Abstract
In mice, ejaculated semen is deposited in the uterus. After ejaculation, the semen changes consistency from gel-like to watery, a process called liquefaction. In this study, we show how to collect the post-ejaculated semen from the female reproductive tract in a mouse model. First, adult female mice in the estrus stage were housed in a male's cage overnight. The next morning, copulation was confirmed by the presence of copulatory plug at the vaginal opening. Female mice with copulatory plugs were euthanized, and each reproductive tract was collected as a whole (vagina, uterus, oviducts, ovaries), ensuring a closed system to contain the semen. The reproductive tract was placed in a 1.5 mL microcentrifuge tube, and the vagina was cut off to release the semen into the tube. To ensure maximum semen volume for analysis, toothless forceps were used to squeeze the uterine horns from ovarian end to vaginal end expelling remaining semen. The whole reproductive tract was then discarded. The semen-containing tube was briefly spun down. A 25 μL capillary pipette was placed into the tube at a 180° angle (parallel to the tube wall). The amount of time used to fill the capillary tube to the 25 μL line was recorded. Semen from a proven male breeder usually takes approximately 60-180 s to fill a 25 μL capillary tube. This semen collection technique can also be used in other downstream applications such as sperm imaging and motility analysis.
Keywords: Developmental Biology, Issue 129, uterus, post-ejaculation, semen viscosity, liquefaction, sperm transport, mouse, female reproductive tract, capillary tube, sperm motility
Introduction
The female reproductive tract is comprised of the upper and lower tracts. The upper reproductive tract includes Fallopian tubes, the uterus, and the endocervix. The lower reproductive tract includes the ectocervix and the vagina. In humans, the ejaculated semen is deposited at the anterior wall of the vagina, adjacent to the ectocervix1. In mice, however, the semen is swept into the uterus within minutes after mating2.
Semen contains seminal gel that is solidified within seconds of ejaculation, causing the sperm to be entrapped and immobilized3. Liquefaction releases the immobilized sperm by changing semen from a gel-like to a watery consistency. This process is essential for sperm motility and mammalian reproduction. Knowledge of semen liquefaction is mostly based on in vitro studies where semen becomes liquefied in a culture dish. In humans, the enzymatic activity of prostate specific antigen or PSA (also known as kallikrein-related peptidase 3 or KLK3) is the major contributor for liquefaction through hydrolysis of semenogelins (gel-forming proteins present in the semen)4,5,6. Degradation of semenogelins liberates the sperm from the seminal coagulum, increasing sperm mobility. Freed sperm swim toward the Fallopian tube to fertilize the egg. Liquefaction (or semen viscosity) tests are one of the standard initial screenings for semen analysis in fertility clinics to assess male fertility7.
A recently published article shows that analysis of seminal fluid collected from the female mouse reproductive tract post-mating can be used to illustrate a deficiency in liquefaction that can subsequently cause fertility defects8. Defective liquefaction such as semen hyperviscosity contributes to 11.8-32.3% of infertile cases in men9, suggesting that normal liquefaction is indispensable for mammalian reproduction. However, studies and treatment of liquefaction defects have focused exclusively on the male7. The possibility that the female reproductive tract plays a role in semen liquefaction has not been explored. Therefore, the methods for semen collection and measuring liquefaction time will provide researchers a novel diagnostic tool to determine whether liquefaction could be one of the causes of infertility in the model organism of interest.
Protocol
All animals and procedures used in this study were handled according to Washington State University (WSU) Animal Care and Use Committee guidelines and in compliance with WSU-approved animal protocols #4702 and #4735.
1. Mice
- Use standard C57BL/6J adult male mice or other strains of interest. Maintain animals under a temperature- and humidity-controlled environment, with ad libitum access of water and food.
- Use male breeders ranging from 8 weeks old to 10 months old. Use only proven breeders in the experiments.
- Use adult female mice with a tissue selective deletion of reproductive tract estrogen receptor α (encoded by Esr1 gene) in the epithelial cells10 (Wnt7aCre/+;Esr1f/f, called knockout or "KO") and control littermates (Esr1f/f, called "CTRL") in this study.
- Use 8 week-old females. Ages of females varied depending on the experiment of interest.
Determine the stages of the estrous cycle in the morning at 08:00h. Use only females at the estrus stage of the cycle. For detailed methods of vaginal smearing and cytological analysis, refer to the previously published procedures11.
2. Mating and Assessing Copulatory Plugs
At the day of estrus (16:00h), house the female in a male's cage.
The next morning (08:00h), separate the female from the male mouse.
Use an adaptation of a previously published method12 to access the presence or absence of a vaginal or copulatory plug as an indication of mating.
Hold the female mouse from the base of the tail to briefly lift the hind limbs.
Find the copulatory plug by the presence of an off-white seminal coagulum at the vaginal opening using a toothpick. If the copulatory plug is present, the toothpick will not be able to be inserted into the vaginal canal.
3. Preparation Before Tissue Collection
Prepare 10 mL of Leibovitz's L-15 media, supplemented with 1% fetal bovine serum (called L15 media + 1% FBS) in a 15 mL tube. Incubate the media at 37 °C for 15 min in a waterbath.
In order to preserve the viability of the tissue and sperm for downstream applications, aliquot 2 mL of pre-warmed L15 media + 1% FBS in a 2 mL microcentrifuge tube for tissue collection.
Warm the stage of the stereomicroscope to 37 °C. Set the heat-block to 37 °C and place an empty 1.5 mL microcentrifuge tube in the block.
If the downstream applications require imaging of sperm motility, warm the glass slide to 37 °C.
4. Tissue Collection
Bring pre-warmed L15 media + 1% FBS aliquot to the tissue collection area.
Euthanize the female (~08:30h) using carbon dioxide asphyxiation followed by cervical dislocation. NOTE: This is approximately 8 h after mating, assuming that mating takes place at midnight.
Place the animal in a supine position to expose the abdominal area.
Spray 70% alcohol on the abdomen near hind limbs.
Use the dissecting scissors to make a small cut (~1 cm) on the skin of the lower abdomen.
Use the dominant hand to pull the tail (and hind limbs if necessary) while non-dominant hand pulls the skin in the opposite direction (toward the head) to expose the abdominal muscle. This method is used to minimize the amount of hair contamination to the visceral organs.
Cut the abdominal muscle in a V-shape at the base of the abdomen, adjacent to the vagina, to expose the visceral organs.
Move intestines and abdominal fat to the side to expose the reproductive tract.
Trim off bladder and other tissues above the vaginal area.
Cut pubic symphysis apart to gain access to the vaginal tissue.
Lift the vaginal tissue at the vaginal opening and using scissors to separate the vaginal canal from the rectal tissue.
Carefully trim off the mesenteric fat from both sides of the uterus using spring scissors. CAUTION: The uterus is very fragile with the semen inside. Be careful not to puncture the uterus, or else the semen will be lost.
Lift the lower reproductive tract and cut the ovarian fat pad off of both sides.
Transfer the whole reproductive tract into the tube of pre-warmed Leibovitz's L-15 media + 1% FBS.
5. Measurement of Semen Liquefaction (Viscosity) from Uterine Content Collection
On the heated stage of the stereomicroscope, transfer the tissues into a 35 mm sterile tissue culture dish filled with 3.5 mL pre-warmed L-15 media + 1% FBS to clean the tissues of blood and other contaminants. CAUTION: While washing, grab the tissues by the fat, not the uterus. At this point, the semen is expected be intact inside the uterus.
Blot the tissues of excess media using laboratory wipes.
Hold the tissue at the vaginal end while placing the ovarian ends in the pre-heated microcentrifuge tube. While holding the vaginal end, cut the uterine horns at the base of the uterus, allowing the uterine horns to fall into the microcentrifuge tube.
Let the semen flow out of the uterine horns into the tube. Use toothless forceps to gently squeeze the uterine horns from the ovarian end to the vaginal end, expelling out leftover semen.
Discard the tissues in biohazard bag and incinerate.
Briefly spin down the semen in a minicentrifuge (~1-2 s).
Lay the tube, parallel to the heated stage - semen will not spill out.
Have the timer ready and set to 'count up'.
Insert a 25 µL capillary tube into the semen sample at a 180° angle (parallel to the tube wall). This is to minimize the variability between each investigator from holding the capillary tube at different angles.
Press the timer as soon as the capillary tube makes contact with the semen.
Record the time (s) it takes for the semen to reach the 25 µL line.
When the measurement is finished, immediately put the tube containing semen back in the dry heat block for further functional analysis, such as video imaging of sperm motility.
Disinfect the capillary tube containing semen by immersing the tube in 10% bleach solution for 20 min and discard the capillary tube in the sharps container.
6. Video Imaging of Sperm Motility
Turn on the microscope and video imaging software.
Pipette 20 µL of the remaining semen onto the pre-warmed glass slide, while the glass slide is placed on the heated stage.
Cover with a glass coverslip.
Place the slide coverslip side down on the microscope.
Use 20X objective lens to find area of interest.
Change to 100X objective lens for video imaging.
Record the video at 12 frames/s (fps) for 30 s.
Randomly record the videos in at least 3 different areas per animal.
Perform the imaging quickly and minimize light exposure to the slide in order to preserve sperm motility and viability. NOTE: This imaging procedure should be done within 2-3 min.
Perform the data analysis using ImageJ software as previously described8.
Representative Results
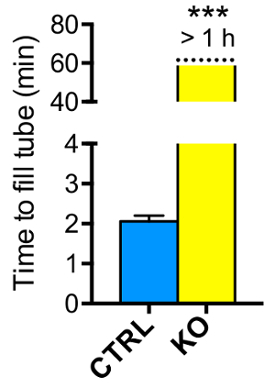
Semen was collected from CTRL and KO females approximately 8 h after mating with fertile CTRL males. Liquefaction (or semen viscosity) is quantified by the time taken to fill 25 μL capillary tube with semen. Semen collected from CTRL uteri took 2.06 ±0.12 min (mean±S.E.M, n = 5) to fill the capillary tube (Figure 1). However, the semen collected from KO uteri took longer than 60 min (60.0 ±0.0, mean±S.E.M, n = 5) of experimental time. These data suggest that the semen is significantly more liquefied or less viscous in the CTRL compared to KO uteri.
To illustrate one of the downstream applications of collecting semen from the female reproductive tract, sperm motility was assessed using video imaging. The semen collected from the CTRL uteri showed freely-swimming sperm within the microscopic field, represented in the snapshot in Figure 2A. The semen collected from the KO uteri showed that the majority of the sperm were clustered together with minimal mobility (Figure 2B). These findings indicate that the semen collected from the female reproductive tract 8 h after mating can be used for further sperm analysis.
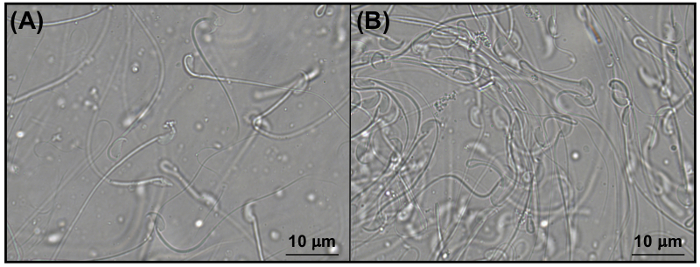
Figure 1: Liquefaction time (semen viscosity) from the semen collected from mouse uteri. Graphical representation of the liquefaction time (min) of semen collected from CTRL and KO uteri approximately 8 h after mating. Data represent mean±S.E.M., n = 5 females/genotype. ***p <0.001, unpaired Student t-test.Adapted from previously published results8 with permission. Please click here to view a larger version of this figure.
Figure 2: Representative images of sperm within the semen collected from mouse uteri. Representative snapshots from the video imaging taken at 1000X magnification of sperm in the semen collected from (A) CTRL and (B) KO uteri. Adapted from previously published results8 with permission. Please click here to view a larger version of this figure.
Discussion
Collecting semen from mouse uteri can provide advantages over semen analysis in vitro as the former can illustrate the physiological interactions between the semen and the secretions from the female reproductive tract. The techniques presented in this study allow researchers to determine semen quality as well as sperm viability and motility in ideal physiological conditions. Moreover, there are various downstream analyses that can be performed using the semen collected from the uteri, for example, the sperm can be counted to determine the actual sperm number entering the uterus and the sperm can also be imaged for the motility assays. The ideal time point to collect the semen for fertilization capability should be within 1 to 2 h after mating1.
In addition to the previously mentioned downstream applications, investigators can also evaluate the effects of inhibitors/compounds of interest on sperm transport processes, such as semen liquefaction (or semen viscosity), sperm number in the tract, sperm motility, or sperm migration to different areas of the female reproductive tract. The compounds can be applied in the female reproductive tract prior to mating8. Then, the impact of those inhibitors/compounds can be assessed from the semen sample post-mating. These methods can be useful to test the practicality of contraceptive drugs that inhibit sperm transport.
Crucial points of consideration for semen collection and liquefaction/viscosity measurements are the sensitivity of the sperm and the angle of the capillary tube. Sperm are sensitive to temperature change. If downstream analysis of sperm function and motility is essential for experimental outcomes, the tissue and semen need to be kept at 37 °C at all times, with the minimal exposure to light. If there are several samples to collect from multiple females, euthanize the animals one at a time to minimize wait. For the liquefaction measurement, different angles of the capillary tube generate inconsistencies in liquefaction time. Therefore, the investigators have to hold the capillary tube parallel to the tube wall (at a 180° angle) to minimize the variability created by each investigator. Semen sample should not be collected later than 09:00h, this is due to the fluid reabsorption in the uterus. The investigator may not obtain sufficient fluid material for the liquefaction measurement.
It is established that the semen liquefaction is mainly mediated by the enzymatic activity of PSA secreted from the prostate gland13. However, there are limited studies to determine the roles of female reproductive tract during in vivo liquefaction process8. Therefore, collection of the semen from the female reproductive tract provides a crucial advantage to study the liquefaction processes in vivo, after the semen has been exposed to secretions from the female reproductive tract14. In conclusion, post-ejaculated semen liquefaction/viscosity measurement allows the researchers to decipher the interplay between semen and the female reproductive tract.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is supported by College of Veterinary Medicine (WSU) start-up fund to WW. The authors thank Sierra Olsen (WSU) for critical reading of this manuscript.
References
- Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12(1):23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Bedford JM, Yanagimachi R. Initiation of sperm motility after mating in the rat and hamster. J Androl. 1992;13(5):444–449. [PubMed] [Google Scholar]
- Lilja H. Cell biology of semenogelin. Andrologia. 1990;22 Suppl 1:132–141. doi: 10.1111/j.1439-0272.1990.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Lilja H, Abrahamsson PA, Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem. 1989;264(3):1894–1900. [PubMed] [Google Scholar]
- Veveris-Lowe TL, Kruger SJ, Walsh T, Gardiner RA, Clements JA. Seminal fluid characterization for male fertility and prostate cancer: kallikrein-related serine proteases and whole proteome approaches. Semin Thromb Hemost. 2007. pp. 87–99. [DOI] [PubMed]
- Emami N, Deperthes D, Malm J, Diamandis EP. Major role of human KLK14 in seminal clot liquefaction. J Biol Chem. 2008;283(28):19561–19569. doi: 10.1074/jbc.M801194200. [DOI] [PubMed] [Google Scholar]
- Gerhard I, Roth B, Eggert-Kruse W, Runnebaum B. Effects of kallikrein on sperm motility, capillary tube test, and pregnancy rate in an AIH program. Arch Androl. 1990;24(2):129–145. doi: 10.3109/01485019008986873. [DOI] [PubMed] [Google Scholar]
- Li S, Garcia M, Gewiss RL, Winuthayanon W. Crucial role of estrogen for the mammalian female in regulating semen coagulation and liquefaction in vivo. PLoS Genet. 2017;13(4):e1006743. doi: 10.1371/journal.pgen.1006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, et al. Human semen hyperviscosity: prevalence, pathogenesis and therapeutic aspects. Asian J Androl. 2009;11(5):609–615. doi: 10.1038/aja.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107(45):19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012. p. e4389. [DOI] [PMC free article] [PubMed]
- JoVE Science Education Database. Lab Animal Research. Fundamentals of Breeding and Weaning. JoVE. 2017. Cambridge, MA.
- Prassas I, Eissa A, Poda G, Diamandis EP. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat Rev Drug Discov. 2015;14(3):183–202. doi: 10.1038/nrd4534. [DOI] [PubMed] [Google Scholar]
- Muytjens CM, Vasiliou SK, Oikonomopoulou K, Prassas I, Diamandis EP. Putative functions of tissue kallikrein-related peptidases in vaginal fluid. Nat Rev Urol. 2016;13(10):596–607. doi: 10.1038/nrurol.2016.161. [DOI] [PubMed] [Google Scholar]


