Abstract
Background
Trypanocidal drugs have been used to control African animal trypanosomosis for several decades. In Ethiopia, these drugs are available from both authorized (legal) and unauthorized (illegal) sources but documentation on utilization practices and quality of circulating products is scanty. This study looked at the practices of trypanocidal drug utilization by farmers and the integrity of active ingredient in trypanocides sold in Gurage zone, south western Ethiopia. The surveys were based on a structured questionnaire and drug quality determination of commonly used brands originating from European and Asian companies and sold at both authorized and unauthorized markets. One hundred farmers were interviewed and 50 drug samples were collected in 2013 (Diminazene aceturate = 33 and Isometamidium chloride = 17; 25 from authorized and 25 from unauthorized sources). Samples were tested at the OIE-certified Veterinary Drug Control Laboratory (LACOMEV) in Dakar, Senegal, by using galenic standards and high performance liquid chromatography.
Results
Trypanosomosis was found to be a major threat according to all interviewed livestock keepers in the study area. Diminazene aceturate and isometamidium chloride were preferred by 79% and 21% of the respondents respectively, and 85% of them indicated that an animal receives more than six treatments per year. About 60% of these treatments were reported to be administered by untrained farmers. Trypanocidal drug sources included both unauthorized outlets (56%) and authorized government and private sources (44%). A wide availability and usage of substandard quality drugs was revealed. Twenty eight percent of trypanocidal drugs tested failed to comply with quality requirements. There was no significant difference in the frequency of non-compliance between diminazene-based and isometamidium chloride products (P = 0.87) irrespective of the marketing channel (official and unofficial). However, higher rates of non-compliant trypanocides were detected for drugs originating from Asia than from Europe (P = 0.029).
Conclusion
The findings revealed the presence of risk factors for the development of drug resistance, i.e. wide distribution of poor quality drugs as well as substandard administration practices. Therefore, it is strongly recommended to enforce regulatory measures for quality control of veterinary drugs, to expand and strengthen veterinary services and to undertake trypanocidal drug efficacy studies of wider coverage.
Electronic supplementary material
The online version of this article (10.1186/s12917-017-1327-6) contains supplementary material, which is available to authorized users.
Keywords: Diminazene, Isometamidium, Trypanocide, Drug quality assessment, Drug utilization practice, Ethiopia
Background
Ethiopia has the largest livestock population in Africa with 53.9 million cattle, 24.6 million goats, 25.5 million sheep 6.8 million donkeys and 1.9 million horses [1]. Hence, livestock is a significant contributor to economic and social development of the country. Livestock accounts for 15 to 17% and 35 to 49% of the total and agricultural GDP respectively [1]. Unfortunately, the development and intensification of livestock production is hampered by transboundary epizootic diseases such as African animal trypanosomosis (AAT). It is estimated that, should AAT be eliminated in Ethiopia, direct benefits would exceed 800 million USD over a 20-year period [2].
Various efforts to control the disease and the associated economic losses have been directed mainly against the parasite through trypanocidal drugs and against the vector through odour-baited and insecticide- impregnated targets/traps and insecticide-treated cattle [3–5]. The main drugs used for treating the disease, i.e. diminazene aceturate (DA) and isometamidium chloride (ISM) have been on the market for more than half a century and the parasites’ resistance to both trypanocidal drugs is increasing [6].
In Ethiopia, several authors reported prevalence of drug resistance against one or both of the drugs (DA and ISM) in several AAT-affected areas [7–12]. It is believed that the emergence of drug resistance is linked to bad handling and utilization practices as well as to poor drug quality [3, 13, 14], which severely reduces the effectiveness of chemotherapy. Drug resistance to ISM is more widespread than to DA [15], but multiple drug resistance is being increasingly reported from different parts of Africa [16, 17].
None of the previous studies has assessed the quality of trypanocidal drugs in Ethiopia. The present study aimed to fill this knowledge gap. The study was initiated as part of a joint action for trypanocidal drug quality control in Africa whose partners include the Global Alliance for Livestock Veterinary Medicine (GALVmed), the Food and Agriculture Organization of the United Nations (FAO), the International Federation of Animal Health (IFAH), the International Atomic Energy Agency (IAEA) and the Trypanosomosis Rational Chemotherapy (TRYRAC) project, funded by the European Commission. The specific objectives of this study were to assess trypanocidal drug utilization practices in south-western Ethiopia and evaluate the quality of diminazene aceturate- and isometamidium chloride-based brands.
Methods
Study sites
The study was conducted in south-western Ethiopia, at the boundary between the Southern Nations, Nationalities and Peoples’ Region (Gurage zone) and Oromia Region (Jimma zone). In these areas, three species of tsetse flies are present (G. pallidipes, G. fuscipes fuscipes, G. morsitans submorsitans) [18]. According to information obtained from local authorities, there were 124 tsetse- and trypanosome-affected villages in the study areas. After randomly assessing 40 villages among the 124, five villages with trypanosome prevalence ≥10% were identified for the questionnaire survey: Borer 4, Borer 5, Misreta, Wolaita and Wuhalimat. Trypanocidal drugs were collected from authorized (veterinary clinics and drug stores) and unauthorized markets of the selected areas (Fig. 1) and wholesalers supplying the areas. The drugs were analysed at the Veterinary Drug Control Laboratory (LACOMEV) in Dakar, Senegal.
Fig. 1.
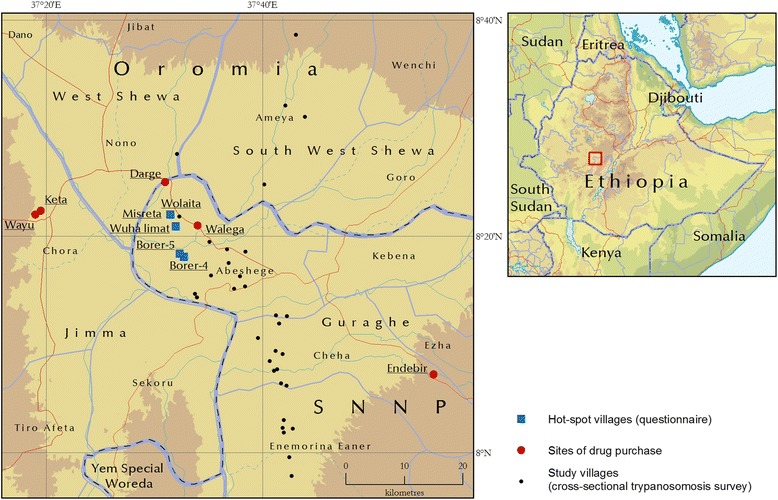
Map of the study area showing sampling sites for crossesctional study (black dotes), trypanocidal drug sampling sites (red bullets) and the five hot spot villages where drug treatment trial and questionnaire survey were done (blue bullets). Bullets for Wolaita and Misreta villages are overlaping
Questionnaire survey
A structured questionnaire was used to collect data on the practice of trypanocidal drug usage amongst farmers in the five selected study villages. A total of 100 farmers selected by systematic random sampling technique (20 farmers in each village) were interviewed. The respondents were selected from farmers who were voluntarily presenting their cattle for trypanosomosis screening during the study period.
Purchase of trypanocidal products for quality assessment
Fifty samples of trypanocidal drugs were collected from different vendors (authorized and unauthorized) and wholesalers in October 2013. The sampling points were purposely selected for consistency with the drug supply facilities mentioned in the questionnaire survey. Sampling points included authorized markets (Veterinary Clinics, pharmacies and wholesalers) and unauthorized sources (open markets) (Table 1). The drugs were sealed in plastic bags identified by a unique number. Information such as trade name, manufacturer, origin, date of manufacture, expiry date, and place of purchase were recorded. To ensure sufficient quantity, each sample contained at least 5–10 sachets depending on the content per sachet (Five for the 23.6 g DA and 1 g Ism, 10 for the 2.36 g DA and 125 mg ISM) to ensure sufficient sample for analysis.
Table 1.
Sources of trypanocidal drugs used for quality analysis (drug samples were collected from nearby authorized vendors and suppliers as well as open markets/illegal sources)
| Wholesaler | Governmental clinica | Private veterinary pharmacya | Open marketb | Total | |
|---|---|---|---|---|---|
| ISM | 5 | 3 | 2 | 7 | 17 |
| DA | 5 | 2 | 8 | 18 | 33 |
| Total | 10 | 5 | 10 | 25 | 50 |
aauthorized, bunauthorized
ISM Isometamidium chloride, DA Diminazene aceturate
Assessment of trypanocidal drug quality
Drug quality was assessed by (i) galenic tests, (ii) identification and (iii) measurement of concentration of the respective active ingredient according to standard operating procedures prepared by GALVmed, FAO and IFAH in collaboration with Manchester Metropolitan University and IAEA [19]. The galenic testing included pH measurement, solubility/limpidity of solutions prepared from DA granules or ISM powder according to the manufacturers’ recommendations. The pH was measured using a Metler MP 230 pH meter with a pH between 4 and 7 considered as compliant. The limpidity of solutions was assessed visually with the naked eye for the absence of visible solid particles Identification and concentration of the active ingredient were assessed using high performance liquid chromatography (HPLC) [19]. Each sample was simultaneously measured with a reference standard. The standards for diminazene and isometamidium were manufactured by VETOQUINOL (Paris, France) and CEVA (Libourne, France), respectively [19] and were provided to LACOMEV by the consortium GALVmed/FAO/IAEA/IFAH. They were stored in a refrigerator at 4° +/− 1 °C until use.
Trypanocide samples were dissolved in ultrapure water for DA and 25% acetonitrile in ultrapure water for ISM to obtain a solution of 0.1 mg/ml of active ingredient. The solution was poured into vials and introduced into the HPLC machine that was programmed to automatically conduct the process of identification and concentration measurement. The mobile phase for the analysis of DA used a mix of 10% methanol, 10% acetonitrile and 80% ammonium formate buffer (20 mM, pH 4.0). Similarly, the mobile phase for ISM used 25% acetonitrile and 75% ammonium formate buffer (50 mM, pH 2.8). A Water Kromasil C18® HPLC column (150 × 4.6 mm, 5 μm - AkzoNobel, Separation Products, Bohus, Sweden) was used to run the test. The procedure was performed twice with a maximum acceptable divergence between analyses of 2%. In case of higher divergence, the procedure was repeated until it fell within the 2% threshold. For DA, a measured concentration within ±10% variation from the manufacturers’ label claim was considered as compliant [20]. For ISM, the following criteria were used to declare compliance: (i) presence of the four isomers (I, II, III, IV), (ii) a proportion of isomer I (principal component) equal to or greater than 55%, (iii) a proportion of isomers II, III and IV equal to or less than 40% and (iv) a proportion of the four isomers falling between 95 and 102%. All along the quality assessment process, names of pharmaceutical companies producing the drugs were kept anonymous.
Statistical analysis
Confidence intervals of proportions were calculated assuming binomial distributions of the variables. For drug quality compliance study, a logistic regression was employed. The response was drug compliance whereas binary explanatory variables were the drugs (DA/ISM), the marketing channel (official/unofficial) and the origin of the drugs (Asia/Europe). Non-significant explanatory variables (p > 0.05) were removed from the model. In the event that a category contained no observation, the exact test was used to evaluate the significance of the difference and the exact method was used to calculate the confidence interval.
Ethical considerations
The objectives of this study were well explained to all selected farmers and those who expressed their consent to participate in the questionnaire survey were recruited. The identity of study participants and data on their livestock population were kept confidential.
Results
Trypanocidal drug utilization practices
For all respondent farmers communal or free grazing is the predominant livestock management practice in the study villages and trypanosomosis was ranked as biggest animal health constraint.
For the control of trypanosomosis, all respondents reported to depend mainly on the two trypanocidal drugs diminazene aceturate and isometamidium chloride rather than other control methods (Additional file 1: Annex I). The majority of respondents in all villages (56%; 95% CI = 45.7–65.9%) get trypanocidal drugs from unauthorized markets (Fig. 2). Diminazene aceturate was the preferred drug (79%) over ISM (21%). Respondents explained that diminazene was cheaper and available from all sources in single dose/sachet. On the other hand, significant numbers of farmers in the study villages (59%; 95%; CI = 48.7–68.7%) administer trypanocidal drugs by themselves or through family members (Fig. 3) and about 85% of them (95% CI = 76.5–91.4%) indicated that they treat their animals seven or more times per year (Fig. 4). All respondents perceived treatment failures as common. In this respect, 58% of the interviewed farmers believe that treatments were more likely to be successful when the drugs are sourced from government veterinary clinics and authorized private sources than from unauthorized open markets.
Fig. 2.
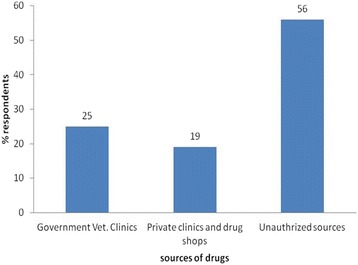
Questionnaire survey response on the source of trypanocidal drugs for treatment of cattle in the study areas
Fig. 3.
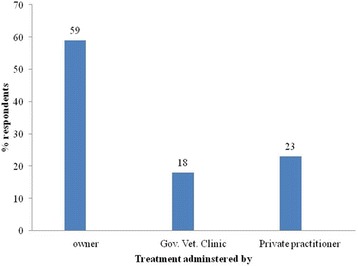
Questionnaire survey response on the administration (injection) of trypanocidal drugs to cattle in the study area
Fig. 4.
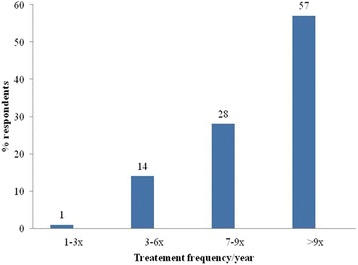
Annual trypanocidal drug treatment frequency per individual cattle based on the questionnaire survey response
Trypanocidal drug quality
Of the 50 trypanocidal drug samples collected, 26 were from European companies and 24 from Asian companies. The samples represent 19 different trade names (9 for ISM and 10 for DA). Overall, result showed that 28% (14/50) of the drugs were non-compliant due to insufficient active ingredient detected by HPLC. The difference in non-compliance between the two drugs was not significant (P = 0.87 in univariate model), being 27.3% for DA and 29.4% for ISM. Also, clients of authorized and non-authorized markets are at similar risk of purchasing non-compliant trypanocides (Table 2; P = 0.53 in univariate model). On the other hand, logistic regression analysis shows that the proportion of non-compliant drugs was significantly more important amongst Asian drugs (P = 0.01 in multivariate model) especially for ISM (interaction not tested since all European ISM samples were found compliant) (Fig. 5). Among the 14 non-compliant samples, nine were from Indian origin (four brands) and two were from UK (one brand). The correlation between marketing channel (official and unofficial) and trypanocidal origin (Asia and Europe) is not significant (P = 0.26 in a Chi Square test).
Table 2.
Proportions of non-compliant samples according to the marketing channel and drug type
| Molecule | Illegal(unauthorized) | Legal(authorized) | ||
|---|---|---|---|---|
| Complies | Not complies | Complies | Not complies | |
| Diminazene Aceturate | 13 | 5 | 11 | 4 |
| Isometamidium chloride | 4 | 3 | 8 | 2 |
Number of observations
Fig. 5.
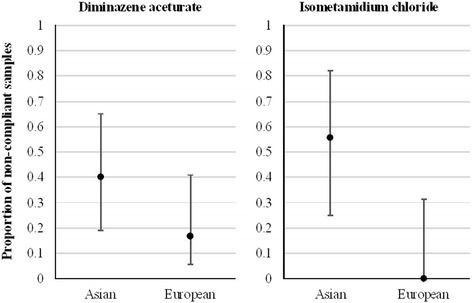
Proportions of non-compliant samples according to continental origin (Asian and European) of the drugs with 95% confidence intervals (based on a logistic regression using drug, origin and the interaction between them as explanatory variables; the exact method was used for categories with no observations)
Discussion
Several control approaches are available to overcome the impacts of AAT [21]. Treatment by the use of trypanocidal drugs such as diminazene aceturate and isometamidium chloride is oftentimes the only method available to farmers for containing the disease in many parts of Ethiopia. In this respect, there is a growing risk of drug resistant trypanosomes as already reported by previous studies [7–12]. In this study, it was observed that risk factors for drug resistance such as the presence of unofficial drug sources and frequent treatments are widespread in the study areas. Diminazene aceturate was reported to be the most frequently used compared to isometamidium chloride. This is believed to expose the drug to more risk of trypanocidal resistance. Inadequate veterinary services and the higher price of drugs from legal sources might have contributed for the greater frequency of respondents opting for unauthorized/illegal sources to easily access trypanocidal drugs. A similar report was documented by Zewdu et al. [22] where annual treatment frequencies range between one and 12 injections/animal and where a significant number of farmers directly gave injections without counseling from a veterinarian. According to Uilenberg [14] and Holmes et al. [3], a high number of annual trypanocidal treatments is suggestive of drug resistance in a given area. Therefore, the high frequency of trypanocidal treatments (more than six times per year), access to drugs from unauthorized sources and the practice of treating animals by untrained personnel is likely to increase the risk of trypanocidal resistance in the study areas and neighbouring localities.
Quality assessment of trypanocides is a prerequisite to ensure better management of trypanosomosis and the prevention of drug resistance [23]. Therefore, trypanocidal drugs were bought anonymously in order to avoid attracting the attention of vendors, who otherwise might have denied access to the products. The 28% of non-compliance observed in this study was below the 71.4% reported in Ivory Coast [24], 100% in Cameroon [25], 70% in Senegal [26], 40% in Togo [27] and 42.3% observed in Burkina Faso [28]. However, since the drugs were supplied by big companies who are national distributers, non-compliant products pose a serious threat to successful AAT treatments countrywide.
The non-compliant veterinary products identified by this study were found in both authorized and unauthorized markets which is different from reports from Togo and Mali [27–29] where non-conform drugs were significantly more often found on unauthorized markets. The non-compliance may be attributed to poor storage conditions and the doubtful sources of supply in this market channel. The 2012 Proclamation for Veterinary Drugs and Feed Administration and Control of Ethiopia capitalizes on regulating “the production, distribution and use of veterinary drugs to ensure safety, efficacy and quality of the products”. It also focuses on “prevention and control of the illegal production, distribution and use of veterinary drugs” [30]. Therefore, the observation of such a significant frequency of non-compliant trypanocides undermines the basic objective of the existing law. Although there are encouraging signs, the existing situation signals the urgent need to enforce the legislative framework at all levels to reduce and prevent illegal marketing and use of veterinary drugs. A similar failure to adequately regulate and control medicinal products for human use has already been reported in the country [31].
Although this study did not demonstrate significant variation in non-compliance between the two drugs, coupled with the high pressure on diminazene due to its short biological half-life and hence more frequent administration [32, 33], this loss of quality would mean a greater risk for the development of resistance. Various brands of trypanocides originating from several countries in Europe and Asia have shown different levels of compliance to drug quality casting doubt on the reputability of some of the manufacturers of veterinary products [27]. In this respect, the detection of non-compliant drugs within samples sourced from wholesalers strongly suggests that some of these drugs might be defective right from their origin and in such case unauthorized vendors in rural areas might have obtained them from legal importers/suppliers. On the other hand, since some of the brand names are identical between both authorized and unauthorised sources, it is also possible that counterfeit drugs are being sold carrying brand names of genuine manufacturers.
The impact of such quality defects in veterinary trypanocidal drug products can be very significant. A lack of efficacy will cause financial harm due to under dosage and residues in the muscle tissue damaging consumers when the concentration is above the prescribed limit [34, 35]. Some specialists [20] quite rightly believe that the circulation of counterfeit drugs in most sub-Saharan African countries inevitably leads to the persistence of animal diseases. This is proof of the urgent need to introduce systematic controls on the supply, distribution and utilization channels for veterinary trypanocidal products in Ethiopia.
Conclusion
Although the results obtained by this study may not represent trypanocidal drug use of the entire country, they clearly show the effects of poor quality veterinary trypanocidal drugs which are a result of the lack of a proper import control and country administration of veterinary controls in the study area and probably beyond. Unsafe utilization practices and poor handling of the drugs by farmers is also believed to aggravate the situation. Non-compliant trypanocidal drugs are known to compromise the efficacy of treatment and escalate the development of drug resistance, which has already been reported in different parts of Ethiopia. Corrective measures must therefore be taken towards effective implementation of regulatory and legislative frameworks, quality control of veterinary trypanocidal drugs and improving the coverage and quality of veterinary services and regular monitoring of trypanocidal drug efficacy.
Additional files
Questionnaire survey data: trypanocidal drug utilization practices, perception of risks of bovine trypanosomosis and efficacy of existing trypanocidal drugs. (DOCX 42 kb)
Acknowledgements
Special thanks go to the National Animal Health Diagnostic and Investigation Center of Ethiopia including Mr. Tesfaye Mulatu and Ms. Genet Bogale for their great assistance during data collection, LACOMEV in Senegal for drug quality analysis, and the CVMA of Addis Ababa University and Mr. Ayalew Zelelew of the District Bureau of Agriculture in Gurage Zone for their all rounded assistance.
Funding
This research was funded by the European Union through the European Commission-funded Trypanosomosis Rational Chemotherapy (TRYRAC) project, DCI-FOOD/2011/279–754. FAO’s assistance to this study was provided in the framework of the Programme against African Trypanosomosis (PAAT), and supported by the Government of Italy (Project ‘Improving food security in sub-Saharan Africa by supporting the progressive reduction of tsetse-transmitted trypanosomosis in the framework of the NEPAD’, codes GTFS/RAF/474/ITA and GCP/RAF/502/ITA).The European Commission and the FAO did not participate in the study process.
Availability of data and materials
The data sets of the current study are not publicly available due to the fact that they contain trypanocidal drugs brand and their manufacturing company names. However, they can be made available from the corresponding author on reasonable request.
Abbreviations
- AAT
African animal trypanosomosis
- DA
Diminazene aceturate
- FAO
Food and Agriculture Organization
- GALVmed
Global Alliance for Livestock Veterinary Medicine
- GDP
Gross domestic product
- HPLC
High performance liquid chromatography
- IAEA
International Atomic Energy Agency
- IFAH
International Federation for Animal Health
- ISM
Isometamidium
- LACOMEV
Laboratoire de Contrôle des Médicaments Vétérinaires
- TRYRAC
Trypanosomosis Rational Chemotherapy
Authors’ contributions
TT collected the data during field work analyzed and interpreted the results and drafted the manuscript. TG Developed a questionnaire, supervised the project and edited the manuscript. CT: Developed and supervised the project. HA Supervised the project and edited the manuscript. AKG and TAA analyzed drug samples in OIE lab in Dakar. CP-H and HAN developed the project and edited the manuscript. GG supported field data collection. MRC and PR facilitated laboratory analysis and revised the manuscript. MT analyzed the data and edited the manuscript. CG proof read the manuscript and constructed map of the study sites. VDAJ and DV developed the project and the study protocol, proof read the manuscript. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The objectives of this study were well explained to all selected farmers and those who expressed their consent to participate in the questionnaire survey were taken. The identity of study participants was kept confidential. Similarly, the identities of trypanocidal drug sources and company names remain confidential. The Research Ethics Review Committee (RERC) of the College of Veterinary Medicine and Agriculture of the Addis Ababa University (Ref. No. VM/ERC/05/07/06/2013) reviewed the study protocol. The committee waived the need for ethics approval and stated that the questionnaire survey part does not involve collecting information about the respondents and their wealth and hence does not require proper clearance.
Consent for publication
Not applicable
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12917-017-1327-6) contains supplementary material, which is available to authorized users.
Contributor Information
T. Tekle, Email: tilahunhgd@gmail.com
G. Terefe, Email: getachew_terefe@yahoo.com, Email: getachew.terefe@aau.edu.et
T. Cherenet, Email: thomascherenet@gmail.com
H. Ashenafi, Email: hagos83@yahoo.com
K. G. Akoda, Email: gilbert_akoda@yahoo.fr
A. Teko-Agbo, Email: tekoagbo2001@yahoo.fr
J. Van Den Abbeele, Email: jvdabbeele@itg.be
G. Gari, Email: Getachew.Gari@fao.org
P.-H. Clausen, Email: Clausen@fu-berlin.de
A. Hoppenheit, Email: Antje.Hoppenheit@fu-berlin.de
R. C. Mattioli, Email: Raffaele.Mattioli@fao.org
R. Peter, Email: rose.peter@galvmed.org
T. Marcotty, Email: tanguy.marcotty@gmail.com
G. Cecchi, Email: Giuliano.Cecchi@fao.org
V. Delespaux, Email: vdelespa@vub.ac.be
References
- 1.Central Statistical Agency. Agricultural sample survey. Report on livestock and livestock characteristics. 2013; Volume II and IV, Addis Ababa, Ethiopia.
- 2.Shaw APM, Cecchi G, Wint GRW, Mattioli RC, Robinson TP. Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in eastern Africa. Prev Vet Med. 2014;113:197–210. doi: 10.1016/j.prevetmed.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Holmes PH, Eisler MC, Geerts S. Current chemotherapy of animal trypanosomiasis. In: Maudlin I, Holmes PH, Miles MA, editors. The trypanosomiasis. Wallingford, United Kingdom, CABI Publishing, 2004. p. 431.
- 4.Meyer A, Holt HR, Selby R, Guitian J. Past and ongoing tsetse and animal trypanosomiasis control operations in five African countries: a systematic review. PLoS Negl Trop Dis. 2016;10:e0005247. doi: 10.1371/journal.pntd.0005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AP, Wint GR, Cecchi G, Torr SJ, Mattioli RC, Robinson TP. Mapping the benefit-cost ratios of interventions against bovine trypanosomosis in eastern Africa. Prev Vet Med. 2015;122:406–416. doi: 10.1016/j.prevetmed.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Giordani F, Morrison LJ, Rowan TG, De Koning HP, Barrett MP. The animal trypanosomiases and their chemotherapy: a review. Parasitology. 2016;143:1862–1889. doi: 10.1017/S0031182016001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimelis D, Getachew T, Getachew A, Dave Barry D, McCulloch R, Goddeeris B. In vivo experimental drug resistance study in Trypanosoma vivax isolates from tsetse infested and non-tsetse infested areas of Northwest Ethiopia. Acta Trop. 2015;146:95–100. doi: 10.1016/j.actatropica.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moti Y, Fikru R, Van Den Abbeele J, Buscher P, Duchateau L, Delespaux V. Gibe river basin in Ethiopia: present situation of trypanocidal drug resistance in T.congolense using tests in mice and PCR-RFLP. Vet Parasitol. 2012;189:197–203. doi: 10.1016/j.vetpar.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Desalegn WY, Etsay K, Getachew A. Study on the assessment of drug resistance on Trypanosoma vivax in Selemti wereda, Tigray, Ethiopia. Ethiop Vet J. 2010;14:15–30. [Google Scholar]
- 10.Shimelis D, Sangwan AK, Getachew A. Assessment of trypanocidal drug resistance in cattle of the Abay (Blue Nile) basin areas of north West Ethiopia. Ethiop Vet J. 2008;12:45–59. [Google Scholar]
- 11.Tewelde N, Abebe G, Eisler MC, Mcdermott J, Grainer M, Afework Y, et al. Application of field methods to assess isometamidium resistance of trypanosomes in cattle in western Ethiopia. Acta Trop. 2004;90:163–170. doi: 10.1016/j.actatropica.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Afework Y, Clausen PH, Abebe G, Tilahun G, Mehlitz D. Multiple drug resistant Trypanosoma congolense population in village cattle of Metekel district, north-West Ethiopia. Acta Trop. 2000;76:231–238. doi: 10.1016/S0001-706X(00)00108-X. [DOI] [PubMed] [Google Scholar]
- 13.Van Den Bossche P, Doran M, Connor RJ. An analysis of trypanocidal drug use in Eastern Province of Zambia. Acta Trop. 2000;75:247–258. doi: 10.1016/S0001-706X(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 14.Uilenberg G. A field guide for the diagnosis, treatment and prevention of African animal trypanosomosis. Rome: Food and Agriculture Organization of the United Nations; 1998. p. 158. [Google Scholar]
- 15.Geerts S, Holmes PH, Eisler MC, Diall O. African bovine trypanosomosis: the problem of drug resistance. Trends Parasitol. 2001;17:25–28. doi: 10.1016/S1471-4922(00)01827-4. [DOI] [PubMed] [Google Scholar]
- 16.Mungube EO, Vitouley HS, Allegye-Cudjoe E, Diall O, Boucoum Z, Diarra B, et al. Detection of multiple drug resistant Trypanosoma congolense populations in village cattle of south east Mali. Parasit Vectors. 2012;5:155. doi: 10.1186/1756-3305-5-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geerts S, Holmes PH. Drug management and parasite resistance in bovine trypanosomiasis in Africa. PAAT Technical Science Series, No. 1, FAO, Rome, 1998. p.31.
- 18.Cecchi G, Paone M, Argiles Herrero R, Vreysen MJ, Mattioli RC. Developing a continental atlas of the distribution and trypanosomal infection of tsetse flies (Glossina species) Parasit Vectors. 2015;8:284. doi: 10.1186/s13071-015-0898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutcliffe OB, Skellern GG, Araya F, Cannavan A, Sasanya JJ, Dungu B, et al. Animal trypanosomosis: making quality control of trypanocidal drugs possible. RevSci Tech Int des Epizoot. 2014;33:1–42. doi: 10.20506/rst.33.3.2320. [DOI] [PubMed] [Google Scholar]
- 20.Tettey J, Chizyuka G, Atsriku C, Slingenbergh J. Non-conformance of diminazene preparations to manufacture's label claims: an extra factor in the development of parasite resistance? ICPTV Newsl. 2002;5:24–25. [Google Scholar]
- 21.Diall O, Cecchi G, Wanda G, Argilés-Herrero R, MJB V, Cattoli G, et al. Opinion: developing a progressive control pathway for African animal trypanosomosis. Trends Parasitol. 2017;33:499–509. doi: 10.1016/j.pt.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Zewdu S, Getachew T, Hagos A. Farmers’ perception of impacts of bovine trypanosomosis and tsetse fly in selected districts in Baro-Akobo and Gojeb river basins. South-western Ethiopia BMC Vet Res. 2013;9:214. doi: 10.1186/1746-6148-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clausen P-H, Bauer B, Zessin KH, Diall O, Bocoum Z, Sidibe I, et al. Preventing and containing trypanocide resistance in the cotton zone of West Africa. Transbound Emerg Dis. 2010;57:28–32. doi: 10.1111/j.1865-1682.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 24.Assoumy AM, Teko-Agbo A, Akoda K, Niang EMM, Oulai J. Qualité pharmaceutique des médicaments vétérinaires en Côte d’Ivoire: cas du district d'Abidjan. Rev Africaine Santé Prod Anim. 2010;8:149–153. [Google Scholar]
- 25.Teko-Agbo A, Akoda K, Assoumy AM, Kadja MC, Niang EMM, Messomo Ndjana F, et al. Qualité des médicaments vétérinaires en circulation au Cameroun et au Sénégal. Dakar Med. 2009;54:226–234. [Google Scholar]
- 26.Akoda K, Walbadet L, Niang EMM, Teko-Agbo A. Qualité des médicaments vétérinaires en circulation au Sénégal. Rev Africaine Santé Prod Anim. 2008;6:29–33. [Google Scholar]
- 27.Tchamdja E, Kulo AE, Akoda K, Teko Agbo A, Assoumi AM, Niang E, et al. Drug quality analysis through high performance liquid chromatography of isometamidium chloride hydrochloride and diminazene diaceturate purchased from official and unofficial sources in northern Togo. Prev Vet Med. 2016;126:151–158. doi: 10.1016/j.prevetmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Teko-Agbo A, Assoumy AM, Akoda K, EMM N, PLJ BH. Qualité pharmaceutique des médicaments antiparasitaires vétérinaires au Burkina-Faso. Rev Africaine Santé Prod Anim. 2011;9:3–7. [Google Scholar]
- 29.Abiola FA. Qualité des anthelminthiques et des trypanocides au Mali (étude préliminaire par sondage limité). Rapport d’expertise. Dakar: EISMV; 2002. [Google Scholar]
- 30.Federal Democratic Republic of Ethiopia. A proclamation to provide for veterinary drug and feed administration and control, Proclamation No. 728/2011. Federal Negarit Gazeta, Addis Ababa. 2012. 18th year, No. 14.
- 31.Suleman S, Woliyin A, Woldemichael K, Tushune K, Duchateau L, Degroote A, et al. Pharmaceutical regulatory framework in Ethiopia: a critical evaluation of its legal basis and implementation. Ethiop J Health Sci. 2016;26:259–276. doi: 10.4314/ejhs.v26i3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur G, Chaudhary RK, Srivastava AK. Pharmacokinetics, urinary excretion and dosage regimen of diminazene in cross bred calves. Acta Vet Hung. 2000;48:187–192. [PubMed] [Google Scholar]
- 33.Eisler MC, Maruta J, Nquindi J, Connor RJ, Ushewokunze-Obatolu U, Holmes PH, et al. Isometamidium concentrations in the sera of cattle maintained under a chemo-prophylactic regime in a tsetse-infested area of Zimbabwe. Tropical Med Int Health. 1996;1:535–541. doi: 10.1046/j.1365-3156.1996.d01-87.x. [DOI] [PubMed] [Google Scholar]
- 34.Monteiro AM. Pharmaceutical quality of veterinary anthelminthics sold in Kenya. Vet Rec. 1998;142:392–398. doi: 10.1136/vr.142.15.396. [DOI] [PubMed] [Google Scholar]
- 35.Boisseau J. Contrôle de la qualité pharmaceutique des médicaments vétérinaires. In: Séminaire sur le contrôle de la qualité des médicaments vétérinaires Niamey, 8–12 December 1997. Paris: OIE. p. 1997, 93.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questionnaire survey data: trypanocidal drug utilization practices, perception of risks of bovine trypanosomosis and efficacy of existing trypanocidal drugs. (DOCX 42 kb)
Data Availability Statement
The data sets of the current study are not publicly available due to the fact that they contain trypanocidal drugs brand and their manufacturing company names. However, they can be made available from the corresponding author on reasonable request.


