Abstract
Adolescence is a critical developmental period associated with both increased vulnerability to substance abuse and maturation of certain brain regions important for learning and memory such as the hippocampus. In this study, we employed a hippocampus-dependent learning context pre-exposure facilitation effect (CPFE) paradigm in order to test the effects of acute nicotine on contextual processing during adolescence (post-natal day (PND) 38) and adulthood (PND 53). In Experiment 1, adolescent or adult C57BL6/J mice received either saline or one of three nicotine doses (0.09, 0.18, and 0.36 mg/kg) prior to contextual pre-exposure and testing. Our results demonstrated that both adolescent and adult mice showed CPFE in the saline groups. However, adolescent mice only showed acute nicotine enhancement of CPFE with the highest nicotine dose whereas adult mice showed the enhancing effects of acute nicotine with all three doses. In Experiment 2, to determine if the lack of nicotine’s effects on CPFE shown by adolescent mice is specific to the age when they are tested, mice were either given contextual pre-exposure during adolescence or adulthood and received immediate shock and testing during adulthood after a 15 day delay. We found that both adolescent and adult mice showed CPFE in the saline groups when tested during adulthood. However, like Experiment 1, mice that received contextual pre-exposure during adolescence did not show acute nicotine enhancement except at the highest dose (0.36 mg/kg) whereas both low (0.09 mg/kg) and high (0.36 mg/kg) doses enhanced CPFE in adult mice. Finally, we showed that the enhanced freezing response found with 0.36 mg/kg nicotine in the 15-day experiment may be a result of decreased locomotor activity as mice that received this dose of nicotine traveled shorter distances in an open field paradigm. Overall, our results indicate that while adolescent mice showed normal contextual processing when tested both during adolescence and adulthood, they are less sensitive to the enhancing effects of nicotine on contextual processing.
Keywords: Nicotine, Context, Fear conditioning, Adolescence
1. Introduction
Adolescence is a critical developmental period associated with increased vulnerability to substance abuse including nicotine addiction (Giovino, 2002; Nelson et al., 2008; see Spear (2000) for a review). For example, the majority of smokers try their first cigarettes before the age of 18 (Everett et al., 1999; Johnston et al., 2009; Lantz, 2003). Furthermore, there is evidence demonstrating that earlier onset of smoking is predictive of more severe nicotine dependence later in life (Everett et al., 1999), suggesting that nicotine use starting in this period has a higher impact on lifelong nicotine addiction when compared to nicotine use started during adulthood.
Adolescence is also a key developmental stage for the maturation of the brain regions important for learning and memory (Benes, 1989; Wolfer and Lipp, 1995; Dumas and Foster, 1998; Eriksson et al., 1998). One such brain region is the hippocampus, a unique brain structure heavily involved in episodic memory, spatial learning, contextual learning, and spatial working memory (Aggleton et al., 1986; Jung and McNaughton, 1993; Burgess et al., 2002; Daumas et al., 2005). Using animal models, numerous studies have shown that acute nicotine enhances hippocampus-dependent forms of learning and memory such as contextual and trace fear conditioning (Gould and Higgins, 2003; Davis et al., 2005, 2006, 2007; Davis and Gould, 2006; Raybuck and Gould, 2010), spatial object recognition (Kenney et al., 2011), spatial learning in Morris Water Maze (Abdulla et al., 1996; Sharifzadeh et al., 2005), and spatial working memory in Radial Arm Maze (Levin et al., 1997, 1998; Levin and Torry, 1996). Importantly, Portugal et al. (2012) investigated the effects of nicotine exposure during adolescence on hippocampus-dependent learning and showed that mice had altered sensitivity to the enhancing effects of nicotine on contextual fear conditioning across adolescence. Together, the studies mentioned above suggest that acute nicotine enhances hippocampus-dependent learning and memory but the cognitive-enhancing effects of acute nicotine is altered during adolescence.
Another hippocampus-dependent learning paradigm that can be used to examine the effects of nicotine on contextual processing separate from context-shock learning is context pre-exposure facilitation effect (CPFE; Rudy et al., 2002; Matus-Amat et al., 2007; Schiffino et al., 2011). CPFE is a learning task where pre-exposure to the context facilitates contextual fear conditioning induced by a single immediate foot-shock (Fanselow, 1990) up to 28 days after pre-exposure (Rudy and Wright-Hardesty, 2005). Importantly, Kenney and Gould (2008) showed that in adult mice, acute nicotine administrations prior to both context exposure and retrieval test enhanced CPFE whereas nicotine injections prior to context-shock learning and testing had no effect. This suggests that acute nicotine specifically enhances contextual learning but not the context-shock association. However, the effects of acute nicotine on CPFE in adolescent mice are unknown. Therefore, in this study, employing a CPFE paradigm, we investigated the effects of acute nicotine on contextual processing during adolescence and adulthood. Moreover, we tested nicotine’s effects on CPFE when mice were given nicotine exposure during adolescence but trained and tested during adulthood. This study informs on the age-specific effects of acute nicotine on short-term (24 h) and long-term (15 days) contextual processing.
2. Results
2.1. Experiment 1: Acute nicotine enhances CPFE in adult but not in adolescent mice
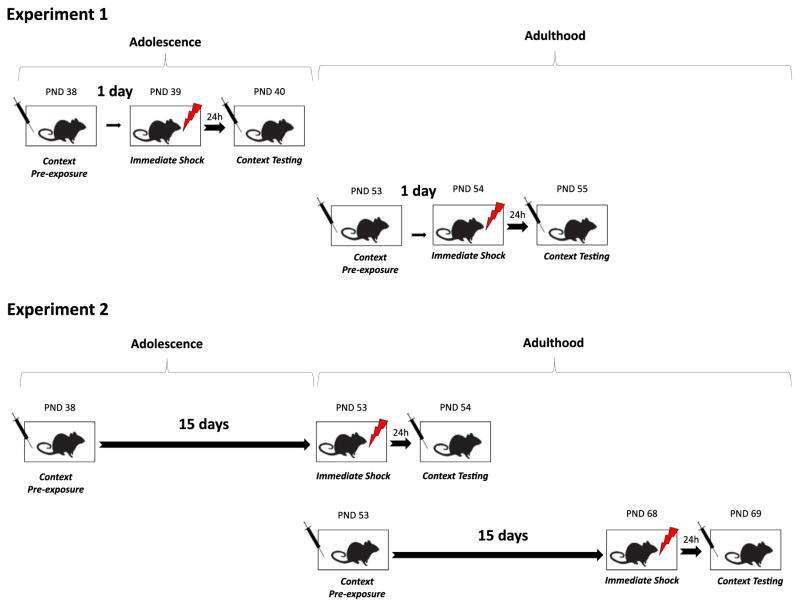
In Experiment 1, adolescent (PND 38) and adult mice (PND 53) were given pre-exposure to the conditioning context (PRE groups) following intraperitoneal injections of either nicotine (0.09, 0.18, or 0.36 mg/kg) or saline. Another group of mice stayed in their homecages after the injections (No-PRE groups). Twenty-four hours later, mice were trained in an immediate shock paradigm and the following day the mice were tested for freezing behavior to the same context (Fig. 1, Upper Panel).
Fig. 1.
The schematic experimental designs of Experiments 1 and 2. Each box represents a phase of the experiment, the syringes represent nicotine or saline injections, and the lightning bolt symbol indicates the presentations of the foot-shocks.
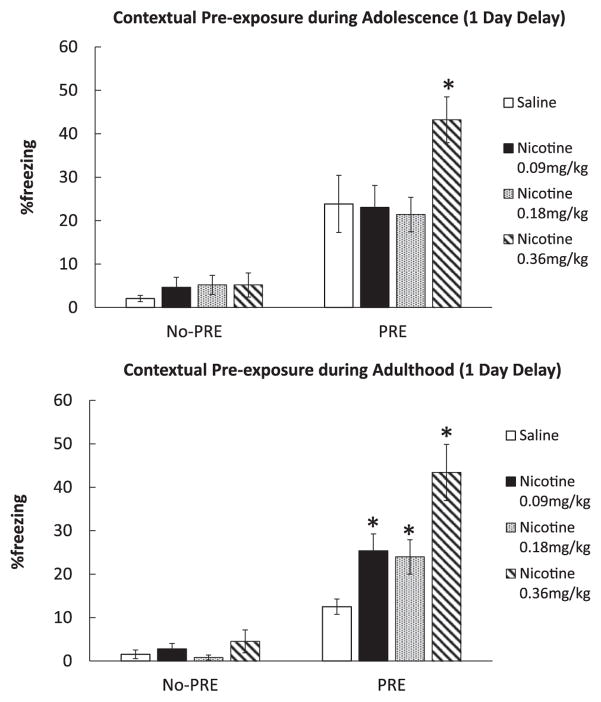
In order to assess the effects of nicotine injections on CPFE in adolescent and adult mice, we conducted a 4 (Drug; 0.09, 0.18, 0.36 mg/kg Nicotine vs. Saline) × 2 (Pre-exposure; No-PRE vs. PRE) ANOVAs for each age group. This way we aimed to identify the dose-response curve for each age group. Our results showed that the adolescent mice were less sensitive to the effects of acute nicotine on CPFE. A 4 × 2 ANOVA showed that the Drug × Pre-exposure interaction was significant for the adult group, F(3,58)= 7.58, p<0.001. Another 4 × 2 ANOVA did not yield a significant Drug × Pre-exposure interaction (F(3,65)=2.45, p>0.05) for the adolescent group but both drug (F(3,65)=3.08, p=0.033) and Pre-exposure (F(1,65)=63.86, p<0.001) main effects were significant. Separate planned t-tests showed a significant difference between Sal PRE and Nic 0.09 mg/kg PRE (t(16)=3.42, p<0.05), Sal PRE and Nic 0.18 mg/kg PRE (t(16)=3.02, p<0.05), and Sal PRE and Nic 0.36 mg/kg PRE (t(16)=5.41, p<0.01) groups in the adults, which shows a significant enhancement of CPFE by all doses of nicotine in adult mice. In adolescent PRE groups, there was only a significant difference between groups that received saline and 0.36 mg/kg nicotine (t(17)=2.12, p<0.05) while 0.09 mg/kg (t (20)=0.96, p>0.05) and 0.18 mg/kg (t(17)=0.27, p>0.05) nicotine groups showed no difference from the saline group. No significant differences were found between the saline and nicotine No-PRE conditions (ps>0.05). Also, both adult and adolescent saline group mice showed significant differences between PRE and No-PRE groups (Adults, t(17)=5.54, p<0.01; Adolescents, t(22)= 3.42, p<0.01), which indicates CPFE in both age groups. Finally, we found a significant difference of baseline CPFE levels between saline treated adolescent and adult mice (t(18)=2.22, p<0.05). However, no interaction between age and pre-exposure was found for saline treated animals (F(3,39)=2.139, p>0.05), which suggests that although baseline Sal PRE group responses differed between ages this effect was not strong enough to affect overall CPFE between age groups. These results replicate Kenney and Gould’s (2008) study showing acute nicotine-induced enhancement of CPFE in adult mice. However, our results also show that adolescent mice did not display the acute nicotine-induced enhancement of CPFE except at the highest dose of nicotine (0.36 mg/kg), suggesting that the dose response for nicotine effects on CPFE in adolescent mice is shifted to the right (Fig. 2).
Fig. 2.
Effects of acute nicotine (0.09 0.18, and 0.36 mg/kg) on CPFE in adult and adolescent mice (n=7–12). Upper Panel: Adolescent mice (PND 38) showed CPFE in the saline treated groups with 1 day delay but showed acute nicotine enhancement of CPFE only with the highest dose of nicotine (0.36 mg/kg). Lower Panel: Adult mice (PND 53) showed both basic CPFE in the saline treated mice with 1 day delay and the acute nicotine-induced enhancement of CPFE at all three nicotine doses. Error bars indicate Standard Error of the Mean (SEM) and asterisks represent differences from the saline group at the p<0.05 level.
2.2. Experiment 2: Acute nicotine enhances the effects of adult but not adolescent contextual pre-exposure when tested during adulthood
The results of Experiment 1 showed that adolescent mice were less sensitive to the enhancing effects of acute nicotine on CPFE. However, it is unclear whether the lack of acute nicotine effects on CPFE shown by adolescent mice was specific to the age when they were tested. Therefore, in Experiment 2, mice were given pre-exposure to the context during adolescence but received immediate shock and were tested during adulthood (Fig. 1, Lower Panel) following acute nicotine (0.09 and 0.36 mg/kg) or saline injections.
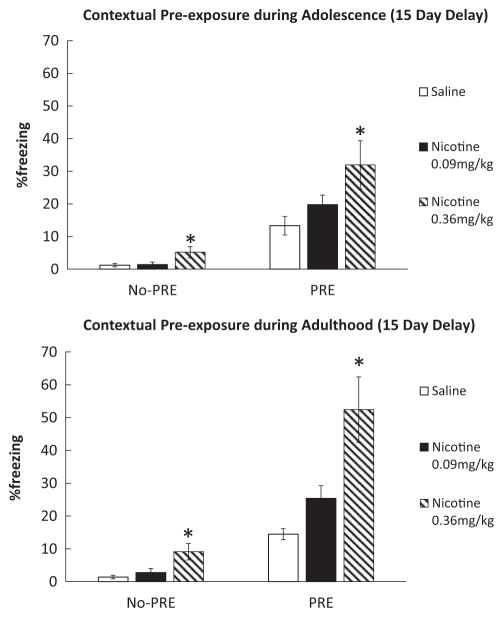
In order to identify the effective doses of acute nicotine enhancing CPFE following a 15 day delay, we conducted a 3 (Drug; 0.09, 0.36 mg/kg Nicotine vs. Saline) × 2 (Pre-exposure; No-PRE vs. PRE) ANOVAs for each age group. Our results showed that the mice that received pre-exposure to the training context during adolescence required a higher acute nicotine dose (0.36 mg/kg) for the effects of acute nicotine on CPFE whereas both acute nicotine doses were effective in the group that received pre-exposure during adulthood. Two 3 × 2 ANOVAs showed that the Drug × Pre-exposure interaction was significant for the group that received pre-exposure as adults, F(2,43)=6.65, p<0.05, but not for the group that received pre-exposure as adolescents F(2,45)=2.661, p>0.05. For the group that received adolescent pre-exposure, the main effect of Pre-exposure was significant (F(1,45)=52.72, p<0.05) but the main effect of drug was not significant (F(2,45)= 6.54, p>0.05). Separate planned t-tests showed significant difference between Sal PRE and Nic 0.09 mg/kg PRE (t(16)=2.94, p<0.05), and Sal PRE and Nic 0.36 mg/kg PRE groups that received adult pre-exposure (t(16)=4.48, p<0.01). In the groups that received pre-exposure as adolescents, the difference between Sal PRE and Nic 0.36 mg/kg PRE groups was significant (t(17)=2.72, p<0.05) but not between Sal PRE and Nic 0.09 mg/kg PRE (t(16)= 1.66, p>0.05). Also, both groups that received pre-exposure and saline treatment during adolescence and adulthood showed a significant difference between PRE and No-PRE groups (Adults, t (16)=7.05, p<0.01; Adolescents, t(17)=4.17, p<0.05), indicating basic CPFE in both age groups whereas there was no significant difference between baseline CPFE levels between those pre-exposed as adults and adolescents (t(18)=0.08, p>0.05). Furthermore, no significant differences were found between the Sal No-PRE and Nic 0.09 mg/kg No-PRE conditions in the groups that received pre-exposure either during adolescence and adulthood (t (15)=0.28, p>0.05 and t(14)=0.30, p>0.05, respectively). However, Nic 0.36 mg/kg No-Pre groups’ freezing was significantly higher than the Sal No-Pre groups that received pre-exposure both as adolescents and adults (t(15)=2.48, p<0.05 and t(13)=3.46, p<0.05, respectively) suggesting a locomotor effect at the 0.36 mg/kg dose. Therefore, following the same timeline for the contextual pre-exposure experiments, we administered 0.36 mg/mg nicotine or saline to adult mice twice, one dose after handling and another dose 15 days later, and tested locomotor activity in an open field paradigm. The results yielded a significant difference in the total distance traveled between saline and nicotine treated mice (Fig. 4; t(14)=4.29, p<0.05). This suggests that the enhanced freezing response found in the No-Pre mice that received 0.36 mg/kg nicotine may be due to decreased locomotor activity rather than an enhanced context pre-exposure effect. Overall, these results show that while both groups of mice that received context pre-exposure during adolescence and adulthood showed long-term CPFE, only mice that received context pre-exposure during adulthood showed the acute nicotine-induced enhancement of CPFE.
Fig. 4.
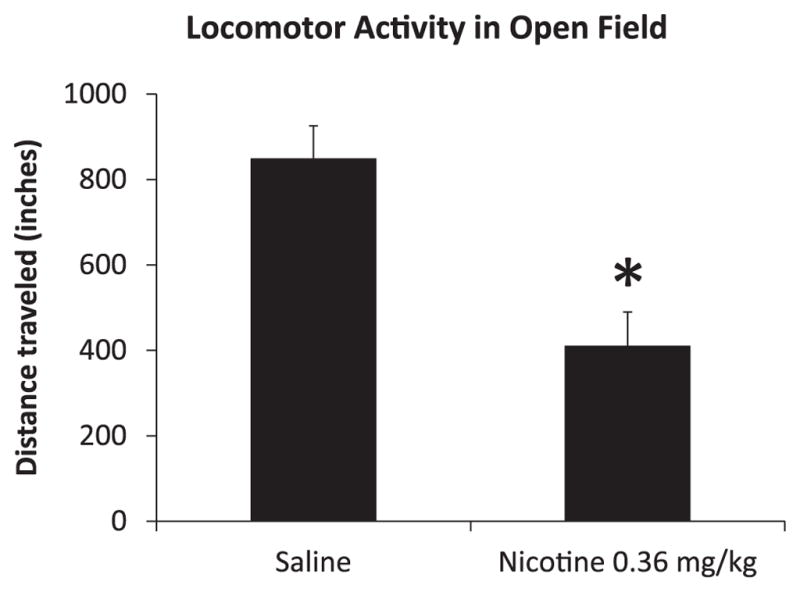
Effects of the high dose of nicotine (0.36 mg/kg) on locomotor activity in open field (n=8). Adult mice (PND 53) showed decreased locomotor activity following 2 injections of 0.36 mg/kg with 15 day delay during adulthood. Error bars indicate Standard Error of the Mean (SEM) and asterisks represent differences from the saline group at the p<0.05 level.
3. Discussion
Our results showed that context pre-exposure facilitated contextual fear learning in adolescent mice when tested 1 day later and when tested in adulthood (15 days later) and adult mice when tested both 1 day and 15 days later. Comparable levels of CPFE in saline treated adult and adolescent mice are in line with a previous report suggesting CPFE emerges during early adolescence and remain stable across developmental stages in rodents (Schiffino et al., 2011; Robinson-Drummer and Stanton, 2014). These results are also consistent with Portugal et al.’s (2012) results, which found no differences in baseline contextual fear conditioning between saline-treated adolescent and adult mice. However, our results appear in conflict with other reports showing that PND37-39 adolescent mice exhibited somewhat reduced contextual fear conditioning compared to adult mice (Pattwell et al., 2011) and that adolescent rats showed higher baseline contextual fear conditioning compared to adults (Esmorís-Arranz et al., 2008), which suggests that procedural differences might strongly influence the age-specific levels of contextual fear conditioning. Specifically, Pattwell et al. (2011) study used 3 tone-footshock pairings with a shock intensity of 0.7 mA whereas in the present study we used a single footshock with an intensity of 0.57 mA in the absence of a tone cue. Importantly, Fanselow (1990) showed that the presence of a conditioned stimulus such as a tone during contextual fear conditioning significantly altered contextual fear conditioning, which suggests that additional mechanisms may be recruited for contextual fear learning in the presence of a conditioned stimulus.
In addition to the basic CPFE shown in both adolescent and adult mice, results of the present study replicated and extended Kenney and Gould’s (2008) study by showing acute nicotine administered prior to context pre-exposure and testing enhanced CPFE in adults with both 1 day (Experiment 1) and 15 (Experiment 2) days between context pre-exposure and immediate shock training. In contrast, mice that received context pre-exposure during adolescence did not display the acute nicotine-induced enhancement of CPFE when tested during adolescence (Experiment 1) or adulthood (Experiment 2) except at the highest dose of nicotine (0.36 mg/kg). This suggests a shifted dose-response in this age group. The CPFE enhancement achieved with 0.36 mg/kg nicotine may potentially be a result of reduced locomotor activity rather than enhanced conditioned response to the context as mice that received 0.36 mg/kg nicotine but no context pre-exposure (Nic 0.36 mg/kg no-PRE group) showed increased freezing behavior in Experiment 2 (Fig. 3). This possibility was confirmed in our open field experiment where adult mice received 0.36 mg/kg nicotine twice with a 15 day delay in between showed reduced locomotor activity. This suggests that enhanced freezing in the 0.36 mg/kg PRE group may be a result of reduced locomotor activity rather than enhanced learning. Nevertheless, given that the freezing levels of the No-PRE group that received 0.36 mg/kg acute nicotine are significantly lower than the freezing observed in both the adolescent and adult PRE Nicotine.36 mg/kg groups, the decreased locomotor activity in this group seems to be a confounding variable but it can only partially explain the results. Overall, our results indicated that while adolescent mice showed normal contextual processing when tested both during adolescence and adulthood, they were less sensitive to the enhancing effects of nicotine on CPFE as opposed to the potential effects of acute nicotine on locomotor activity. In addition, the results of Experiment 2 demonstrated that the lack of acute nicotine’s effect was not specific to the age that mice were tested in as mice that received contextual pre-exposure during adolescence but tested during adulthood still showed reduced sensitivity to acute nicotine enhancing effects on CPFE. Finally, although CPFE levels (comparing No PRE Saline to PRE Saline freezing levels between age groups) were comparable in both age groups, adolescent PRE Saline mice showed higher levels of freezing compared to their adult counterparts. However, the higher freezing levels found in this group did not result in a ceiling effect for the nicotine treated groups as the PRE Nicotine 0.36 mg/kg group showed significantly higher levels of freezing compared to saline the controls. Therefore, it is not possible to attribute the lack of effects of acute nicotine on CPFE in the adolescent group in Experiment 1 to a potential celling effect driven by the higher levels of freezing in the PRE Saline controls.
Fig. 3.
Effects of acute nicotine (0.09 and 0.36 mg/kg) on contextual encoding in adult and adolescent mice (n=7–10). Upper Panel: Adolescent mice (PND 38) showed CPFE in the saline treated groups with 15 day delay but only showed acute nicotine enhancement of CPFE only at the highest dose (0/36 mg/kg). Lower Panel: Adult mice (PND 53) showed both basic CPFE in the saline treated mice with 15 day delay and the acute nicotine-induced enhancement of CPFE at all three nicotine doses. Error bars indicate Standard Error of the Mean (SEM) and asterisks represent differences from the saline group at the p<0.05 level.
It is also important to note that the main advantage of using a CPFE paradigm is that, unlike simple contextual fear conditioning, in CPFE contextual learning and context-shock association learning occur in separate phases of the experiment. Although Portugal et al. (2012) showed that adolescent mice were less sensitive to the enhancing effects of acute nicotine in a simple contextual fear conditioning paradigm, it was not clear which learning process was differentially altered between adolescent and adult mice. Given that our results showed that 0.09 and 0.18 mg/kg acute nicotine doses did not enhance CPFE when either contextual pre-exposure and immediate shock (Experiment 1) or only contextual pre-exposure (Experiment 2) were given during adolescence, the present study suggests that nicotine’s altered modulation of contextual processing but not context-shock learning during adolescence may be responsible for adolescent mice’s reduced sensitivity to nicotine’s enhancing effects of contextual fear learning.
These results add to the growing body of evidence showing that adolescents may have an altered response to nicotine. For example, there is evidence from numerous studies using rodent models of nicotine addiction showing that adolescents are more sensitive to the rewarding effects of nicotine (see Schramm-Sapyta et al. (2009) for a review). Studies found that adolescent rats acquire nicotine self-administration faster and sustain a higher level of stable nicotine self-administration (Levin et al., 2003, 2007; Chen et al., 2007). Also, adolescent rats seem to require lower doses of nicotine for its rewarding effects (Vastola et al., 2002) and show less severe somatic signs during withdrawal compared to adult rats (O’Dell et al., 2006). Furthermore, there is evidence showing that adolescents are less vulnerable to the aversive effects of nicotine (Wilmouth and Spear, 2004; Shram et al., 2006). Although these results suggest that adolescent rodents have increased sensitivity to the rewarding effects of nicotine, importantly, Portugal et al. (2012) demonstrated that while adult mice (PND 53) showed acute nicotine enhancement of contextual fear conditioning with lower doses but not with higher doses of nicotine, early adolescent mice (PND23) showed acute nicotine enhancement with both low and high doses of nicotine whereas late adolescent mice (PND 38) required higher doses of nicotine for the enhancement effects. These results show a rightward shift of dose-response in the late adolescent mice suggesting a decreased sensitivity to the effects of nicotine on learning and memory. Portugal et al.’s (2012) results are in line with the results of the present study showing that late adolescent mice require higher doses of nicotine for the enhancement of CPFE. Finally, in Portugal et al.’s (2012) study, no differences were detected in plasma nicotine or cotinine levels between late adolescent and adult mice. This suggests that the differential sensitivity to acute nicotine’s effects on CPFE between these age groups is not likely to be due to nicotine metabolism. Overall, together with the results of the present study, studies investigating potential age-specific differences in the effects of nicotine suggest that while the adolescent mice showed higher sensitivity to the rewarding effects of nicotine and suffer less from the aversive effects, they are less sensitive to the enhancing effects of nicotine on fear learning and memory.
4. Experimental procedure
4.1. Subjects
Subjects were C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). For both experiments, adolescent mice were shipped to our animal facility post-natal day (PND) 31 whereas adult mice were shipped at PND 46. Although a specific age is not indicated for adolescence in mice, there is evidence that male mice show puberty-like signs, such as increased impulsivity and transition to social behavior characteristic of adults, starting at PND 30 (Johnston et al., 2009; Keene et al., 2002; Terranova et al., 1993,1998). Both adolescent and adult mice were acclimated to the animal facilities for 1 week prior to the start of experiments and given context pre-exposure at PND 38 and PND 53, respectively. All subjects were group-housed in a colony room maintained on a 12 h light/dark cycle with access to food and water ad libitum. All training and testing occurred between 9:00 am and 6:00 pm. Behavioral procedures used in this study were approved by the Temple University Institutional Animal Care and Use Committee.
4.2. Apparatus
Context pre-exposure, immediate shock training, and testing took place in four identical conditioning chambers (18.8 × 20 × 18.3 cm) placed in sound-attenuating boxes (MED Associates, St. Albans, VT). Ventilations fans were located at the back of the boxes providing a background noise (65 dB). The front wall and ceiling of the chambers were composed of Plexiglas and the floors were metal grids (0.20 cm and 1.0 cm apart) connected to a shock generator, which produced a 2 s long, 0.57 mA foot-shock unconditioned stimulus (US). The stimuli were controlled by a custom made MED-PC program. The open field test took place in a plexiglass arena (49.5 cm × 59.7 cm). All chambers and the open field arena were cleaned with 70% ethanol before and between each subject.
4.3. Drugs and administration
Nicotine hydrogen tartrate salt (0.09, 0.18, or 0.36 mg/kg free-base, Sigma, St. Louis, MO) dissolved in saline or saline alone was injected intraperitoneally (i.p.) 5 mins prior to contextual pre-exposure and testing. The 0.09 mg/kg dose and injection time were selected based on Kenney and Gould’s (2008) study showing enhancement of CPFE with this dose. Additional doses (0.18 and 0.36 mg/kg) were also used to determine dose-response. Both saline and nicotine injection volumes were 10 ml/kg.
4.4. Behavioral procedures
4.4.1. Contextual pre-exposure facilitation paradigm
For both experiments, freezing behavior was measured, converted to percent freezing, and used as the dependent variable. A time sampling procedure was used to score freezing behavior where each subject was observed every 5 sec for a duration of 1 sec and scored as either freezing or active. During scoring, experimenters were blinded to the drug conditions. As in previous studies (e.g., Kenney and Gould, 2008), freezing was defined as the absence of voluntary movement except respiration. Prior to context pre-exposure, each mouse was handled and habituated to the transport cages daily for 5 min for a total of 2 days. Transport cages were identical to the homecages and fresh bedding material was mixed with bedding material from their homecages. Context pre-exposure, immediate shock training, and testing were identical to Kenney and Gould (2008) (Fig. 1). During the context pre-exposure phase, Pre-exposure (PRE) group mice were placed in the conditioning chambers and allowed to explore the chambers for 10 mins while No Pre-exposure (No-PRE) mice remained in the transport cages. Then all mice received either a 1 day (Experiment 1) or 15 day (Experiment 2) delay before the immediate shock treatment. All mice were placed in the conditioning chambers and received a 2 sec, 0.57 mA footshock 5 sec after placement and stayed in the chambers for 60 sec. Finally, 24 h after the immediate shock, mice were transported to the conditioning chambers again, and freezing behavior was assessed for 3 mins as described above during testing. Nicotine-treated (Nic) mice were given nicotine (0.09, 0.18, or 0.36 mg/kg) injections 5 min prior to both context pre-exposure and testing sessions whereas saline-treated mice (Sal) were given saline vehicle injections. Consequently, there were 8 groups for each age condition in Experiment 1, Sal PRE, Nic 0.09 mg/kg PRE, Nic 0.18 mg/kg PRE, Nic 0.36 mg/kg PRE, Sal No-PRE, Nic 0.09 mg/kg No-PRE, Nic 0.18 mg/kg No-PRE, and Nic 0.36 mg/kg No-PRE. In Experiment 2, we only used the lowest (0.09 mg/kg) and highest (0.36 mg/kg) nicotine doses. Therefore, we had 6 groups for each age condition in this experiment, Sal PRE, Nic 0.09 mg/kg PRE, Nic 0.36 mg/kg PRE, Sal No-PRE, Nic 0.09 mg/kg No-PRE, and Nic 0.36 mg/kg No-PRE.
4.4.2. Open-field paradigm
To test the potential effects of the highest nicotine dose (0.36 mg/kg) on locomotor activity in mice we employed an open field paradigm. Adult mice were first handled for 2 days as described for the CPFE paradigm. The next day, mice received either nicotine (0.36 mg/kg) or saline injections. Fifteen days later, mice received either 0.36 mg/kg nicotine or saline again and were tested in the open field 5 min later. Specifically, individual mice were placed in the center quadrant of the open field box and locomotor activity, defined as distance traveled in inches, was recorded for 5 mins using a tracking software (Smart Tracking Software, Panlab).
4.5. Statistical analysis
Freezing response to the context during testing was examined using 4 (Drug; 0.09, 0.18, 0.36 mg/kg Nicotine vs. Saline) × 2 (Pre-exposure; No-PRE vs. PRE) ANOVAs for each age group for Experiment 1. Similarly, data from Experiment 2 were also analyzed using a 3 (Drug; 0.09, 0.36 mg/kg Nicotine vs. Saline) × 2 (Pre-exposure; No-PRE vs. PRE) ANOVA. We chose to run separate 4 × 2 ANOVAs for adolescent and adult groups in order to directly identify if the nicotine doses tested have any effect on CPFE in different age groups as opposed to comparing the degree of nicotine’s effects on CPFE between adolescent and adult mice. Planned comparison t-tests were used for analysis between individual groups at α=0.05 level (Ruxton and Beauchamp, 2008) as critical comparisons were determined a-priori. A total of 4 mice (1 Adolescent No-PRE 0.09 mg/kg; 1 Adolescent PRE 0.18 mg/kg; 1 Adolescent PRE 0.36 mg/kg; and 1 Adult No-PRE 0.18 mg/kg) from Experiment 1 and 3 mice (1 Adolescent No-PRE Saline; 1 Adult No-PRE Saline; and 1 Adult No-PRE 0.36 mg/kg) from Experiment 2 were removed from the analysis as their testing freezing levels were 2 standard deviations above the mean. Distance traveled in inches was used as the dependent variable for the open field experiment. A t-test was computed for the difference between groups. Group sizes were indicated in All statistical analyses were run using SPSS 16.0.
Acknowledgments
This work was funded with grant support from the National Institute on Drug Abuse (T.J.G., DA017949).
References
- Abdulla FA, Gray JA, Sinden JD, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology. 1996;124(4):323–331. doi: 10.1007/BF02247437. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Rawlins JNP. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19(2):133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15(4):585. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Francés B, Lassalle JM. Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem. 2005;12(4):375–382. doi: 10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25(38):8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394(3):202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184(3–4):345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal α4β2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27(40):10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC, Foster TC. Late developmental changes in the ability of adenosine A1 receptors to regulate synaptic transmission in the hippocampus. Dev Brain Res. 1998;105(1):137–139. [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Esmorís-Arranz FJ, Méndez C, Spear NE. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav Process. 2008;78(3):340–350. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of cigarette smoking and subsequent smoking behavior among US high school students. Prev Med. 1999;29(5):327–333. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18(3):264–270. [Google Scholar]
- Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21(48):7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80(2):147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary School Students. I. National Institute on Drug Abuse; Bethesda, MD: 2009. Monitoring the Future National Survey Results on Drug Use, 1975–2008. [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Keene DE, Suescun MO, Bostwick MG, Chandrashekar V, Bartke A, Kopchick JJ. Puberty is delayed in male growth hormone receptor gene-disrupted mice. J Androl. 2002;23(5):661. [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci. 2008;122(5):1158. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology. 2011;217(3):353–365. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM. Smoking on the rise among young adults: implications for research and policy. Tob Control. 2003;12(Suppl 1):i60–i70. doi: 10.1136/tc.12.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169(2):141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kaplan S, Boardman A. Acute nicotine interactions with nicotinic and muscarinic antagonists: Working and reference memory effects in the 16-arm radial maze. Behav Pharmacol. 1997 [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Weaver T, Christopher NC. Nicotine–dizocilpine interactions and working and reference memory performance of rats in the radial-arm maze. Pharmacol Biochem Behav. 1998;61(3):335–340. doi: 10.1016/s0091-3057(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology. 1996;123(1):88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29(4):458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Mowery P, Asman K, Pederson LL, O’Malley PM, Malarcher A, Pechacek TF. Long-term trends in adolescent and young adult smoking in the United States: metapatterns and implications. Am J Public Health. 2008;98(5):905–915. doi: 10.2105/AJPH.2007.115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186(4):612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci. 2011;108(3):1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012;97(4):482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94(3):353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Stanton ME. Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol Behav. 2014;148:22–28. doi: 10.1016/j.physbeh.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Wright-Hardesty K. The temporal dynamics of retention of a context memory: something is missing. Learn Mem. 2005;12(2):172–177. doi: 10.1101/lm.84005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008;19(3):690–693. [Google Scholar]
- Sharifzadeh M, Tavasoli M, Naghdi N, Ghanbari A, Amini M, Roghani A. Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats. J Neurochem. 2005;95(4):1078–1090. doi: 10.1111/j.1471-4159.2005.03454.x. [DOI] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95(2):190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206(1):1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186(2):201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev Psychobiol. 1993;26:467–481. doi: 10.1002/dev.420260805. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, de Acetis L, Alleva E. A description of the ontogeny of mouse agonistic behavior. J Comp Psychol. 1998;112:3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77(1):107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann NY Acad Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Lipp HP. Evidence for physiological growth of hippocampal mossy fiber collaterals in the guinea pig during puberty and adulthood. Hippocampus. 1995;5(4):329–340. doi: 10.1002/hipo.450050406. [DOI] [PubMed] [Google Scholar]