploded view shows temporal arrangement of cues during training, with a 30 s light co-terminating with 2 s footshock (dark gray with thunderbolt) followed by a 20 s trace interval and a 30 s tone (light gray). To test for learned safety, summation testing consisted of alternating presentations of light or light/tone. (b) To assess backwards trace conditioned fear mice where trained similarly, but on testing where only presented with the backward paired tone conditioned stimulus.
Behavioral testing for all experiments occurred in an alternate context 24 h after the last training session. Learned safety was assessed using summation testing in which learning of the CS as a conditioned inhibitor of fear was measured. Summation testing consisted of three alternating presentations of Light and Light/ Tone compound cues (60 s each), with 60 s intertrial intervals (Figure 1). Freezing was scored for the entire duration of cue presentation and data were collapsed into Light-Danger and Light/Tone-Safety for statistical analysis. Finally, in order to explicitly test whether nicotine caused the formation of a fear association with the backward CS, mice were tested for freezing to the backwards CS alone (Figure 1). Specifically, similar to Gould et al. (2004), mice were placed in an altered context and exposed to a 3 min stimulus free period, Pre-CS, followed by a 3 min presentation of the backward trace CS. Freezing was scored for the entire duration of testing during the first 3 min, Pre-CS, and the latter 3 min, CS, for statistical analysis.
Minipump surgeries
For the chronic nicotine study, mice were anesthetized with isoflurane (5% induction, 2.5% maintenance) and implanted with osmotic minipumps (Alzet, Model 1002, Durect Co, Cupertino, CA, USA). Minipumps delivered chronic saline or nicotine (12.6 mg/kg per day) for 14 days (testing occurring on the 14th day). Osmotic minipumps were surgically implanted subcutaneously via an incision posterior to the scapulae. The incision site was closed with surgical staples.
Cannula surgeries
After being anesthetized with isoflurane (5% induction, 2.5% maintenance), mice were placed in a stereotaxic apparatus and implanted with a guide cannula. The hippocampus is genetically and functionally differentiated along its septotemporal axis (Fanselow and Dong, 2010; Gould et al., 2014b). Therefore, bilateral guide cannulas (Plastics One) were placed for the dorsal hippocampus (A/P −1.7, M/L ±3.0, D/V −2.3 mm) and the ventral hippocampus (A/P −2.8, M/L ±3.0, D/V −4.0 mm) (Raybuck and Gould, 2010). The rodent prefrontal cortex is anatomically different from that of the primate, lacking granularization; however, the prelimbic cortex appears to mediate working memory and attention functions (Seamans et al., 2008; Uylings et al., 2003). In addition, the prelimbic cortex is critically involved in working memory-like activity during trace acquisition (Gilmartin et al., 2013b). Thus, prelimbic cortex was the target for PFC infusion. Coordinates were based on the stereotaxic location of prelimbic cortex (A/P +1.7, M/L ±0.5, D/V −2.5 mm) (Paxinos and Franklin, 2008). Mice were allowed to recover for at least five days prior to initiation of behavioral experimental procedures.
Histology
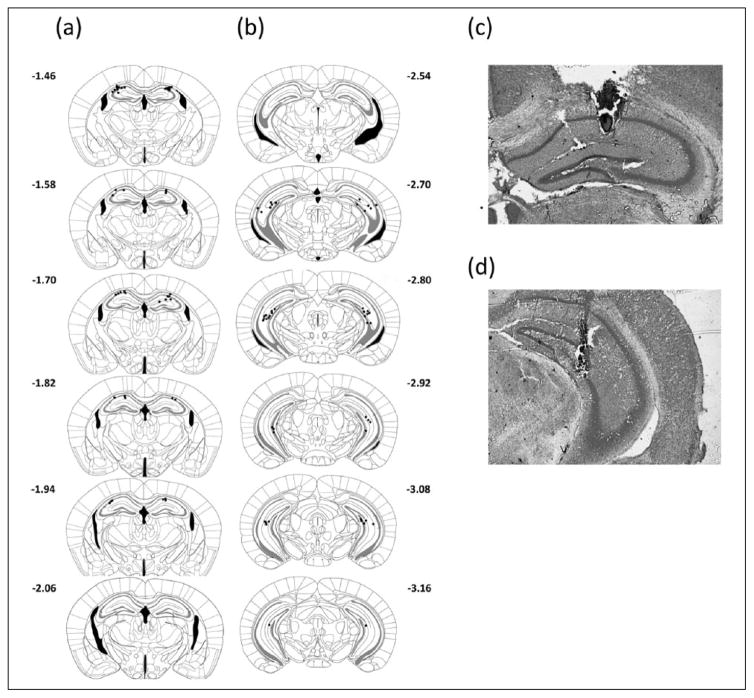
Following behavioral procedures mice were euthanized by cervical dislocation and brains were extracted. All brains were postfixed in formalin for a minimum of 24 h. Following fixation, brains were sectioned at 50 μm on cryostat and stained with cresyl violet. Confirmation of infusion sites was done using brightfield microscopy at 10× magnification to identify cannula tracks (Figures 2 and 3). Placements determined to fall outside of the target regions (23 mice total) were excluded from all analysis.
Figure 2.
Cannula placement in hippocampus. Cannula tips location in relation to bregma in coronal sections (a) dorsal and (b) ventral hippocampus. Representative images of placements in dorsal (c) and ventral (d) hippocampus.
Figure 3.
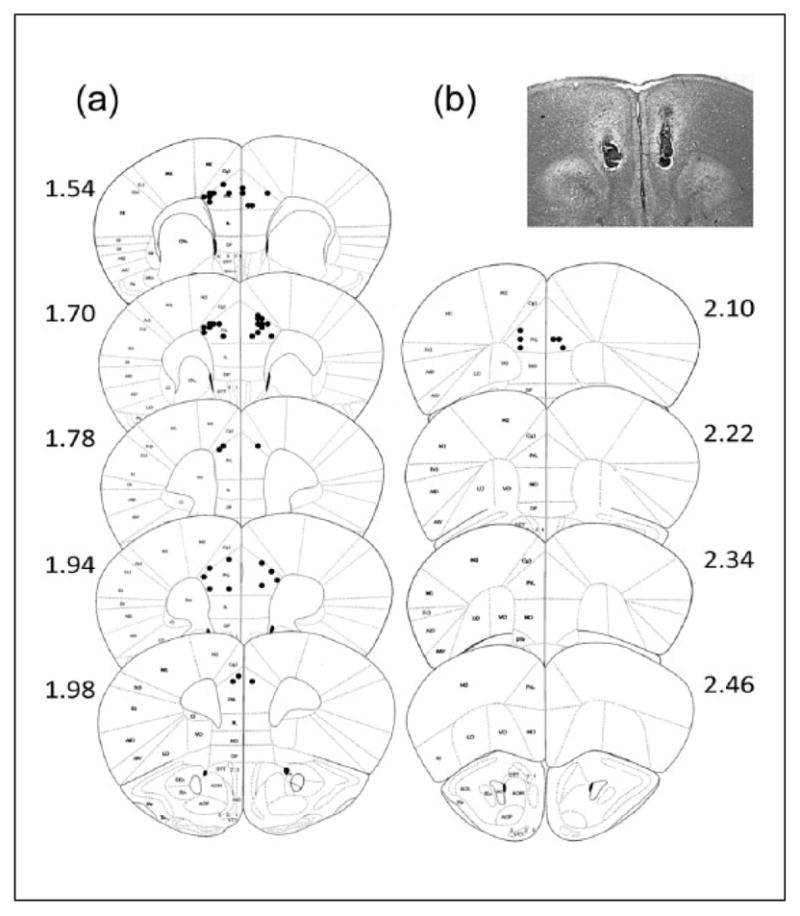
(a) Cannula placement in prelimbic cortex with (b) representative image. Cannula tips location in relation to bregma in coronal sections.
Data analysis
For all experiments freezing data were analyzed using mixed-design analyses of variance (ANOVAs) (SPSS 16.0). Exploratory analysis of prelimbic cortex infusion results also included one-way ANOVAs. Results were considered significant at p < 0.05. All data are presented as means ± SEM. One animal from the backward trace fear conditioning experiment was removed from analysis due to freezing over two standard deviations from the mean. One animal from the chronic nicotine experiment and one from the dorsal hippocampus infusion experiment were removed for Pre-CS baseline freezing two standard deviations higher than the rest of the group.
Results
The effects of acute nicotine on backward trace conditioned safety
Mice were administered systemic nicotine on all sessions, training and testing, to assess changes in backward trace conditioned safety. A mixed-design ANOVA found a significant interaction (Drug (Nicotine vs. Saline) × Condition (Light-Danger vs. Light/Tone-Safety)) F(1,16) = 7.910, p < 0.05 and a main effect of drug, F(1,16) = 8.21, p < 0.05. A planned comparison paired t-test indicated that saline treated control animals froze less during Light/Tone-Safety (M = 29.01%, SD = 14.64%) compared with Light-Danger (M = 46.91%, SD = 17.59%), t(8) = 2.42, p < 0.05. Thus, saline treated mice demonstrated conditioned inhibition of fear in response to the backward CS, indicating learned safety. In contrast, animals treated with nicotine showed no difference in freezing to Light-Danger (M = 46.91%, SD = 9.66%) versus Light/Tone-Safety trials (M = 55.55%, SD = 13.03%), p > 0.05. These data show that nicotine prevents responding to the backward CS as a conditioned inhibitor and disrupted backward trace conditioned safety. Finally, a planned independent-samples t-test found no difference between saline and nicotine treatment on freezing during baseline period, p > 0.05. Thus, both groups showed no differences in non-associative freezing behaviors (Figure 4).
Figure 4.
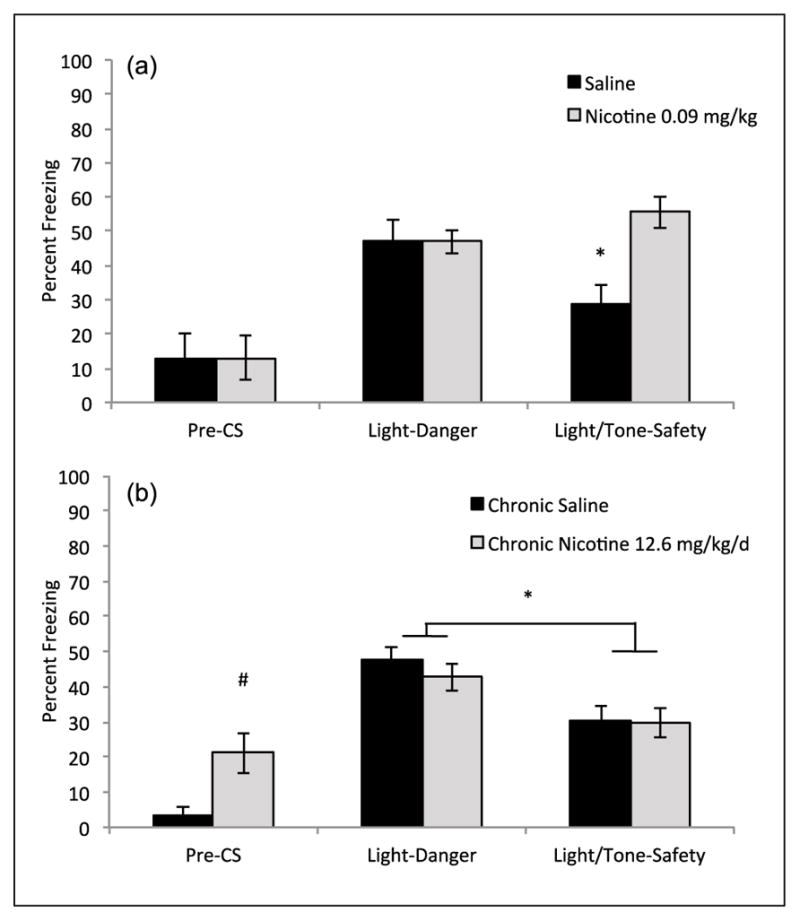
The effects of acute and chronic nicotine on backward trace conditioned safety. (a) Mice treated with acute nicotine, 0.09 mg/kg, did not freeze less during summation testing of the Light/Tone-Safety compound cue, but saline treated mice froze significantly less (data shown as % freezing in mice) during compound Light/Tone compared with Light alone (n = 9 per group). Error bars indicate SEM, * indicates within drug group difference between Light-Danger and Light/Tone-Safety, p < 0.05. (b) Mice in both chronic saline and chronic nicotine, 12.6 mg/kg/ per day, demonstrated reduced freezing during presentation of Light/Tone-Safety compared with Light alone (n = 14 per group). Mice treated with chronic nicotine showed greater generalized freezing during the Pre-CS period prior to stimulus presentation. Error bars indicate SEM, * indicates a main effect of testing condition Light-Danger versus Light/ Tone-Safety, p < 0.05. # indicates significant difference between chronic saline and nicotine during Pre-CS, p < 0.05.
CS: conditioned stimulus
The effects of chronic nicotine on backward trace conditioned safety
To assess whether chronic nicotine might also disrupt learned safety we performed the same backward trace conditioned safety procedure on mice chronically administered nicotine via osmotic mini-pumps. Mixed-design ANOVA showed a within subjects main effect of testing condition (Light-Danger vs. Light/Tone-Safety) F(1,27) = 28.52, p < 0.01, but no significant between subjects main effect of drug (Nicotine vs. Saline) F(1,28) = 0.46, p > 0.05 or interaction was observed (Condition × Drug) F(1,27) = 0.53, p > 0.05. The significant between subjects main effect indicates that both saline and nicotine treatment groups learned safety. Specifically, chronic saline treated mice froze more during the Light-Danger (M = 47.62%, SD = 12.65%) compared with Light/Tone-Safety (M = 30.55%, SD = 14.24%). Similarly, chronic nicotine treated mice also showed freezing at lower levels during Light/Tone-Safety (M = 29.63%, SD = 15.24%) compared with the Light-Danger (M = 42.59%, SD = 13.87%) (Figure 4). Finally, an independent samples t-test found that nicotine treated mice froze significantly more during the Pre-CS period compared with saline treated mice t(27) = 2.93, p < 0.05. However, because nicotine and saline treated mice both showed similar high levels of freezing during Light-Danger, indicating formation of a fear association with the forward CS, differences in Pre-CS freezing did not alter our interpretation of the mixed ANOVA results, which suggest that both groups learned the safety association.
The effects of nicotine on backward trace fear conditioning
In order to assess whether acute nicotine disrupts backward trace conditioned safety by causing a fear association with the backward conditioned cue an alternative test was implemented. Rather than a summation test, mice were placed in an alternative context and presented with the backward conditioned cue alone. Thus, if mice learned the cue as safe they should show no freezing, while freezing to the cue would indicate a fear association. A mixed-design ANOVA found a significant effect of condition (Pre-CS vs. CS) F(1,13) = 5.82, p < 0.05. In addition, a trending but non-significant interaction was found, F(1,13) = 4.19, p = 0.061, while no significant between subjects effect of drug was observed, p > 0.05. Importantly, as hypothesized, a planned comparison paired-samples t-test found that nicotine treated mice froze significantly more during presentation of CS (M = 29.16%, SD = 14.47%) than during the Pre-CS period (M = 9.72%, SD = 14.16%) t(7) = 2.65, p < 0.05 (Figure 5). Therefore, nicotine treated mice formed a fear association with the backward conditioned cue. In contrast, there was no difference observed in the saline group: Pre-CS (M = 8.73%, SD = 12.36%) and CS (M = 10.31%, SD = 8.74%), p > 0.05. These data support the claim that acute nicotine can disrupt learned safety by facilitating maladaptive associations between the CS and the footshock US.
Figure 5.
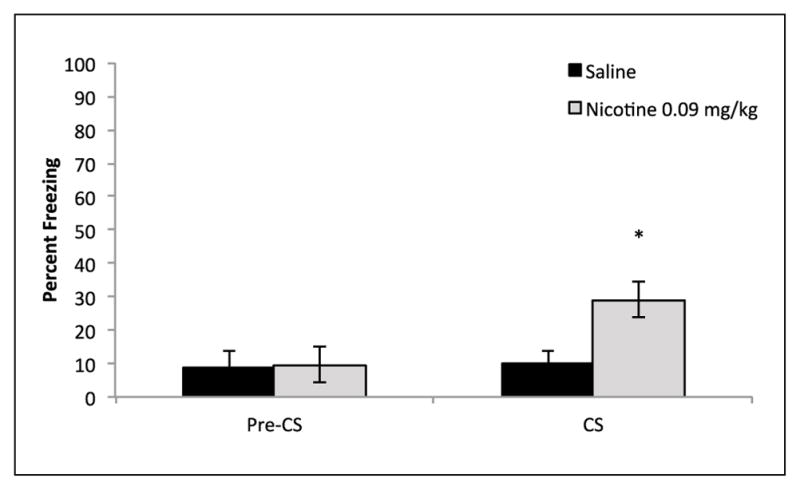
Backward trace fear conditioning control mice did not show any difference in freezing during the Pre-CS versus CS conditioning, indicating no fear association with the backward trace conditioned CS. In contrast, acute nicotine, 0.09 mg/kg, treatment resulted in greater freezing to the CS compared with Pre-CS, indicating a fear association with the backward trace conditioned CS (n = 7–8). Error bars indicate SEM, * indicates significant planned comparison paired samples t-test between Pre-CS and CS, p < 0.05.
CS: conditioned stimulus
The effects of local administration of nicotine into the dorsal hippocampus on backward trace conditioning
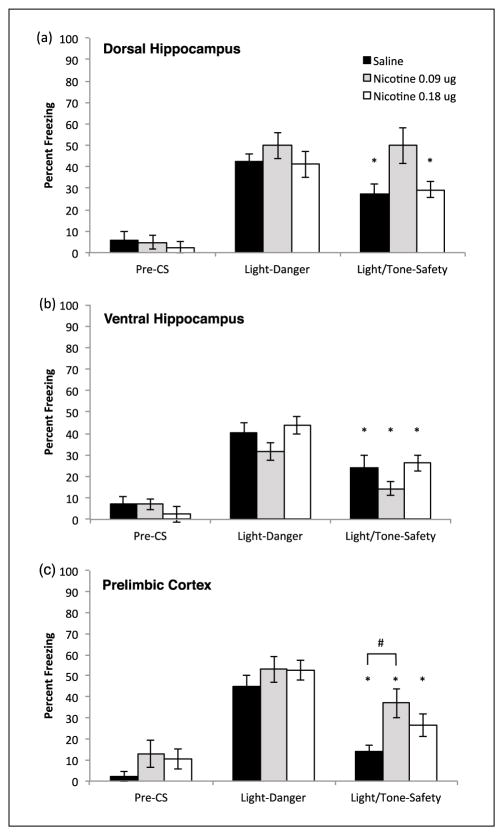
Similar to the previously described systemic experiments, backward trace conditioned safety was assessed using a summation test. Nicotine (0.09 and 0.18 μg/side) or saline was infused into the dorsal hippocampus prior to training and testing. A mixed-design ANOVA found a significant interaction (Drug × Condition), F(2,20) = 3.79, p < 0.05. Also a within subjects main effect of testing condition was found (Danger vs. Safe), F(1,20) = 14.46, p < 0.01, but no between subjects main effect was observed (Saline vs. 0.09 μg vs. 0.18 μg), F(2,20) = 3.145, p = 0.07. A priori planned contrasts using paired samples t-test found that mice administered saline showed significant decreased freezing during Light/Tone (27.16%, SD = 14.28%) compared with Light alone (42.59%, SD = 10.39%), t(8) = 3.22, p < 0.05. Similarly, mice infused with 0.18 μg/side nicotine also froze significantly less during Light/Tone (29.37%, SD = 8.91%) compared with Light alone (41.27%, SD = 14.99%), t(6) = 3.19, p < 0.05. In contrast, mice administered 0.09 μg/side nicotine did not freeze differently during Light/Tone (50.00%, SD = 20.54%) compared with Light alone (50.00%, SD = 15.04%), p > 0.05. Finally, a one-way ANOVA revealed no significant variation in Pre-CS freezing among the different drug groups, F(2,22) = 0.237, p > 0.05. Thus, no group differed in innate freezing or generalized freezing to the testing context.
The effects of local administration of nicotine into the ventral hippocampus on backward trace conditioning
To examine whether the ventral hippocampus plays a role in the effects of acute nicotine on backward trace conditioning, nicotine (0.09 and 0.18 μg/side) or saline was infused into the ventral hippocampus prior to training and testing. A mixed-design ANOVA found a significant within subjects main effect of testing condition (Danger vs. Safety), F(1, 20) = 43.02, p < 0.01, indicating that mice responded differently to Light-Danger and Light/Tone-Safety presentations. No between subjects main effect of drug (Saline vs. 0.09 μg vs. 0.18 μg), F(2, 20) = 2.665, p > 0.05 or interaction was observed, F(2, 20) = 0.036, p > 0.05. The significant within subjects main effect of testing condition indicates that all saline and nicotine treatment groups learned safety. Specifically, mice administered saline froze less during compound Light/ Tone-Safety (24.07%, SD = 16.43%) compared with Light-Danger (40.12%, SD = 13.26%). This effect of testing condition was also observed in mice infused with 0.09 μg nicotine, Light/ Tone-Safety (14.29%, SD = 8.99%) compared with Light-Danger (31.75%, SD = 9.99%), and 0.18 μg nicotine, Light/Tone-Safety (26.19%, SD = 7.67%) compared with Light-Danger (43.65%, SD = 10.36%). Finally, assessment of Pre-CS freezing using a one-way ANOVA was not significant, F(2,22) = 0.883, p > 0.05. Therefore, groups did not differ in innate freezing or show differences in generalized freezing from the testing context (Figure 6).
Figure 6.
(a) Nicotine, in the dorsal hippocampus dose-dependently disrupts safety learning. Mice treated with 0.09 μg/side nicotine fail to show learned safety, with freezing levels similar between Light-Danger and Light/Tone-Safety. Mice treated with saline and nicotine, 0.18 μg/side, showed learned safety with decreased freezing during Light/Tone-Safety (n = 9–7 per group). Error bars indicate SEM, * indicates significant within group planned comparison paired samples t-test between Light-Danger and Light/Tone-Safety, p < 0.05. (b) Nicotine in the ventral hippocampus has no effect on backwards trace conditioned safety; all mice showed decreased freezing during Light/ Tone-Safety compared with Light-Danger (n = 9–7 per group). Error bars indicate SEM, * indicates significant within group difference between Light and Light/Tone, p < 0.05. (c) Nicotine in prelimbic cortex dose-dependently alters freezing to Light/Tone compound. Mice administered saline 0.09 μg/side and nicotine 0.18 μg/side all showed significantly less freezing during presentation of compound Light/Tone-Safety compared with Light-Danger. However, mice treated with 0.09 μg/side nicotine froze significantly more than saline treated mice during compound Light/Tone-Safety presentation (n = 8–9 per group). Error bars indicate SEM, * indicates planned comparison paired samples t-test p < 0.05, # indicates post-hoc Tukey’s HSD contrast p < 0.05.
CS: conditioned stimulus; HSD: honest significant difference
The effects of local administration of nicotine into prelimbic cortex on backwards trace conditioning
To assess whether the prelimbic cortex is sufficient to mediate the effects of acute nicotine on backward trace conditioning, nicotine (0.09 and 0.18 μg/side) or saline was directly infused into the prelimbic cortex prior to training and testing. A mixed-design ANOVA revealed a significant interaction (Condition × Drug), F(2,22) = 4.48, p < 0.05. In addition, a main effect of testing condition was found (Danger vs. Safety), F(1,22) = 130.29, p < 0.01, but there was no significant between subjects main effect of drug (Saline vs. 0.09 μg vs. 0.18 μg), F(2,22) = 2.68, p > 0.05. Therefore, the effect cue presentation on freezing was moderated by nicotine treatment. A priori planned paired samples t-tests were performed and saline treatment was associated with significantly reduced freezing during Light/Tone-Safety trials (13.89%, SD = 8.91%) compared with Light-Danger (45.14%, SD = 13.09%), t(7) = 9.00, p < 0.01. Additionally, mice treated with 0.09 μg/side nicotine showed significantly reduced freezing during Light/Tone-Safety (37.04%, SD = 19.64%) compared with Light-Danger (53.09%, SD = 17.37%), t(8) = 4.73, p < 0.01. Similarly, mice treated with 0.18 μg/side nicotine showed significantly reduced freezing during Light/Tone-Safety (26.38%, SD = 14.47%) compared with Light-Danger (52.77%, SD = 12.59%), t(7) = 6.14, p < 0.01 (Figure 6).
Our a priori comparisons found that all drug groups inhibited fear during Light/Tone-Safety compared with Light-Danger. However, visual inspection of the data, as well as a significant interaction from the mixed ANOVA, suggested that drug groups froze differently during presentation of compound Light/Tone. To further examine this, we performed a one-way ANOVA on freezing data from Light/Tone-Safety and found a significant effect of drug, F(2,25) = 4.88, p < 0.05. Furthermore, follow up post-hoc contrasts using Tukey’s HSD (honest significant difference) showed that treatment with nicotine 0.09 μg/side resulted in significantly higher freezing compared with saline treatment, p < 0.05, during Light/Tone trials. No significant difference was found between saline and 0.18 μg/side nicotine, p > 0.05. We considered that this difference could be the result of a global reduction in freezing within the control group. To assess this, we also performed a one-way ANOVA for Light-Danger and found a non-significant effect of drug, F(2,25) = 0.77, p > 0.05. Finally, a one-way ANOVA found no significant differences in freezing during the Pre-CS period, F(2,24) = 1.48, p > 0.05, indicating that mice did not differ in innate freezing. These data suggest a modest effect of nicotine within the prelimbic cortex on inhibitory summation of fear.
Discussion
The present study examined the effects of nicotine on backward trace conditioned safety and found that acute nicotine disrupted safety learning, observed as a failure to reduce freezing to a danger CS+ in the presence of the backward CS− during summation testing. Next, we assessed whether acute nicotine-dependent disruption of learned safety was the result of a US–CS danger association. We found that mice treated with acute nicotine displayed a fear response to the backward CS in a novel context. Thus, acute nicotine prevented learning of the backward CS as a safety cue by facilitating the formation of a maladaptive fear association with a backward CS.
Within the context of prior work investigating the effects of acute nicotine on learning, the finding that acute nicotine disrupts safety learning initially appears divergent. For example, acute nicotine has been repeatedly shown to enhance hippocampus-dependent forms of learning (Davis et al., 2005; Gould and Wehner, 1999; Kenney et al., 2011). However, our findings suggest that this disruption is actually due to nicotine facilitating the formation of a maladaptive US–CS association; that is, the backward trace conditioning. Normally, backward trace conditioning either does not produce learning or produces weak associations (Quinn et al., 2002). Nicotine enhances hippocampus-dependent learning, including trace fear conditioning (Gould et al., 2004; Raybuck and Gould, 2009), and backward trace conditioning may engage the hippocampus (Quinn et al., 2002). In support, a previous study found that post-training lesions of the dorsal hippocampus abolished recall of forward as well as backward trace fear associations (Quinn et al., 2002), which suggests that dorsal hippocampus-dependent processes may similarly mediate forward trace fear conditioning as well as backward trace conditioned fear associations. Thus, acute nicotine may facilitate backward trace fear associations via hippocampal learning processes that become engaged when stimuli are discontiguous, which results in the disruption of a competing safety association.
In contrast to the effects of acute nicotine, we observed no effect of chronic nicotine on backward trace conditioned safety. This null finding supports prior studies showing that while initial nicotine exposure leads to changes in learning, chronic administration results in tolerance to such cognitive effects (Davis et al., 2005; Portugal et al., 2012a). Tolerance is thought to be mediated by neural adaptations induced by continuous nicotine exposure, including desensitization and upregulation of nAChRs (Marks et al., 1992, 1993). Behavioral work supports this view as the rate at which tolerance develops has been shown to temporally align with nAChR upregulation within the hippocampus (Gould et al., 2014a). Furthermore, acute nicotine has been shown to facilitate trace conditioning, but chronic nicotine does not (Raybuck and Gould, 2009). Therefore, the lack of an effect of chronic nicotine on backward trace conditioned safety is consistent with our interpretation that acute nicotine facilitates a maladaptive hippocampus-dependent trace US–CS association. A caveat is that only a single dose of nicotine was tested, but this dose falls within a range of doses resulting in similar plasma nicotine and cotinine levels as observed in active human smokers (Cole et al., 2014; Hukkanen et al., 2005; Kutlu et al., 2016b).
In follow up to experiments that revealed an effect of acute nicotine on backward trace conditioning, we found that nicotine infused into the dorsal hippocampus, a region involved in trace conditioning, also resulted in similar disruption of backward trace conditioned safety learning. Specifically, we found that infusion of nicotine at 0.09 μg/side into the dorsal hippocampus disrupted inhibitory summation. These data suggest that nicotine may disrupt safety via changes in dorsal hippocampus process underlying associative learning. In support, post-training lesions of the dorsal hippocampus abolished backward trace conditioned fear (Quinn et al., 2002). In addition, local administration of nicotine within the dorsal hippocampus during training enhanced trace fear conditioning (Raybuck and Gould, 2010). Thus, nicotine may act to enhance the US–CS association by altering signaling within the dorsal hippocampus that mediates learning temporally discontiguous trace memories. Therefore, our results give evidence that nicotine can alter associative learning by acting directly within the dorsal hippocampus and that this can alter adaptive safety learning by facilitating maladaptive associations.
In contrast to dorsal hippocampus infusions, ventral hippocampus administration had no effect on backward conditioned safety. This null effect supports the interpretation that nicotine’s effects on backward trace conditioning are mediated by enhanced maladaptive associative learning. For example, previous studies have found that infusion of nicotine into the ventral hippocampus actually disrupts associative learning (Kenney et al., 2012b; Raybuck and Gould, 2010). Therefore infusion of nicotine into the ventral hippocampus likely has no effect on safety because under normal conditions backward trace conditioning results in no excitatory US–CS association. Our results suggest that the ventral hippocampus may not be a critical mediator of learned safety. This conclusion is also directly supported by work showing that inactivation of the ventral hippocampus does not prevent discrimination of dangerous and safe cues (Chen et al., 2016). Thus, while the ventral hippocampus plays an important role in fear expression, as observed in other fear conditioning and fear extinction work (Kjelstrup et al., 2002; Sierra-Mercado et al., 2011), our data support a view that it may not similarly modulate learned safety. In sum, these data suggest that the ventral hippocampus is not important in the effect of systemic nicotine on backward conditioned safety.
Given that the prelimbic cortex may play a role in cue discrimination as well as trace conditioning (Connor and Gould, 2016; Gilmartin et al., 2013a, 2013b; Likhtik et al., 2014; Meyer and Bucci, 2014), we infused nicotine into the prelimbic cortex to assess nicotine’s effect on backward trace conditioned safety. We found that all groups showed inhibitory summation, indicating nicotine did not prevent learned safety. However, we also observed a small increase in freezing during summation testing in mice receiving the lower dose, 0.09 μl/side. This modest effect of nicotine within the prelimbic cortex appears consistent with other investigations of conditioned safety, which indicate that lesions or inactivation of the mPFC result in null (Christianson et al., 2008; Gewirtz et al., 1997) or small effects (Sangha et al., 2014). Alternatively, cells within the prelimbic cortex maintain sustained firing during the trace interval during forward trace fear conditioning, suggesting that the prelimbic cortex mediates learning non-overlapping events by maintaining a representation of separate events across time (Gilmartin and McEchron, 2005) and inhibition of prelimbic cortex neurons during the trace interval disrupted trace fear learning (Gilmartin et al., 2013b). Moreover, nicotine can modulate theta and mPFC-amygdala theta connectivity is associated with danger/safe cue discrimination (Bueno-Junior et al., 2012; Likhtik et al., 2014). Similarly, nicotine can enhance working memory, which involves short-term maintenance of information (Levin and Torry, 1996; Provost and Woodward, 1991). Therefore, during backward trace conditioning, nicotine may have altered prelimbic cortex activity during the trace interval increasing the salience of the US across the trace interval and decreasing the salience of the safety cue.
Our results from the direct infusions studies demonstrating efficacy at the lower dose are in agreement with previous behavioral work showing that the effects of nicotine are associated with an inverse U-shaped dose–response curve (Picciotto, 2003). For example, systemic treatment with acute nicotine results in enhanced contextual fear conditioning in a dose-dependent inverted-U shaped fashion (Gould and Higgins, 2003). Similarly, enhancement of forward trace fear conditioning by dorsal hippocampus and mPFC nicotine infusions also showed an inverted U-shaped dose–response (Raybuck and Gould, 2010). One possible reason for a U-shaped dose–response to nicotine is that nAChRs may activate both inhibitory and excitatory processes (Picciotto, 2003), mediated by different nAChR subtypes that have differential affinity for nicotine. Indeed, lower levels of nicotine, similar to that seen in smokers, are thought to act via high-affinity nAChRs (Wooltorton et al., 2003). Therefore, a higher dose of nicotine might additionally activate low-affinity nAChRs, changing the pharmacological effects of nicotine.
This present study was not designed to determine whether the effects of nicotine on safety learning depend on changes of acquisition or recall. However, prior work has shown that the enhancing effects of nicotine on fear learning are dependent on molecular substrates of long-term memory consolidation (Kenney et al., 2012a), and blockade of protein synthesis prevents long-term enhancement of contextual fear memory by systemic nicotine administration (Gould et al., 2014b). Additionally, administration of nicotine prior to testing did not enhance hippocampal fear memory recall (Gould and Wehner, 1999). Similarly, direct infusion of nicotine into the mPFC prior to testing did not alter freezing after trace fear conditioning (Raybuck and Gould, 2010) and pre-testing administration of nicotine into dorsal hippocampus did not alter freezing after contextual fear conditioning (Kenney et al., 2012b). Thus, the effects of nicotine on learned safety could be due to changes in memory acquisition/consolidation; however, future studies are needed to explicitly examine this.
This study shows for the first time that nicotine can alter cued safety learning, a process that appears highly adaptive, but dysfunctional in individuals with PTSD. Thus, these results may have implication for understanding the relationship between nicotine use and PTSD, particularly with regard to nicotine exposure during smoking initiation in naïve individuals. In support, clinical work suggests that the rates of smoking initiation are higher in individuals with PTSD (Fu et al., 2007). Other preclinical studies have found that acute nicotine can also disrupt fear extinction and contextual safety discrimination (Gould and Kutlu, 2014; Kutlu et al., 2014). Together these data indicate that smoking initiation during or after a traumatic event may facilitate or exacerbate PTSD symptomology. In sum, while there are likely multiple ways in which nicotine use may alter cognition to facilitate PTSD symptomology, we show that under some conditions nicotine can alter learning of cues that indicate safety by facilitating formation of maladaptive danger associations with cues that actually predict the absence of threat.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Drug Abuse (grant number DA017949 TG).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Amitai N, Markou A. Chronic nicotine improves cognitive performance in a test of attention but does not attenuate cognitive disruption induced by repeated phencyclidine administration. Psychopharmacology. 2009;202:275–286. doi: 10.1007/s00213-008-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, et al. Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, et al. Fear conditioning in post-traumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bueno-Junior LS, Lopes-Aguiar C, Ruggiero RN, et al. Muscarinic and nicotinic modulation of thalamo-prefrontal cortex synaptic pasticity in vivo. PLoS One. 2012;7:e47484. doi: 10.1371/journal.pone.0047484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VM, Foilb AR, Christianson JP. Inactivation of ventral hippocampus interfered with cued-fear acquisition but did not influence later recall or discrimination. Behav Brain Res. 2016;296:249–253. doi: 10.1016/j.bbr.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Benison AM, Jennings J, et al. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J Neurosci. 2008;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Fernando ABP, Kazama AM, et al. Inhibition of fear by learned safety signals: A mini-symposium review. J Neurosci. 2012;32:14118–14124. doi: 10.1523/JNEUROSCI.3340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RD, Poole RL, Guzman DM, et al. Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology. 2014;232:1207–1217. doi: 10.1007/s00213-014-3754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor DA, Gould TJ. The role of working memory and declarative memory in trace conditioning. Neurobiol Learn Mem. 2016;134:193–209. doi: 10.1016/j.nlm.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Zvolensky MJ, Fitch KE, et al. The role of comorbidity in explaining the associations between anxiety disorders and smoking. Nicotine Tob Res. 2010;12:355–364. doi: 10.1093/ntr/ntq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. β2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology. 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, et al. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci Society. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal α4β2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Falls WA, Gewirtz J. Neural systems involved in fear inhibition: Extinction and conditioned Inhibition. In: Myslobodsky MS, Weiner I, editors. Contemporary Issues in Modeling Psychopathology. Springer: Neurobiological Foundation of Aberrant Behaviors; 2000. [accessed 22 April 2016]. pp. 113–141. Available at: http://link.springer.com/chapter/10.1007/978-1-4757-4860-4_8. [Google Scholar]
- Der Velden PGV, Grievink L, Olff M, et al. Smoking as a risk factor for mental health disturbances after a disaster: A prospective comparative study. J Clin Psychiatry. 2007;68:87–92. doi: 10.4088/jcp.v68n0112. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clin Psychol Rev. 2007;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SS, McFall M, Saxon AJ, et al. Post-traumatic stress disorder and smoking: A systematic review. Nicotine Tob Res. 2007;9:1071–1084. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- Gazendam FJ, Kamphuis JH, Kindt M. Deficient safety learning characterizes high trait anxious individuals. Biol Psychol. 2013;92:342–352. doi: 10.1016/j.biopsycho.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioning inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medial prefrontal cortex in rats. Behav Neurosci. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17:289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013a;20:290–294. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Miyawaki H, Helmstetter FJ, et al. Prefrontal activity links nonoverlapping events in memory. J Neurosci. 2013b;33:10910–10914. doi: 10.1523/JNEUROSCI.0144-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Kutlu GG. Acute nicotine delays extinction of contextual fear in mice. Behav Brain Res. 2014;263:133–137. doi: 10.1016/j.bbr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, et al. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62:2118–2125. doi: 10.1016/j.neuropharm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wilkinson DS, Yildirim E, et al. Dissociation of tolerance and nicotine withdrawal-associated deficits in contextual fear. Brain Res. 2014a;1559:1–10. doi: 10.1016/j.brainres.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wilkinson DS, Yildirim E, et al. Nicotine shifts the temporal activation of hippocampal protein kinase A and extracellular signal-regulated kinase 1/2 to enhance long-term, but not short-term, hippocampus-dependent memory. Neurobiol Learn Mem. 2014b;109:151–159. doi: 10.1016/j.nlm.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CAI. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hammond LJ. A traditional demonstration of the active properties of Pavlovian inhibition using differential CER. Psychon Sci. 2013;9:65–66. [Google Scholar]
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, et al. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Res. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, et al. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology. 2011;217:353–365. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Poole RL, Adoff MD, et al. Learning and nicotine interact to increase CREB phosphorylation at the jnk1 promoter in the hippocampus. PLoS ONE. 2012a;7:e39939. doi: 10.1371/journal.pone.0039939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012b;22:1681–1690. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach H-A, et al. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Kong E, Monje FJ, Hirsch J, et al. Learning not to fear: Neural correlates of learned safety. Neuropsychopharmacology. 2014;39:515–527. doi: 10.1038/npp.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem Pharmacol. 2015;97:498–511. doi: 10.1016/j.bcp.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Holliday E, Gould TJ. High-affinity α4β2 nicotinic receptors mediate the impairing effects of acute nicotine on contextual fear extinction. Neurobiol Learn Mem. 2016a;128:17–22. doi: 10.1016/j.nlm.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Oliver C, Gould TJ. The effects of acute nicotine on contextual safety discrimination. J Psychopharmacol. 2014;28:1064–1070. doi: 10.1177/0269881114552743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Oliver C, Huang P, et al. Impairment of contextual fear extinction by chronic nicotine and withdrawal from chronic nicotine is associated with hippocampal nAChR upregulation. Neuropharmacology. 2016b;109:341–348. doi: 10.1016/j.neuropharm.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. J Am Med Assoc. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology. 1996;123:88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lee C, Rose JE, et al. Chronic nicotine and withdrawal effects on radial-arm maze performance in rats. Behav Neural Biol. 1990;53:269–276. doi: 10.1016/0163-1047(90)90509-5. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, et al. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biol Psychol. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- MacLeod JE, Potter AS, Simoni MK, et al. Nicotine administration enhances conditioned inhibition in rats. Eur J Pharmacol. 2006;551:76–79. doi: 10.1016/j.ejphar.2006.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod JE, Vucovich MM, Bucci DJ. Differential effects of nicotinic acetylcholine receptor stimulation on negative occasion setting. Behav Neurosci. 2010;124:656–661. doi: 10.1037/a0020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266:1268–1276. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Bucci DJ. The contribution of medial prefrontal cortical regions to conditioned inhibition. Behav Neurosci. 2014;128:644–653. doi: 10.1037/bne0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, et al. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Elsevier Academic Press; 2008. [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: Beyond the inverted U. Trends Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Lubec G. The learned safety paradigm as a mouse model for neuropsychiatric research. Nat Protoc. 2010;5:954–962. doi: 10.1038/nprot.2010.64. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, et al. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav Genet. 2012a;42:133–150. doi: 10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, et al. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012b;97:482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost SC, Woodward R. Effects of nicotine gum on repeated administration of the stroop test. Psychopharmacology. 1991;104:536–540. doi: 10.1007/BF02245662. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, et al. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 Mice: A role for high-affinity β2 Subunit-containing nicotinic acetylcholine receptors. Eur J Neurosci. 2009;29:377–387. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychol Bullet. 1969;72:77–94. [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rhodes SEV, Killcross AS. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. Eur J Neurosci. 2007;26:2654–2660. doi: 10.1111/j.1460-9568.2007.05855.x. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, et al. Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology. 2014;39:2405–2413. doi: 10.1038/npp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the pre-frontal cortex of rats and primates: Insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a pre-frontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Kathryn Dahlgren M, Caroline Davis F, et al. From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Overmier JB, LoLordo VM. A reevaluation of Rescorla’s early dictums about Pavlovian conditioned inhibition. Psychol Bull. 1992;111:275–290. [Google Scholar]
- Wilson MA, Fadel JR. Cholinergic regulation of fear learning and extinction. J Neurosci Res. 2016;95:836–852. doi: 10.1002/jnr.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JRA, Pidoplichko VI, Broide RS, et al. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E, Connor DA, Gould TJ. ABT-089, but not ABT-107, ameliorates nicotine withdrawal-induced cognitive deficits in C57BL6/J mice. Behav Pharmacol. 2015;26:241–248. doi: 10.1097/FBP.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]