Abstract
This protocol describes a procedure to assist surgeons in training for the implantation of microelectrode arrays into the neocortex of the human brain. Recent technological progress has enabled the fabrication of microelectrode arrays that allow recording the activity of multiple individual neurons in the neocortex of the human brain. These arrays have the potential to bring unique insight onto the neuronal correlates of cerebral function in health and disease. Furthermore, the identification and decoding of volitional neuronal activity opens the possibility to establish brain-computer interfaces, and thus might help restore lost neurological functions. The implantation of neocortical microelectrode arrays is an invasive procedure requiring a supra-centimetric craniotomy and the exposure of the cortical surface; thus, the procedure must be performed by an adequately trained neurosurgeon. In order to provide an opportunity for surgical training, we designed a procedure based on a human cadaver model. The use of a formaldehyde-fixed human cadaver bypasses the practical, ethical and financial difficulties of surgical practice on animals (especially non-human primates) while preserving the macroscopic structure of the head, skull, meninges and cerebral surface and allowing realistic, operating room-like positioning and instrumentation. Furthermore, the use of a human cadaver is closer to clinical daily practice than any non-human model. The major drawbacks of the cadaveric simulation are the absence of cerebral pulsation and of blood and cerebrospinal fluid circulation. We suggest that a formaldehyde-fixed human cadaver model is an adequate, practical and cost-effective approach to ensure proper surgical training before implanting microelectrode arrays in the living human neocortex.
Keywords: Medicine, Issue 129, Neurosurgery, Microelectrode arrays, Brain-Computer Interfaces, Surgical Training, Human Cadaver Model, Formaldehyde Fixation
Introduction
Recent years have seen the development of technological solutions to the challenge of recording the activity of multiple individual neurons in the living brain1,2,3. Silicon-based microelectrode arrays perform similarly to conventional wire microelectrodes in terms of signal properties, and they can record from dozens to hundreds of neurons in a small patch of cerebral tissue4,5,6,7. Microelectrode arrays have allowed scientists to establish the correspondence between neural activity in the primary motor cortex of monkeys and arm movements8, which in turn has provided a boost to the development of brain-computer interfaces (BCIs)9.
Microelectrode arrays have been used in humans in two situations: as chronic implants to control BCIs and as semi-chronic implants to study the activity of individual neurons in patients suffering from epilepsy. Chronic implants, targeting the functional representation of the hand in primary motor cortex, have allowed patients suffering from tetraplegia to control the motion of a robotic arm or of computer cursors10,11,12,13. Semi-chronic implants, inserted together with subdural electrocorticography (ECOG) electrodes in patients with drug-resistant epilepsy who are candidates for epilepsy surgery14, allow single-unit recordings before, during and after seizures, and have begun to shed light on the activity of single neurons during and in between epileptic seizures15,16,17,18,19. Microelectrode arrays have the potential to significantly improve our understanding of how the brain functions by establishing a link between the activity of neurons, on the one hand, and the perceptions, movements and thoughts of human beings, both in health and in disease, on the other20,21.
Silicon-based microelectrode arrays are now available commercially and their use in humans has been approved by regulatory authorities in the USA in the semi-chronic epilepsy indication. However, these devices are invasive and must be inserted into the brain. The negative consequences of improper insertion technique, beyond the failure of the device to record neuronal activity, include cerebral hemorrhage and infection, with the potential for long-lasting or permanent neurological dysfunction. Although the complication rate of microelectrode array implantation is currently unknown, the rate of potentially serious complications during the implantation of intracranial electroencephalography (EEG) macroelectrodes is 1-5%22,23. Therefore, the proper implantation of microelectrode arrays requires both extensive neurosurgical skills and procedure-specific training.
The approaches available for surgeons to hone their skills with microelectrode arrays in a safe environment include non-human mammals and human cadavers. The ideal training model would faithfully reproduce the size and thickness of the human skull; the toughness and vascular ramification of the dura; the gyrification pattern, consistency and pulsation of the human brain; the presence of circulating blood and cerebrospinal fluid; and the overall positioning of the subject in an operating room (OR)-like environment. Thus, animal models need to be of a sufficient size to provide a meaningful experience to the surgeons. Large non-human primates come closest, but their use for surgical training is unsustainable both from an ethical perspective and because they are expensive. Rodents do not enter consideration because of their small size; using even cats or rabbits implies diverging significantly from an OR-like environment.
Human cadavers represent an attractive alternative. Their advantages include the life-like size and shape of the head and brain and the possibility of setting up surgical training in an OR-like environment. The most obvious departures from a realistic situation are the absence of cerebral pulsations and bleeding and the modifications in the aspect and consistence of body tissues that are specific to the technique employed for cadaver preservation24. Fresh-frozen cadavers preserve the consistency and flexibility of many organs and tissues to some extent, but they have several drawbacks: they start degrading as soon as thawing begins, so that the brain becomes too degraded for the insertion of a microelectrode array to be performed realistically, and they are a relatively rare and expensive resource. Formaldehyde-fixed cadavers, on the other hand, are more affordable and available and much more durable, at the expense of hardened tissue consistency.
Here, we establish a procedure using a formaldehyde-fixed human cadaver model to provide neurosurgical training for the implantation of a neocortical microelectrode array. Our approach allows realistic, OR-like positioning and instrumentation; performing craniotomy and durotomy and exposing the neocortical surface; attaching the electrode pedestal to the skull bone neighboring the craniotomy; and inserting the microelectrode array into the neocortex with a pneumatic impactor25. Critically, it enables surgeons to practice the precise alignment of the microelectrode array (which is connected to the electrode pedestal by a bundle of individually insulated gold wires) parallel to the neocortical surface26. Our protocol faithfully replicates the indication of microelectrode array implantation together with ECOG implantation in patients who are candidates for epilepsy surgery. The particulars of the implantation surgery are influenced significantly by the exact type of microelectrode array; here, we describe the procedure for an array that recently received regulatory approval for use in humans in the USA. The so-called Utah array comprises a 4x4 mm, 100 microelectrode grid; a transcutaneous pedestal that is attached to the external table of the skull; and a wire bundle connecting the two.
Protocol
The human cadaver used in this work was provided under the framework of body donations for medical education. Informed consent for body donation was obtained in writing during the lifetime of the donor. In accordance with the federal and cantonal laws, no review by an ethics committee was necessary.
Note: This protocol assumes that the persons performing the practice surgery are neurosurgeons with training and expertise in standard neurosurgical procedures, including patient positioning and head fixation, craniotomy and durotomy, and suturing. In addition to the tools and equipment specific to the microelectrode array, standard neurosurgical tools and equipment are used.
1. Selection of the cadaver and setup of the operating room
- Select a specimen with no history of disease or injury to the head, skull and brain.
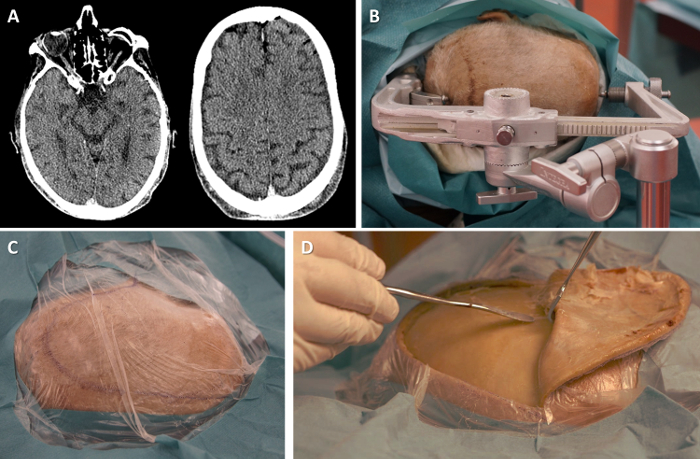
- Optionally, perform a computed tomography (CT) scan of the cadaver's head to ensure that there is no significant intracranial lesion (Figure 1A), e.g. chronic subdural hematoma or an intra-axial expansive lesion. Using the CT scan, identify a target cortical area for the implantation of the microelectrode array (such as the "hand knob" area of the precentral gyrus, corresponding to the representation of the hand in the primary motor cortex27, in the case of training for the implantation of a BCI).
Position cadaver in lateral decubitus on an operating table. Use an operating table rather than a dissection table in order to add to the realism of the OR-like environment and facilitates the fixation of the skull clamp and pneumatic impactor. Position the cadaver in lateral decubitus in order to allow the fronto-temporal approach in a formaldehyde-fixed cadaver, in whom neck rotation is limited.
- Fix the head in the skull clamp (Figure 1B). Cover with surgical drapes (Figure 1C). Note: In our case, the posterior pins of skull clamp are unusually positioned in the sagittal plane of the head (see Figure 1B), because we used a skull clamp that had been modified for surgical training purposes to hold a cadaveric head separated from the rest of the body.
- When using a standard skull clamp on an operating table, place the posterior pins securing the head perpendicular to the sagittal plane.
2. Exposure of the neocortical surface
Incise the scalp using a scalpel, following a question-mark incision to expose the temporal and frontal bones. Dissect the temporalis muscle along the posterior edge of the incision. Recline the scalp and temporalis muscle by blunt dissection (Figure 1D).
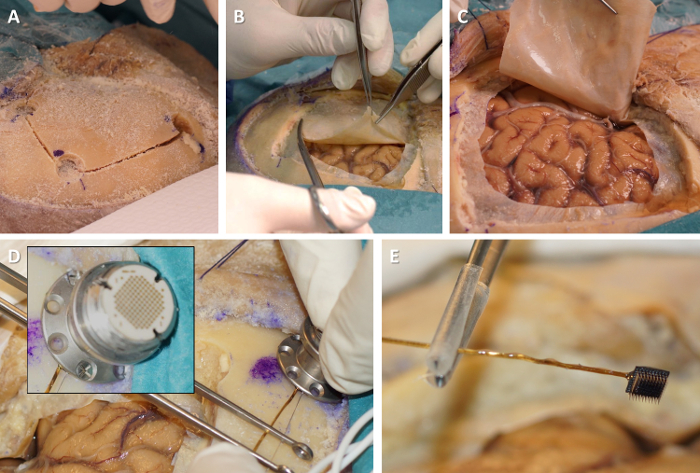
Perform a large square fronto-temporal craniotomy, e.g. 5 x 5 cm (Figure 2A). For that purpose, drill four burr holes at the corners of the intended craniotomy. Then, use the craniotome to connect the burr holes. Remove the bone flap using a spatula, exposing the dura mater. Store the bone flap in saline solution.
Open the dura mater on three sides of the craniotomy using dura scissors (Figure 2B). Recline it and expose the arachnoid membrane and the surface of the cerebral neocortex (Figure 2C).
3. Fixation of the electrode pedestal
Select a cortical gyrus where the microelectrode array will be implanted. Select a gyral surface that is approximately flat so that the microelectrode array will lie flush with it when inserted. Ensure that there is no visible blood vessel coursing on the cortical surface where the microelectrode array will be inserted.
- Select a site for the fixation of the electrode pedestal on the superior edge of the craniotomy, close to the skin incision, and allowing sufficient slack for the wire bundle so that the microelectrode array can reach the target gyrus. Screw the pedestal onto the external table of the skull bone next to the craniotomy (Figure 2D). Use 6 to 8 self-tapping cortical bone screws (6 mm length, 2 mm diameter) to ensure appropriate fixation.
- When manipulating the pedestal, always ensure that the microelectrode array does not touch anything (it may be damaged or could lacerate the neocortical surface) by holding the wire bundle close to the microelectrode array with tweezers with plastic- or rubber-coated tips (Figure 2E).
4. Positioning and insertion of the microelectrode array
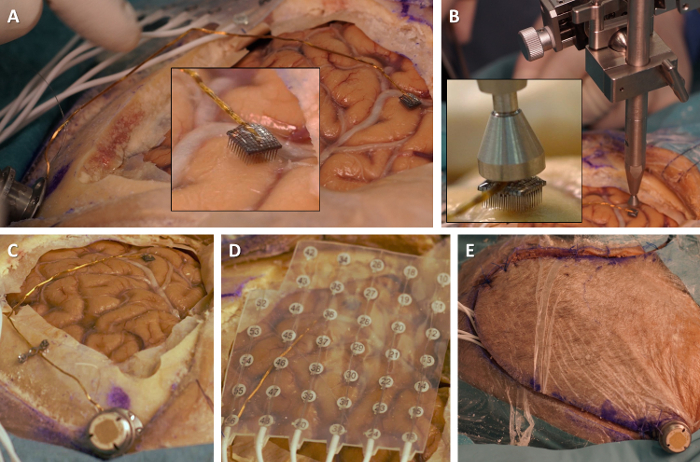
- Position the microelectrode array parallel with the surface of the target gyrus. Bend the wire bundle as necessary for that purpose (Figure 3A). Note: The stiff wire bundle does not easily conform to the surgeon's wishes. Care and patience are required to obtain good alignment of the microelectrode array and cortical surface.
- Optionally, use "dog-bone" titanium straps to secure the wire bundle to the skull and control its course toward the target gyrus. Do not screw the strap too tightly to avoid damaging the wire bundle.
Bring the pneumatic impactor into approximate alignment with the back of the microelectrode array (Figure 3B). Control the connections of the pneumatic impactor to its control box, and then turn on the control box. Note: Make sure that the pneumatic impactor is at least 5 mm away from the array before turning the control box on, as the pneumatic impactor might be triggered when first turned on.
Use the millimetric screws of the pneumatic impactor holder to refine the impactor's alignment with the back of the microelectrode array (Figure 3B, inset). Using the impactor, apply an excursion distance- and pressure-controlled tap to the back of the microelectrode array and insert it into the cortical surface, pushing it through the arachnoid membrane. Note: Check that the microelectrode array is flush with the cortical surface.
5. Positioning of the subdural ECOG grid
Note: This step is optional.
Position a subdural ECOG grid over the exposed cortical surface (Figure 3D). If necessary, remove electrodes by cutting through the grid so that the overall shape of the ECOG grid fits the craniotomy.
Orient the ECOG grid so that its wires will exit the dura mater and skull superiorly or posteriorly.
Irrigate the ECOG grid with saline before placing it into contact with the cortical surface.
Secure the ECOG grid by suturing it to the dura mater at the edges of the durotomy.
6. Repositioning and closure of the dura mater, bone flap, and skin flap
Reflect the dura mater back over the exposed cortical surface and suture it to the edges of the durotomy.
Screw "dog-bone" titanium straps onto the edges of the bone flap using self-tapping cortical bone screws. Reposition the bone flap within the craniotomy. Secure the bone flap to neighboring skull bones with the "dog-bone" titanium straps and self-tapping cortical bone screws. Take care not to crush the wire bundle of the microelectrode array (and those of the optional ECOG grid) between bone edges.
- Reflect and suture the skin flap. Close the skin incision around the neck of the electrode pedestal (Figure 3E).
- Alternatively, allow the pedestal to egress the scalp through a separate stab incision made into the scalp flap.
Representative Results
Our protocol uses a formaldehyde-fixated human cadaver model to allow surgeons to practice the surgical procedure of implanting a microelectrode array into the cerebral neocortex in a realistic, OR-like environment. The option of performing post-mortem neuroimaging, such as head CT, will confirm the absence of any significant intracranial lesion (Figure 1A) and can help with the selection of the implantation site. Working with an entire specimen and setting up for surgery on an operating table increases the realism of the training procedure (Figure 1B-1C). Although formaldehyde fixation somewhat alters the color, texture and stiffness of the body tissues, each step of the surgical procedure to expose the neocortical surface (skin incision, craniotomy and durotomy) can be performed easily according to standard neurosurgical practice (Figure 1D and Figure 2A-2C).
The steps of the surgical procedure that are specific to the microelectrode array proceed very similarly to the in vivo situation. The first step consists of screwing the electrode pedestal to the skull bone near the craniotomy ( Figure 2D-2E). Bringing the microelectrode array into alignment with the neocortical surface is one of the most delicate steps of the procedure (Figure 3A)26. The positioning and operation of the pneumatic impactor are also performed in realistic fashion (Figure 3B). Our training protocol provides ample opportunity for surgeons to experiment with these crucial steps. One departure from lifelike realism is the absence of cerebral pulsation in a cadaver model (the slight upwards and downwards movements of the exposed neocortical surface caused by heartbeats and breathing). Nevertheless, the end result of the training protocol (Figure 3C-3E) closely reproduces the real-life situation26.
If performed by two surgeons, the average operative time for microelectrode array implantation is under 30 minutes, as also reported by others26.
Figure 1. Setting up the operating room-like environment. (A) Head CT scan can confirm the absence of any significant intracranial lesion. (B) Position the head in the skull clamp. (C) Drape the head. The specimen's nose is to the right of the picture, the occiput to the left. (D) Incise and recline scalp and temporalis muscle. Please click here to view a larger version of this figure.
Figure 2. Exposing the neocortical surface and attaching the electrode pedestal. (A) Perform a large square craniotomy. (B) Perform durotomy. (C) Reflect the dura mater and expose the neocortical surface. (D) Screw the electrode pedestal to the skull bone near the edge of the craniotomy (inset: close-up on the fixation of the pedestal with bone screws). (E) Hold the fragile microelectrode array with tweezers to avoid damage from unwanted contact. Please click here to view a larger version of this figure.
Figure 3. Positioning and inserting the microelectrode array. (A) Bend the wire bundle in order to bring the microelectrode array into alignment with the cortical surface (inset: close-up on the alignment of the microelectrode array and cortex). (B) Bring the pneumatic impactor into alignment with the back of the microelectrode array (inset: close-up on the alignment of the impactor and microelectrode array). (C) Overview of the microelectrode array, wire bundle and electrode pedestal. (D) Position ECOG grid over the cortical surface. (E) Close skin around the neck of the electrode pedestal. Please click here to view a larger version of this figure.
Discussion
The formaldehyde-fixed human cadaver model and the surgical protocol described here replicate the surgical procedure of implanting microelectrode arrays into the human cerebral neocortex. Each step of the procedure, including the positioning of the microelectrode array and its insertion with the pneumatic inserter, proceed in almost the same fashion as in a real-life patient, with the exception that cerebral pulsation and circulation are absent. The critical steps in the protocol are the alignment of the microelectrode array with the neocortical surface and its impaction into the cortex using the pneumatic inserter. Care must be taken to approximate the array as parallel to the cortical surface as possible. In the case that the array does not lie flush with the neocortical surface after the first tap of the pneumatic inserter, one additional tap can be delivered. Throughout the procedure, the microelectrode array should be protected from mechanical damage. In the case of implantation in a human patient in clinical conditions, if there is any visible damage to the microelectrodes, bundle or connector, the array should be discarded and another one used.
The Utah array is currently the only neocortical microelectrode array that has received regulatory approval for use in humans. However, other types of microelectrodes have been developed in animals and may be used in humans within specific research projects28. Each approach carries its own advantages and drawbacks, mostly related to the design of the electrodes. For instance, the ballistic insertion technique of the Utah array, which was developed out of necessity25, requires that the array be precisely aligned with the cortical surface; this requirement does not necessarily apply to other microelectrodes, which can be pushed gently into the gray matter. Some electrodes allow access to the activity of all cortical layers29, whereas the Utah array samples from neurons at a single, predetermined depth. One of the major advantages of the Utah array is the large number of neurons that can be recorded simultaneously, making it particularly appropriate for motor BCIs11.
For neurosurgical laboratory training courses, cadaveric specimens are considered as models of high value, allowing haptic feedback in an environment presenting specifically human anatomy30,31. There is no universal cadaver model, however, and the embalming technique must be adapted to each procedure's objectives: are soft tissues (such as the scalp) of importance, or rather the bones, dura mater, cortex, ventricles, or blood vessels32,33,34,35,36? Fresh or fresh-frozen (cryopreserved) specimens, while frequently considered as the best model for a variety of surgical procedures, carry the risk of transmitting infectious diseases. Furthermore, they have a very limited working time because of fast decay31,37,38,39, followed by decreased tissue compliance, ventricular collapse and pneumocephalus35. In the case of our protocol, maintaining a somewhat firm cortical surface was a requirement to enable the insertion of the microelectrode array, thus precluding the use of a fresh-frozen specimen. Embalming solutions providing long-term fixative and germicidal properties are also widely accepted30,33,35,40. Cadavers embalmed according to the Thiel fixation are highly regarded in terms of soft tissue consistency and for developing fascial or internervous planes36, but the preservation of the brain is thought to lack realism41. Formaldehyde-based fixation causes tissue stiffening and retraction as well as discoloration35,36,37. However, formaldehyde fixation is widely available and affordable, and formaldehyde-fixed cadavers are very durable. In the context presented in this paper, the hardening of soft tissues caused by formaldehyde fixation, while being a disadvantage for many surgical training courses (in particular for orthopedic approaches), turned out to be an adequate model, presenting a stable, but not too rigid surface of the brain, thus allowing for a realistic application of the cortical microelectrode array on the post mortem brain. Techniques have been developed to simulate the circulation of blood and cerebrospinal fluid in formaldehyde-fixed cadavers30,31,39 and could complement the present protocol in order to further increase the realism of the OR-like environment.
Three-dimensional (3D) printing has recently become an accessible and affordable means of replicating body parts for medical and surgical education. Novel 3D printing and molding using synthetic gelatinous casts provides a realistic brain model with tactile feedback. This approach has the advantage of providing a deformable structure that can be printed to reproduce a particular individual's cerebral anatomy and is thus more anatomically accurate than more generic models42. On the other hand, there are still reservations concerning the stiffness and the tissue cutting properties of the synthetic material43. In this sense, the cadaveric model gives a broader anatomical framework, including the complete stratigraphy, not only the brain surface itself.
An alternative to surgical training on human cadavers is practicing on live animals. Implanting a microelectrode array on a primate model, for instance a macaque monkey, would reproduce most of the features of the actual procedure in a human patient, including surgical positioning and instrumentation similar to those used in humans, a gyrencephalic brain of a size not very far from that of a human's, and the presence of cerebral pulsation as well as blood and cerebrospinal fluid circulation. However, while it is acceptable to implant microelectrode arrays in monkeys for the purpose of neuroscience research, using monkeys solely for surgical training is widely discouraged, for ethical reasons as well as because of their very high cost. Because few neuroscience centers implant microelectrode arrays in monkeys for research purposes, and because these centers use few animals at a time (due to the cost of the monkeys themselves and the long and labor-intensive training that neuroscience research with monkeys generally entails), training for microelectrode array implantation in monkeys is not an option for most surgeons. Using smaller animals, such as rodents and even cats or rabbits, would depart too much from OR-like realism. One potential advantage of animal models is that tissue healing allows repeating the entire procedure more than once over the lifetime of the animal. In a human cadaver model, the entire procedure can be repeated once per hemisphere. That being said, craniotomy does not present any particular difficulty to a trained neurosurgeon. Provided that the craniotomy is large enough, the specific steps of pedestal fixation and microelectrode positioning and insertion can be repeated as often as desired during a given session, providing an adequate training opportunity for more than one surgeon. Thus, we think that embalmed human cadavers are the most appropriate model to train surgeons to implant microelectrode arrays.
Recent breakthroughs in BCI development suggest that microelectrode arrays could represent a clinically significant addition to the therapeutic and restorative solutions that are available today for patients with severe motor or communicative disabilities11,13,44. In the near future, the implantation of microelectrode arrays might thus become a required part of the training of neurosurgeons. Refinements in the design of the microelectrodes themselves, together with improvements in connecting the electrodes to the computer processing neuronal signals (likely through wireless connections), will reduce the invasiveness of microelectrode arrays and further enhance their usability for both the physicians and the patients and their caregivers.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors are grateful to Dr. Rob Franklin (Blackrock Microsystems), Prof. Margitta Seeck (Division of Neurology, Geneva University Hospitals, Geneva, Switzerland), Dr. Andrea Bartoli and Prof. Karl Schaller (Division of Neurosurgery, Geneva University Hospitals, Geneva, Switzerland), and Mr. Florent Burdin and Prof. John P. Donoghue (Wyss Center for Bio and Neuroengineering, Geneva, Switzerland) for their support in preparing the present work.
References
- Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. A silicon-based, 3-dimensional neural interface - manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991;38(8):758–768. doi: 10.1109/10.83588. [DOI] [PubMed] [Google Scholar]
- Jones KE, Campbell PK, Normann RA. A glass/silicon composite intracortical electrode array. Ann. Biomed. Eng. 1992;20(4):423–427. doi: 10.1007/BF02368134. [DOI] [PubMed] [Google Scholar]
- Maynard EM, Nordhausen CT, Normann RA. The Utah Intracortical Electrode Array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 1997;102(3):228–239. doi: 10.1016/s0013-4694(96)95176-0. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, et al. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J. Neurophysiol. 2003;90(2):1314–1314. doi: 10.1152/jn.00116.2003. [DOI] [PubMed] [Google Scholar]
- Kelly RC, et al. Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. J. Neurosci. 2007;27(2):261–264. doi: 10.1523/JNEUROSCI.4906-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhausen CT, Maynard EM, Normann RA. Single unit recording capabilities of a 100 microelectrode array. Brain Res. 1996;726(1-2):129–140. [PubMed] [Google Scholar]
- Nordhausen CT, Rousche PJ, Normann RA. Optimizing recording capabilities of the Utah Intracortical Electrode Array. Brain Res. 1994;637(1-2):27–36. doi: 10.1016/0006-8993(94)91213-0. [DOI] [PubMed] [Google Scholar]
- Maynard EM, et al. Neuronal interactions improve cortical population coding of movement direction. J. Neurosci. 1999;19(18):8083–8093. doi: 10.1523/JNEUROSCI.19-18-08083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416(6877):141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372, 375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J. Neural Eng. 2011;8(2):25027. doi: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosiewicz B, et al. Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci. Transl. Med. 2015;7(313):313ra179. doi: 10.1126/scitranslmed.aac7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck M, Schomer DL, Niedermeyer E. Intracranial Monitoring: Depth, Subdural, and Foramen Ovale Electrodes. Niedermeyer’s Electroencephalogr. 2011. pp. 677–714.
- Truccolo W, et al. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 2011;14(5):635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, et al. Neuronal ensemble synchrony during human focal seizures. J. Neurosci. 2014;34(30):9927, 9944. doi: 10.1523/JNEUROSCI.4567-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, et al. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain. 2010;133(Pt 6):1668–1681. doi: 10.1093/brain/awq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat. Commun. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain. 2013;136(Pt 12):3796–3808. doi: 10.1093/brain/awt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash SS, Hochberg LR. The Emergence of Single Neurons in Clinical Neurology. Neuron. 2015;86(1):79–91. doi: 10.1016/j.neuron.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP. Bridging the brain to the world: a perspective on neural interface systems. Neuron. 2008;60(3):511–521. doi: 10.1016/j.neuron.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Hader WJ, et al. Complications of epilepsy surgery - A systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54(5):840–847. doi: 10.1111/epi.12161. [DOI] [PubMed] [Google Scholar]
- Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: A systematic review and meta-analysis. Epilepsia. 2013;54(5):828–839. doi: 10.1111/epi.12073. [DOI] [PubMed] [Google Scholar]
- Hayashi S, et al. History and future of human cadaver preservation for surgical training: from formalin to saturated salt solution method. Anat. Sci. Int. 2016;91(1):1–7. doi: 10.1007/s12565-015-0299-5. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann. Biomed. Eng. 1992;20(4):413–422. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- Waziri A, Schevon CA, Cappell J, Emerson RG, McKhann GM, Goodman RR. Initial surgical experience with a dense cortical microarray in epileptic patients undergoing craniotomy for subdural electrode implantation. Neurosurgery. 2009;64(3):540–545. doi: 10.1227/01.NEU.0000337575.63861.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Tóth E, Fabó D, Entz L, Ulbert I, Erőss L. Intracranial neuronal ensemble recordings and analysis in epilepsy. J. Neurosci. Methods. 2016;260:261–269. doi: 10.1016/j.jneumeth.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Cash SS, et al. The human K-complex represents an isolated cortical down-state. Science. 2009;324(5930):1084–1087. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabe J, Olabe J, Sancho V. Human cadaver brain infusion model for neurosurgical training. Surg. Neurol. 2009;72(6):700–702. doi: 10.1016/j.surneu.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Winer JL, et al. Cerebrospinal fluid reconstitution via a perfusion-based cadaveric model: feasibility study demonstrating surgical simulation of neuroendoscopic procedures. J. Neurosurg. 2015;123(5):1316–1321. doi: 10.3171/2014.10.JNS1497. [DOI] [PubMed] [Google Scholar]
- Cardali S, et al. Microsurgical Anatomic Features of the Olfactory Nerve: Relevance to Olfaction Preservation in the Pterional Approach. Oper. Neurosurg. 2005;57:17–21. doi: 10.1227/01.neu.0000144844.72403.7b. [DOI] [PubMed] [Google Scholar]
- Alvernia JE, Pradilla G, Mertens P, Lanzino G, Tamargo RJ. Latex injection of cadaver heads: technical note. Neurosurgery. 2010;67(2 Suppl Operative):362–367. doi: 10.1227/NEU.0b013e3181f8c247. [DOI] [PubMed] [Google Scholar]
- Chowdhury FH, et al. Endoscopic endonasal transsphenoidal exposure of circle of Willis (CW); can it be applied in vascular neurosurgery in the near future? A cadaveric study of 26 cases. Turk. Neurosurg. 2012;22(1):68–76. doi: 10.5137/1019-5149.JTN.4887-11.2. [DOI] [PubMed] [Google Scholar]
- Benet A, Rincon-Torroella J, Lawton MT, González Sánchez JJ. Novel embalming solution for neurosurgical simulation in cadavers. J. Neurosurg. 2014;120(5):1229–1237. doi: 10.3171/2014.1.JNS131857. [DOI] [PubMed] [Google Scholar]
- Tomlinson JE, Yiasemidou M, Watts AL, Roberts DJH, Timothy J. Cadaveric Spinal Surgery Simulation: A Comparison of Cadaver Types. Glob. spine J. 2016;6(4):357–361. doi: 10.1055/s-0035-1563724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, Powers SK. The use of fabric softener in neurosurgical prosections. Neurosurgery. 1995;36(2):420-3-4at. doi: 10.1227/00006123-199502000-00029. [DOI] [PubMed] [Google Scholar]
- Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. 1. Review of the literature and development of a new method of vascular injection-filling in cadaveric controls. Clin. Anat. 1997;10(6):371–379. doi: 10.1002/(SICI)1098-2353(1997)10:6<371::AID-CA1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Tubbs RS, Loukas M, Shoja MM, Wellons JC, Cohen-Gadol AA. Feasibility of ventricular expansion postmortem: a novel laboratory model for neurosurgical training that simulates intraventricular endoscopic surgery. J. Neurosurg. 2009;111(6):1165–1167. doi: 10.3171/2009.3.JNS081653. [DOI] [PubMed] [Google Scholar]
- Aktas U, Yilmazlar S, Ugras N. Anatomical restrictions in the transsphenoidal, transclival approach to the upper clival region: a cadaveric, anatomic study. J. Craniomaxillofac. Surg. 2013;41(6):457–467. doi: 10.1016/j.jcms.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Yiasemidou M, Roberts D, Glassman D, Tomlinson J, Biyani S, Miskovic D. A Multispecialty Evaluation of Thiel Cadavers for Surgical Training. World J. Surg. 2017. [DOI] [PMC free article] [PubMed]
- Ploch CC, Mansi CSSA, Jayamohan J, Kuhl E. Using 3D Printing to Create Personalized Brain Models for Neurosurgical Training and Preoperative Planning. World Neurosurg. 2016;90:668–674. doi: 10.1016/j.wneu.2016.02.081. [DOI] [PubMed] [Google Scholar]
- Del Castillo-Calcáneo J, Donoghue JA. A Novel Method for 3-Dimensional Printing a Brain That Feels and Looks Like One: The Next Step in the Search of the Perfect Neurosurgical Simulator. World Neurosurg. 2016;91:620–622. doi: 10.1016/j.wneu.2016.03.086. [DOI] [PubMed] [Google Scholar]
- Martin S, Millán JDR, Knight RT, Pasley BN. The use of intracranial recordings to decode human language: Challenges and opportunities. Brain Lang. 2016. [DOI] [PMC free article] [PubMed]


