Abstract
Purpose
Age-related macular degeneration (AMD) is a frequent, complex disorder in elderly of European ancestry. Risk profiles and treatment options have changed considerably over the years, which may have affected disease prevalence and outcome. We determined the prevalence of early and late AMD in Europe from 1990 to 2013 using the European Eye Epidemiology (E3) consortium, and made projections for the future.
Design
Meta-analysis of prevalence data.
Participants
A total of 42 080 individuals 40 years of age and older participating in 14 population-based cohorts from 10 countries in Europe.
Methods
AMD was diagnosed based on fundus photographs using the Rotterdam Classification. Prevalence of early and late AMD was calculated using random-effects meta-analysis stratified for age, birth cohort, gender, geographic region, and time period of the study. Best-corrected visual acuity (BCVA) was compared between late AMD subtypes; geographic atrophy (GA) and choroidal neovascularization (CNV).
Main Outcome Measures
Prevalence of early and late AMD, BCVA, and number of AMD cases.
Results
Prevalence of early AMD increased from 3.5% (95% confidence interval [CI] 2.1%–5.0%) in those aged 55–59 years to 17.6% (95% CI 13.6%–21.5%) in those aged ≥85 years; for late AMD these figures were 0.1% (95% CI 0.04%–0.3%) and 9.8% (95% CI 6.3%–13.3%), respectively. We observed a decreasing prevalence of late AMD after 2006, which became most prominent after age 70. Prevalences were similar for gender across all age groups except for late AMD in the oldest age category, and a trend was found showing a higher prevalence of CNV in Northern Europe. After 2006, fewer eyes and fewer ≥80-year-old subjects with CNV were visually impaired (P = 0.016). Projections of AMD showed an almost doubling of affected persons despite a decreasing prevalence. By 2040, the number of individuals in Europe with early AMD will range between 14.9 and 21.5 million, and for late AMD between 3.9 and 4.8 million.
Conclusion
We observed a decreasing prevalence of AMD and an improvement in visual acuity in CNV occuring over the past 2 decades in Europe. Healthier lifestyles and implementation of anti–vascular endothelial growth factor treatment are the most likely explanations. Nevertheless, the numbers of affected subjects will increase considerably in the next 2 decades. AMD continues to remain a significant public health problem among Europeans.
Abbreviations and Acronyms: AMD, age-related macular degeneration; CI, confidence interval; CNV, choroidal neovascularization; E3, European Eye Epidemiology (consortium); EPIC, European Prospective Investigation into Cancer and Nutrition; EUREYE, European Eye Study; GA, geographic atrophy; RS, Rotterdam Study; UK, United Kingdom; VEGF, vascular endothelial growth factor
Age-related macular degeneration (AMD) can cause irreversible blindness and is the leading cause of visual impairment in the elderly of European ancestry.1 Two stages are known for this disease: early AMD, which is characterized by drusen and pigmentary changes, and late AMD, which can be distinguished in 2 subtypes—geographic atrophy (GA) and choroidal neovascularization (CNV).2
Worldwide estimates approximated that 30–50 million people are affected by AMD,3, 4 and these numbers are expected to increase over time because of the aging population.1, 5, 6, 7, 8, 9 Although multiple small studies have assessed the prevalence of AMD and its relation to visual decline at various places in Europe,10, 11, 12 a clear overview for Europe as a whole is lacking.13 Comprehensive epidemiologic figures on AMD in Europe would help proper planning for public health and eye care policy makers.
Recent studies report a decrease in AMD-associated blindness and visual impairment,14, 15 which are likely to be attributable to improved diagnostic procedures and hence earlier diagnosis, and the introduction of anti–vascular endothelial growth factor (VEGF) therapy.14, 15, 16 Anti-VEGF therapy for CNV was introduced in 2004 and, since 2006, it has been widely used for clinical care in Europe.17, 18 However, the impact of anti-VEGF therapy on general visual function of persons with AMD in Europe has not been sufficiently studied.1, 16
In this study, we investigated the prevalence of both early and late AMD in Europe using summary data of population-based cohort studies from the European Eye Epidemiology (E3) consortium. We analyzed changes in prevalence over time, compared geographic regions, and studied differences between men and women. Moreover, we analyzed the visual acuity of affected individuals before and after the introduction of anti-VEGF therapy and predicted the number of persons with AMD by 2040 in Europe.
Methods
Study Population
Fourteen population-based cohort studies participating in the E3 consortium contributed to this analysis. This consortium consists of European studies with epidemiologic data on common eye disorders; a detailed description on the included studies has been published elsewhere.16 For the current analysis, studies with gradable macular fundus photographs (n = 42 080 participants) and participants aged 40 years and over provided summary data. Participants were recruited between 1990 and 2013 from the following countries: Estonia, France, Germany, Greece, Italy, Northern Ireland, Norway, Netherlands, Spain, Portugal,19, 20 and the United Kingdom (UK)16 (Table 1). The composition of AMD in each cohort is shown in Figure 1 (available at www.aaojournal.org). The study was performed in accordance with the Declaration of Helsinki for research involving human subjects and the good epidemiologic practice guideline.
Table 1.
Description of the European Eye Epidemiology Consortium Studies Included in the Meta-analysis
| Region | Study | Data Collection Period | Total Participants (n) | Age Range (yrs) | Median Age (yrs) | Male Gender (%) | European Ethnicity (%) | Crude Prevalence of Early AMD (%) | Crude Prevalence of Late AMD (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| North | United Kingdom | EPIC | 2004–2011 | 5344 | 45–85+ | 60–64 | 43.1 | 99.7 | – | 0.5 |
| Norway | Tromsø | 2007–2008 | 2631 | 65–85+ | 65–69 | 42.5 | 91 | – | 3.5 | |
| West | France | ALIENOR-3C | 2006–2008 | 879 | 70–85+ | 75–79 | 37.7 | – | 16.8 | 5.6 |
| Germany | GHS | 2007–2012 | 3839 | 40–74 | 50–54 | 50.2 | – | 2.3 | 0.2 | |
| Netherlands | RS-I | 1990–1993 | 6419 | 55–85+ | 60–64 | 40.7 | 98.9 | 7.5 | 1.7 | |
| Netherlands | RS-II | 2000–2002 | 2545 | 55–85+ | 55–59 | 45.4 | 97.8 | 6 | 0.7 | |
| Netherlands | RS-III | 2005–2008 | 3449 | 45–85+ | 55–59 | 43.4 | 96.4 | 4.6 | 0.4 | |
| France | Montrachet-3C | 2009–2013 | 1069 | 75–85+ | 80–84 | 37 | 100 | 9.2 | 2.2 | |
| France | POLA | 1995–1997 | 2196 | 60–85+ | 65–69 | 43.5 | – | 8.7 | 1.9 | |
| South | Portugal | Lousa | 2012–2013 | 3021 | 55–85+ | 60–64 | 43.9 | 99.3 | 15.4 | 1.3 |
| Portugal | Mira | 2009–2011 | 2975 | 55–85+ | 65–69 | 43.4 | 99.7 | 6.9 | 0.7 | |
| Thessaloniki | Thessaloniki Eye Study | 2000–2005 | 2107 | 60–85+ | 65–69 | 55.6 | 97.7 | – | 2.7 | |
| Italy | PAMDI | 2005–2006 | 853 | 60–85+ | 65–69 | 45.8 | 100 | 13.5 | 2.1 | |
| Multiple | EUREYE | 2000–2002 | 4753 | 65–85+ | 65–69 | 44.8 | – | 12.6 | 3.3 |
ALIENOR = Antioxydants, Lipids Essentials, Nutrition et maladies OculaiRes Study; AMD = age-related macular degeneration; EPIC = European Prospective Investigation into Cancer; EUREYE = European Eye Study; GHS = Gutenberg Health Study; PAMDI = Prevalence of Age-Related Macular Degeneration in Italy; POLA = Pathologies Oculaires Liées à l'Age Study; RS = Rotterdam Study; – = data not available.
Grading of Age-Related Macular Degeneration
Both eyes of each participant were graded and classified separately by experienced graders or clinicians, and the most severe AMD grade of the worse eye was used for classification of the person. To harmonize classification of AMD, studies were graded or reclassified according to the Rotterdam Classification, as previously described.21 Main outcomes of this study were early AMD (grade 2 or 3 of the Rotterdam Classification) and late AMD (grade 4 of the Rotterdam Classification). Persons with late AMD were stratified as GA and CNV or mixed (both GA and CNV present in one person, either both types in the same eye or one type per eye), which is henceforth in this article referred to as CNV. The Tromsø Eye Study, the Thessaloniki Eye Study, and the European Prospective Investigation into Cancer and Nutrition (EPIC) study had fundus photograph grading that could not be converted to match the definition of early AMD of the Rotterdam Classification. Therefore, these 3 studies only participated in the late AMD analysis.
Visual Impairment
Visual acuity was measured for each eye separately as best-corrected visual acuity in 2 categories: ≥0.3 and <0.3. When best-corrected visual acuity differed in the 2 eyes, visual acuity of the best eye was used to classify the person. Low vision and blindness were defined as visual acuity <0.3 and further referred to as visually impaired.
Projection of Age-Related Macular Degeneration
The projection of AMD cases in Europe from 2013 to 2040 was calculated using the prevalence data for 5-year age categories obtained from the meta-analysis. Two different scenarios were used to calculate the projection. In the first scenario, it was assumed that the prevalence of both early and late AMD will remain stable until 2040. This scenario accounted for changes in population structure only. The second scenario followed the trend of decreasing prevalence based on data from the meta-analysis of the E3 consortium regarding the period 2006–2013. We calculated the rate of decline, with 2013 as the starting point and 2040 as the end point, and made the assumption that the rate of decline was decelerating and zero at the end point. For each projected year, prevalences were calculated for every 5-year age group, for early AMD from 45 years of age and onward and for late AMD 65 years and onward. The projected prevalences were then multiplied by the predicted European population estimates obtained from Eurostat for all 28 countries in Europe, and the sum of individuals from all age groups was calculated.22
Statistical Analysis
The crude prevalence of early and late AMD were calculated per study for each 5-year age group. A random-effects meta-analysis was performed by weighing the studies according to sample size, for early and late AMD separately for 5-year age groups and for people aged 70 years and older. In case of reported zero prevalence, the Haldane correction was used.23 In the case of 100% prevalence, 0.01 was subtracted to prevent exclusion from the analysis. This analysis was repeated, stratified for the midpoint year of the study recruitment period, before and after the year 2006 and for 10-year birth cohorts. Furthermore, it was repeated for gender, and for geographical area in Europe based on the United Nations Geoscheme.24 A chi-square test was used to compare time trends.
In addition, a meta-analysis was performed for eyes with visual impairment owing to late AMD, and per subtype of late AMD. Subsequently, the analysis was stratified for studies conducted before and after 2006, for which the midpoint year of the study recruitment period was used. The number of visually impaired people was calculated before and after 2006. Meta-analysis was performed with Stata software (release 13, version 13.1; StataCorp LP, College Station, TX) using metaprop. Graphical outputs were constructed with GraphPad Prism 7 (for Windows; GraphPad Software, La Jolla, CA; www.graphpad.com).
Results
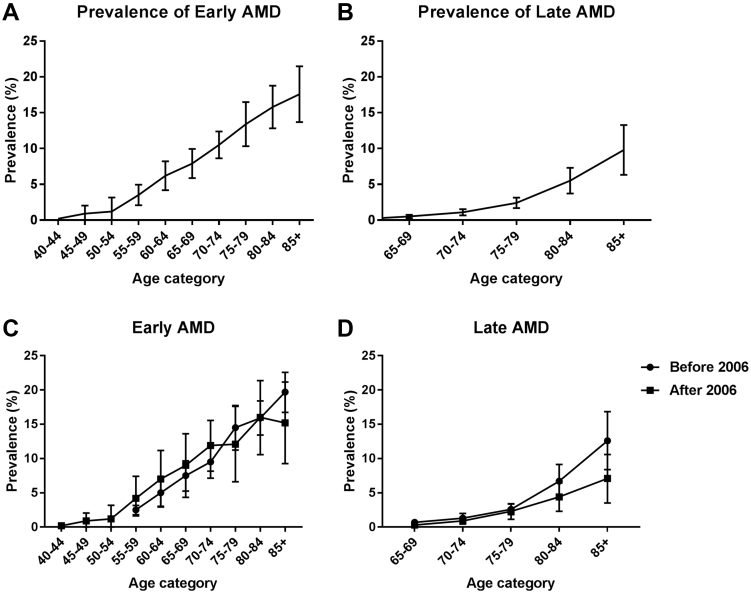
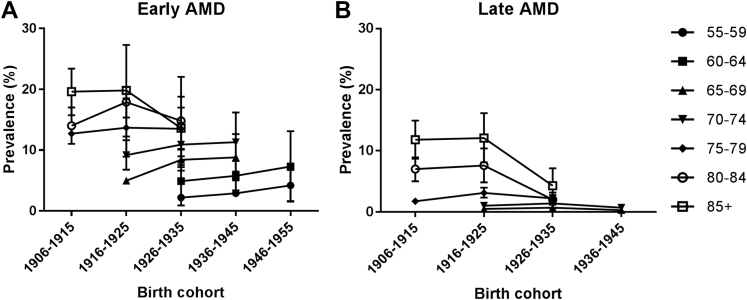
The total study population included in this analysis consisted of 42 080 individuals from 14 studies with a median age of 65–69 years and a slight female predominance (55.8%). The prevalence of all age groups together varied per study between 2.3% and 16.8% for early AMD (total N = 2703) and between 0.2% and 5.6% for late AMD (total N = 664) (Fig 2A and B, available at www.aaojournal.org; to avoid biased estimates only groups larger than 30 individuals are shown; this applied only to the Rotterdam Study 3 age category ≥85 years). Owing to moderate to high heterogeneity (I2 ≥75% in 73 of 141 analyses), which was not related to time trends, we applied a random-effects model for each meta-analysis. This provided a prevalence of early AMD increasing with age from 3.5% (95% confidence interval [CI] 2.1%–5.0%) at 55–59 years to 17.6% (95% CI 13.6%–21.5%) in persons aged ≥85 (Fig 3A, and Table 2, available at www.aaojournal.org). The prevalence of late AMD rose from virtually zero in the youngest age group to 9.8% (95% CI 6.3%–13.3%) for those in the highest age group (Fig 3B). Taking together all people aged ≥70 years, the overall prevalence was 13.2% (95% CI 11.2%–15.1%) for early AMD and 3.0% (95% CI 2.2%–3.9%) for late AMD. We investigated prevalence changes over time by dividing the E3 consortium into studies conducted before and after 2006. The prevalence of early AMD before and after 2006 seemed to rise with age in a similar fashion. For late AMD, a trend of decreasing prevalence was observed for the higher age categories after 2006 (Fig 3C and D). Even after exclusion of the 2 cohorts (Rotterdam Study [RS]-II and European Eye Study [EUREYE]) with the highest prevalences in the highest age category before 2006, results remained similar (data not shown). When we analyzed prevalence data as a function of birth cohort, a relatively stable prevalence of early AMD was visible across all birth cohorts, whereas a decreasing prevalence of late AMD was seen for the more recent birth cohorts (Fig 4A and B).
Figure 3.
Meta-analysis of (A) early and (B) late age-related macular degeneration (AMD) in Europe per age category for the participating studies. Meta-analysis of the prevalence of (C) early and (D) late AMD before and after 2006.
Figure 4.
Meta-analysis of early (A) and late (B) age-related macular degeneration in Europe by 10-year birth cohorts.
Gender and Geographic Region
We studied the relation with gender and found no differences in the prevalence of early and late AMD between men and women except for the age category of 85 years and older for late AMD (Fig 5A and B, available at www.aaojournal.org). This category shows a trend for a higher prevalence in women compared to men, although CIs overlap.
To address differential distribution of AMD in Europe, we stratified studies according to 3 regions defined by the United Nations.24 In older individuals, we observed a trend toward a higher prevalence of early AMD in the North (16% in those ≥70 years; 95% CI 14%–17%) compared to the West (12%; 95% CI 10%–14%) and South (14%; 95% CI 10%–17%) (Fig 6A, available at www.aaojournal.org). Likewise, late AMD had the highest prevalence in the North (4.2%; 95% CI 2%–6%) compared to the West (3.1%; 95% CI 2%–4%) and South (3.1%; 95% CI 2%–4%) (Fig 6B, available at www.aaojournal.org). More detailed analyses showed that a frequency difference was only present for CNV (Fig 6C and D, available at www.aaojournal.org); however, CIs of the regional differences overlapped.
Visual Consequences
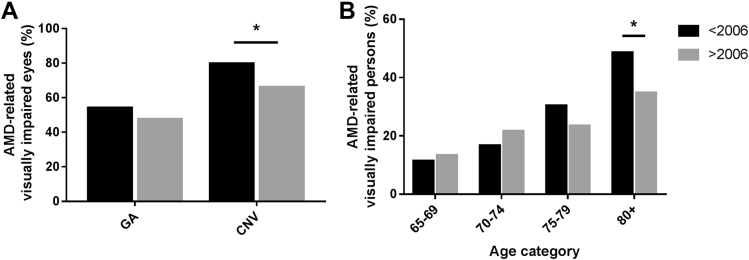
As most countries implemented anti-VEGF therapy for CNV from 2006 onward, we compared visual impairment from AMD in studies carried out before and after this year. Before 2006, 54.2% of eyes with GA were visually impaired, and 79.8% of eyes suffering from CNV were visually impaired. From 2006 onward, the proportion of visually impaired eyes remained the same for GA (47.6%; P = 0.40), but dropped to 66.2% (P = 0.026) for CNV (Fig 7A). This improvement was also observed for the number of bilaterally visually impaired persons; 120 of 345 (34.8%) before 2006 to 75 of 259 (28.9%; P = 0.13) after 2006. The largest drop was seen for people aged 80 years and older; 85 of 175 (48.6%) before 2006 to 46 of 132 (34.8%; P = 0.016) after 2006 (Fig 7B).
Figure 7.
A, Proportion of visually impaired eyes within each subgroup of late age-related macular degeneration (AMD). The proportion of visually impaired eyes remained the same for geographic atrophy (47.6%; P = 0.4), but dropped to 66.2% (P = 0.026) for choroidal neovascularization after 2006. B, Proportion of persons with late AMD with bilateral visual impairment before and after 2006 (P = 0.016). ∗P < 0.05.
Projections of Age-Related Macular Degeneration in Europe for 2040
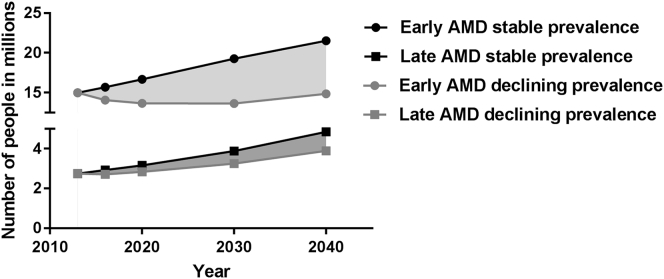
Assuming that the prevalence of early and late AMD will remain stable over time, an increase from 15.0 million in 2013 to 21.5 million for early AMD can be expected by 2040. The number of people with late AMD will almost double during this time period, from 2.7 million in 2013 to 4.8 million in 2040.
Assuming a more realistic scenario for which E3 historic data and a decelerating slope were used, we found that the prevalence of early AMD will first decrease and then slightly increase between 2013 and 2040. The model estimated that the number of people with early AMD would remain almost the same: from 15.0 million in 2013 to 14.9 million in 2040. This model also displayed that the number of people with late AMD in Europe will increase from 2.7 million in 2013 to 3.9 million by 2040 (Fig 8).
Figure 8.
Predicted number of persons with age-related macular degeneration (AMD) in years 2013–2040 as a function of 2 prevalence scenarios.
Discussion
Age-Related Macular Degeneration Prevalence and Its Time Trends
Our study provides insight into the prevalence of both early and late AMD in Europe. Based on meta-analyzed data from 14 population-based cohort studies included in the E3 consortium, the overall prevalence of early and late AMD was 13.2% and 3.0%, respectively, in the age category ≥70 years. These estimates are comparable to those among persons of European descent living in other continents.4, 25
Our data show a trend toward a slightly decreasing prevalence of AMD in the older age categories. It is unlikely that this is explained by differential mortality in AMD patients before and after 2006, although studies have shown conflicting results on death as a competing risk factor for AMD, and we cannot exclude that this plays a role.26, 27, 28 The decreasing trend in time has also been observed in the Beaver Dam Eye Study, indicating that these trends are not confined to Europe.29 Decreasing rates have also been observed for other aging disorders such as cardiovascular disease and dementia,30, 31, 32, 33 and may be related to improved lifestyle among the elderly34, 35, 36; for example, the number of smokers declined by 30.5% from 1990 to 2010 in Europe.37 Taken together, the decline in prevalence suggests that the increases in the number of AMD patients may not be as substantial as previous prediction studies suggested.38
Gender and Geographic Regions
Our data showed no difference in the prevalence of early and late AMD with respect to gender. In the oldest age category of 85 years and older, women seemed to have a higher prevalence of late AMD, but detailed analysis showed that this was mostly owing to imprecision of the estimate in men, caused by a lower number of men in this age group (Fig 9, available at www.aaojournal.org). This has also been observed in other studies.7, 39
As for regional differences, we noticed that the northern region of Europe showed a slightly higher prevalence of early and late AMD. This trend was the result of a higher prevalence of CNV in the north. Our findings are in concordance with the results previously published by the Tromsø Eye Study40 but are in contrast with other studies performed in the north of Europe finding a higher prevalence of GA (EUREYE, Reykjavik Eye Study, and Oslo Macular Study).41, 42, 43 Considering the larger sample size and high response rate of the Tromsø Eye Study compared with the other studies, these findings might be more legitimate. No consistent differences were observed for the western and southern regions of Europe.
Visual Consequences
The proportion of eyes affected by CNV that were visually impaired was reduced after the year 2006. Unfortunately, our study lacked actual data on interventions for CNV, but it is likely that the reduction is attributable to the use of anti-VEGF injections, which were introduced as a therapy for CNV in Europe from 2006 onward.18 This notion is supported by findings from clinical trials44, 45 and other studies, which show an up to 2-fold decrease in legal blindness due to AMD after 2006.14, 15, 46, 47 The public campaigns that were initiated after the introduction of anti-VEGF have undoubtedly contributed to the reduction of visual loss, as they made elderly persons more aware of the symptoms and stimulated prompt therapy.48, 49
Projections of Age-Related Macular Degeneration in Europe
It is unclear whether the prevalence of AMD will decrease even more in the coming years, but an increase is not likely to be expected. Therefore, we projected the estimated number of AMD-affected persons until the year 2040 based on 2 different scenarios: 1 based on stable prevalence and 1 following the trend of declining prevalences. The results of the first scenario suggests that the absolute number of persons with late AMD will increase by 2.1 million, a 1.5-times increase. A Norwegian study predicted, under the assumption of a stable prevalence, the same relative increase of affected subjects, with a total of 328 000 cases of late AMD in Scandinavia by 2040.5, 8 A study in the United States calculated a 2.2-times increase in absolute numbers and estimated a total number of affected subjects to be 3.8 million by 2050.5, 8 Worldwide projections have shown a doubling of late AMD and an increase of 9 million cases by 2040.4
The second scenario was based on declining rates, and showed a small increase in the number of people with early AMD, from 14 million in 2016 to 14.9 million by 2040, and a larger relative increase in the number of people with late AMD, from 2.7 million in 2016 to 3.9 million by 2040. Considering the declining rates of smoking and implementation of healthier diets in elderly persons, the second projection may be more legitimate.
Study Limitations
A limitation to this E3 consortium meta-analysis is the heterogeneity across studies regarding study design and inclusion criteria. For example, age at inclusion and method of recruitment varied between studies. Although in every study AMD was classified according to the Rotterdam Classification, studies differed in AMD grading, especially for pigmentary changes and drusen size. Given the heterogeneity, we therefore performed a random-effects meta-analysis for both early and late AMD. Furthermore, patient management and access to health care may have differed between study sites, resulting in differences in preventive and treatment options.50, 51
When data collection started in 1990, fundus photography was the gold standard for grading AMD. Since 1990, imaging techniques evolved rapidly, greatly improving the diagnosis of AMD features with non-invasive techniques such as optical coherence tomography, autofluorescence, and near-infrared photographs. In addition, multimodal imaging better visualizes edema and subtle changes resulting from CNV, which may not be so apparent when the patient was treated with anti-VEGF therapy.52, 53 Although macular edema due to subretinal neovascularization often coincides with prominent retinal changes such as hemorrhages or hard exudates, our data may have underestimated the true prevalence of CNV.53
In summary, this study estimates the prevalence of early and late AMD per age category in Europe over the past two decades. Prevalence of both these forms remained stable or decreased slightly. Nevertheless, we observed a significant reduction in the proportion of visually impaired eyes attributable to CNV after 2006. Unfortunately, due to the aging population, the number of people with AMD will increase during the next decades, indicating a continuous need to develop comprehensive modalities for prevention and treatment of AMD.
Manuscript no. 2016-1147.
Footnotes
Supplemental material available at www.aaojournal.org.
Financial Disclosure(s): C.D.: Consultant – Allergan, Bausch & Lomb, Laboratoires, Théa, Novartis, and Roche.
R.S.: Consultant – Alimera, Allergan, Alcon, Bayer, Novartis, and Théa.
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. The authors are grateful to the study participants, the staff from the Rotterdam Study, and the participating general practitioners and pharmacists.
The Gutenberg Health Study (GHS) is funded through the government of Rhineland-Palatinate (“Stiftung Rheinland-Pfalz für Innovation,” contract AZ 961-386261/733), the research programs “Wissen schafft Zukunft” and “Center for Translational Vascular Biology (CTVB)” of the Johannes Gutenberg-University of Mainz, and its contract with Boehringer Ingelheim and PHILIPS Medical Systems, including an unrestricted grant for the GHS. The authors thank all study participants for their willingness to provide data for this research project and we are indebted to all coworkers for their enthusiastic commitment.
H2020-RIA, EYE-RISK, grant number: 634479. Uitzicht grant number: 2015-36, Oogfonds, MaculaFonds, LSBS, Novartis Fonds. The sponsors and funding organization had no role in the design or conduct of this research.
Author Contributions:
Conception and design: Khawaja, Korb, Erke, Piermarocchi, Creuzot-Garcher, Pfeiffer, Delcourt, Klaver
Analysis and interpretation: Colijn, Buitendijk, Prokofyeva, Alves, Cachulo, Khawaja, Cougnard-Gregoire, Merle, Korb, Erke, Bron, Anastasopoulos, Segato, Piermarocchi, Vingerling, Topouzis, Creuzot-Garcher, Pfeiffer, Silva, Korobelnik, Delcourt, Klaver
Data collection: Colijn, Buitendijk, Cachulo, Khawaja, Korb, Erke, Bron, Anastasopoulos, Meester-Smoor, Segato, Piermarocchi, de Jong, Vingerling, Topouzis, Creuzot-Garcher, Bertelsen, Fletcher, Foster, Silva, Delcourt, Klaver
Obtained funding: Not applicable
Overall responsibility: Colijn, Buitendijk, Prokofyeva, Khawaja, Cougnard-Gregoire, Merle, Korb, Erke, Anastasopoulos, Topouzis, Bertelsen, Pfeiffer, Fletcher, Foster, Silva, Korobelnik, Delcourt, Klaver
Contributor Information
Caroline C.W. Klaver, Email: c.c.w.klaver@erasmusmc.nl.
EYE-RISK consortium:
Soufiane Ajana, Blanca Arango-Gonzalez, Verena Arndt, Vaibhav Bhatia, Shomi S. Bhattacharya, Marc Biarnés, Anna Borrell, Sebastian Bühren, Sofia M. Calado, Johanna M. Colijn, Audrey Cougnard-Grégoire, Sascha Dammeier, Eiko K. de Jong, Berta De la Cerda, Cécile Delcourt, Anneke I. den Hollander, Francisco J. Diaz-Corrales, Sigrid Diether, Eszter Emri, Tanja Endermann, Lucia L. Ferraro, Míriam Garcia, Thomas J. Heesterbeek, Sabina Honisch, Carel B. Hoyng, Eveline Kersten, Ellen Kilger, Caroline C.W. Klaver, Hanno Langen, Imre Lengyel, Phil Luthert, Cyrille Maugeais, Magda Meester-Smoor, Bénédicte M.J. Merle, Jordi Monés, Everson Nogoceke, Tunde Peto, Frances M. Pool, Eduardo Rodríguez, Marius Ueffing, Karl U. Ulrich Bartz-Schmidt, Elisabeth M. van Leeuwen, Timo Verzijden, and Markus Zumbansen
European Eye Epidemiology (E3) consortium:
Niyazi Acar, Eleftherios Anastosopoulos, Augusto Azuara-Blanco, Arthur Bergen, Geir Bertelsen, Christine Binquet, Alan Bird, Lionel Brétillon, Alain Bron, Gabrielle Buitendijk, Maria Luz Cachulo, Usha Chakravarthy, Michelle Chan, Petrus Chang, Johanna Colijn, Audrey Cougnard-Grégoire, Catherine Creuzot-Garcher, Philippa Cumberland, José Cunha-Vaz, Vincent Daien, Gabor Deak, Cécile Delcourt, Marie-Noëlle Delyfer, Anneke den Hollander, Martha Dietzel, Maja Gran Erke, Sascha Fauser, Robert Finger, Astrid Fletcher, Paul Foster, Panayiota Founti, Arno Göbel, Theo Gorgels, Jakob Grauslund, Franz Grus, Christopher Hammond, Catherine Helmer, Hans-Werner Hense, Manuel Hermann, René Hoehn, Ruth Hogg, Frank Holz, Carel Hoyng, Nomdo Jansonius, Sarah Janssen, Anthony Khawaja, Caroline Klaver, Jean-François Korobelnik, Julia Lamparter, Mélanie Le Goff, Sergio Leal, Yara Lechanteur, Terho Lehtimäki, Andrew Lotery, Irene Leung, Matthias Mauschitz, Bénédicte Merle, Verena Meyer zu Westrup, Edoardo Midena, Stefania Miotto, Alireza Mirshahi, Sadek Mohan-Saïd, Michael Mueller, Alyson Muldrew, Sandrina Nunes, Konrad Oexle, Tunde Peto, Stefano Piermarocchi, Elena Prokofyeva, Jugnoo Rahi, Olli Raitakari, Luisa Ribeiro, Marie-Bénédicte Rougier, José Sahel, Aggeliki Salonikiou, Clarisa Sanchez, Steffen Schmitz-Valckenberg, Cédric Schweitzer, Tatiana Segato, Jasmin Shehata, Rufino Silva, Giuliana Silvestri, Christian Simader, Eric Souied, Henriet Springelkamp, Robyn Tapp, Fotis Topouzis, Virginie Verhoeven, Therese Von Hanno, Stela Vujosevic, Katie Williams, Christian Wolfram, Jennifer Yip, Jennyfer Zerbib, and Isabella Zwiener
Appendix
The E3 Consortium
| First Name | Last Name | Institution | City | Country |
|---|---|---|---|---|
| Niyazi | Acar | Inra-University of Burgundy | Dijon | France |
| Eleftherios | Anastosopoulos | University of Thessaloniki | Thessaloniki | Greece |
| Augusto | Azuara-Blanco | Queen's University | Belfast | UK |
| Arthur | Bergen | Netherlands Institute for Neurosciences-KNAW | Amsterdam | Netherlands |
| Geir | Bertelsen | University of Tromsø | Tromsø | Norway |
| Christine | Binquet | University Hospital of Dijon | Dijon | France |
| Alan | Bird | Moorfields Eye Hospital | London | UK |
| Lionel | Brétillon | Inra-University of Burgundy | Dijon | France |
| Alain | Bron | University Hospital of Dijon | Dijon | France |
| Gabrielle | Buitendijk | Erasmus Medical Center | Rotterdam | Netherlands |
| Maria Luz | Cachulo | AIBILI/CHUC | Coimbra | Portugal |
| Usha | Chakravarthy | Queen's University | Belfast | UK |
| Michelle | Chan | UCL Institute of Ophthalmology | London | UK |
| Petrus | Chang | University of Bonn | Bonn | Germany |
| Johanna | Colijn | Erasmus Medical Center | Rotterdam | Netherlands |
| Audrey | Cougnard-Grégoire | University of Bordeaux Segalen | Bordeaux | France |
| Catherine | Creuzot-Garcher | University Hospital of Dijon | Dijon | France |
| Philippa | Cumberland | UCL Institute of Child Health | London | UK |
| José | Cunha-Vaz | AIBILI/CHUC | Coimbra | Portugal |
| Vincent | Daien | Inserm U1061 | Montpellier | France |
| Gabor | Deak | Medical University of Vienna | Vienna | Austria |
| Cécile | Delcourt | University of Bordeaux Segalen | Bordeaux | France |
| Marie-Noëlle | Delyfer | University of Bordeaux Segalen | Bordeaux | France |
| Anneke | den Hollander | Radboud University | Nijmegen | Netherlands |
| Martha | Dietzel | University of Muenster | Muenster | Germany |
| Maja Gran | Erke | University of Tromsø | Tromsø | Norway |
| Sascha | Fauser | University Eye Hospital | Cologne | Germany |
| Robert | Finger | University of Bonn | Bonn | Germany |
| Astrid | Fletcher | London School of Hygiene and Tropical Medicine | London | UK |
| Paul | Foster | UCL Institute of Ophthalmology | London | UK |
| Panayiota | Founti | University of Thessaloniki | Thessaloniki | Greece |
| Arno | Göbel | University of Bonn | Bonn | Germany |
| Theo | Gorgels | Netherlands Institute for Neurosciences-KNAW | Amsterdam | Netherlands |
| Jakob | Grauslund | University of Southern Denmark | Odense | Denmark |
| Franz | Grus | University Medical Center Mainz | Mainz | Germany |
| Christopher | Hammond | King's College | London | UK |
| Catherine | Helmer | University of Bordeaux Segalen | Bordeaux | France |
| Hans-Werner | Hense | University of Muenster | Muenster | Germany |
| Manuel | Hermann | University Eye Hospital | Cologne | Germany |
| René | Hoehn | University Medical Center | Mainz | Germany |
| Ruth | Hogg | Queen's University | Belfast | UK |
| Frank | Holz | University of Bonn | Bonn | Germany |
| Carel | Hoyng | Radboud University | Nijmegen | Netherlands |
| Nomdo | Jansonius | Erasmus Medical Center | Rotterdam | Netherlands |
| Sarah | Janssen | Netherlands Institute for Neurosciences-KNAW | Amsterdam | Netherlands |
| Anthony | Khawaja | UCL Institute of Ophthalmology | London | UK |
| Caroline | Klaver | Erasmus Medical Center | Rotterdam | Netherlands |
| Jean-François | Korobelnik | University of Bordeaux Segalen | Bordeaux | France |
| Julia | Lamparter | University Medical Center Mainz | Mainz | Germany |
| Mélanie | Le Goff | University of Bordeaux Segalen | Bordeaux | France |
| Sergio | Leal | AIBILI/CHUC | Coimbra | Portugal |
| Yara | Lechanteur | Radboud University | Nijmegen | Netherlands |
| Terho | Lehtimäki | Pirkanmaa Hospital District | Tampere | Finland |
| Andrew | Lotery | University of Southampton | Southampton | UK |
| Irene | Leung | Moorfields Eye Hospital | London | UK |
| Matthias | Mauschitz | University of Bonn | Bonn | Germany |
| Bénédicte | Merle | University of Bordeaux Segalen | Bordeaux | France |
| Verena | Meyer zu Westrup | University of Muenster | Muenster | Germany |
| Edoardo | Midena | University of Padova | Padova | Italy |
| Stefania | Miotto | University of Padova | Padova | Italy |
| Alireza | Mirshahi | University Medical Center | Mainz | Germany |
| Sadek | Mohan-Saïd | Institut de la Vision | Paris | France |
| Michael | Mueller | Pirkanmaa Hospital District | Tampere | Finland |
| Alyson | Muldrew | Queen's University | Belfast | UK |
| Sandrina | Nunes | AIBILI/CHUC | Coimbra | Portugal |
| Konrad | Oexle | Institue of Human Genetics | Munich | Germany |
| Tunde | Peto | Queen's University | Belfast | UK |
| Stefano | Piermarocchi | University of Padova | Padova | Italy |
| Elena | Prokofyeva | Scientific Institute of Public Health (WIV-ISP), Federal Agency for Medicines and Health Products | Brussels | Belgium |
| Jugnoo | Rahi | UCL Institute of Ophthalmology | London | UK |
| Olli | Raitakari | Pirkanmaa Hospital District | Tampere | Finland |
| Luisa | Ribeiro | AIBILI/CHUC | Coimbra | Portugal |
| Marie-Bénédicte | Rougier | University of Bordeaux Segalen | Bordeaux | France |
| José | Sahel | Institut de la Vision | Paris | France |
| Aggeliki | Salonikiou | University of Thessaloniki | Thessaloniki | Greece |
| Clarisa | Sanchez | Radboud University | Nijmegen | Netherlands |
| Steffen | Schmitz-Valckenberg | University of Bonn | Bonn | Germany |
| Cédric | Schweitzer | University of Bordeaux Segalen | Bordeaux | France |
| Tatiana | Segato | University of Padova | Padova | Italy |
| Jasmin | Shehata | Medical University of Vienna | Vienna | Austria |
| Rufino | Silva | AIBILI/CHUC | Coimbra | Portugal |
| Giuliana | Silvestri | Queen's University | Belfast | UK |
| Christian | Simader | Medical University of Vienna | Vienna | Austria |
| Eric | Souied | University Hospital of Créteil | Créteil | France |
| Henriet | Springelkamp | Erasmus Medical Center | Rotterdam | Netherlands |
| Robyn | Tapp | Pirkanmaa Hospital District | Tampere | Finland |
| Fotis | Topouzis | University of Thessaloniki | Thessaloniki | Greece |
| Virginie | Verhoeven | Erasmus Medical Center | Rotterdam | Netherlands |
| Therese | Von Hanno | University of Tromsø | Tromsø | Norway |
| Stela | Vujosevic | University of Padova | Padova | Italy |
| Katie | Williams | King's College London | London | UK |
| Christian | Wolfram | University Medical Center | Mainz | Germany |
| Jennifer | Yip | UCL Institute of Ophthalmology | London | UK |
| Jennyfer | Zerbib | University Hospital of Créteil | Créteil | France |
| Isabella | Zwiener | University Medical Center | Mainz | Germany |
The EYE-RISK Consortium‡
Soufiane Ajana,1 Blanca Arango-Gonzalez,2 Verena Arndt,3 Vaibhav Bhatia,4 Shomi S. Bhattacharya,4 Marc Biarnés,5 Anna Borrell,5 Sebastian Bühren,6 Sofia M. Calado,4 Johanna M. Colijn,7,8 Audrey Cougnard-Grégoire,1 Sascha Dammeier,2 Eiko K. de Jong,9 Berta De la Cerda,4 Cécile Delcourt,1 Anneke I. den Hollander,9,10 Francisco J. Diaz-Corrales,4 Sigrid Diether,2 Eszter Emri,11 Tanja Endermann,3 Lucia L. Ferraro,5 Míriam Garcia,5 Thomas J. Heesterbeek,9 Sabina Honisch,2 Carel B. Hoyng,9 Eveline Kersten,9 Ellen Kilger,2 Caroline C.W. Klaver,7,8,9 Hanno Langen,12 Imre Lengyel,11 Phil Luthert,13 Cyrille Maugeais,12 Magda Meester-Smoor,7,8 Bénédicte M.J. Merle,1 Jordi Monés,5 Everson Nogoceke,12 Tunde Peto,14 Frances M. Pool,15 Eduardo Rodríguez,5 Marius Ueffing,2,16 Karl U. Ulrich Bartz-Schmidt,2,16 Elisabeth M. van Leeuwen,7,8 Timo Verzijden,7,8 Markus Zumbansen17
1University Bordeaux, Inserm, Bordeaux Population Health Research Center, Team LEHA, UMR 1219, Bordeaux, France.
2Centre for Ophthalmology, Institute for Ophthalmic Research, Eberhard Karls University Tübingen, University Clinic Tübingen, Tübingen, Germany.
3Assay Development, AYOXXA Biosystems GmbH, Cologne, Germany.
4Department of Regeneration and Cell Therapy, Andalusian Molecular Biology and Regenerative Medicine Centre (CABIMER), Seville, Spain.
5Barcelona Macula Foundation, Barcelona, Spain.
6Business Development, AYOXXA Biosystems GmbH, Cologne, Germany.
7Department of Epidemiology, Erasmus Medical Center, Rotterdam, Netherlands.
8Department of Ophthalmology, Erasmus Medical Center, Rotterdam, Netherlands.
9Department of Ophthalmology, Radboud University Medical Center, Nijmegen, Netherlands.
10Department of Human Genetics, Radboud University Medical Center, Nijmegen, Netherlands.
11Centre for Experimental Medicine, Queen's University Belfast, Belfast, United Kingdom.
12Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
13Institute of Ophthalmology, University College London, London, United Kingdom.
14Centre for Public Health, Queen's University Belfast, Belfast, United Kingdom.
15Ocular Biology, UCL Institute of Opthalmology, London, United Kingdom.
16Department of Ophthalmology, University Medical Centre Tübingen, Tübingen, Germany.
17Research and Development, AYOXXA Biosystems GmbH, Cologne, Germany.
Supplementary Data
References
- 1.Bourne R.R., Jonas J.B., Flaxman S.R. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. Br J Ophthalmol. 2014;98(5):629–638. doi: 10.1136/bjophthalmol-2013-304033. [DOI] [PubMed] [Google Scholar]
- 2.de Jong P.T. Age-related macular degeneration. N Engl J Med. 2006;355(14):1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki E., Campbell M., Kiang A.S. Inflammation in age-related macular degeneration. Adv Exp Med Biol. 2014;801:229–235. doi: 10.1007/978-1-4614-3209-8_30. [DOI] [PubMed] [Google Scholar]
- 4.Wong W.L., Su X., Li X. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 5.Lindekleiv H., Erke M.G. Projected prevalence of age-related macular degeneration in Scandinavia 2012-2040. Acta Ophthalmol. 2013;91(4):307–311. doi: 10.1111/j.1755-3768.2012.02399.x. [DOI] [PubMed] [Google Scholar]
- 6.Bauer P., Barthelmes D., Kurz M. The potential effect of population development, smoking and antioxidant supplementation on the future epidemiology of age-related macular degeneration in Switzerland. Klin Monbl Augenheilkd. 2008;225(5):376–379. doi: 10.1055/s-2008-1027264. [DOI] [PubMed] [Google Scholar]
- 7.Friedman D.S., O'Colmain B.J., Munoz B. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 8.Rein D.B., Wittenborn J.S., Zhang X. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 9.Owen C.G., Jarrar Z., Wormald R. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012;96(5):752–756. doi: 10.1136/bjophthalmol-2011-301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaver C.C., Wolfs R.C., Vingerling J.R. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116(5):653–658. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 11.Korb C.A., Kottler U.B., Wolfram C. Prevalence of age-related macular degeneration in a large European cohort: results from the population-based Gutenberg Health Study. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1403–1411. doi: 10.1007/s00417-014-2591-9. [DOI] [PubMed] [Google Scholar]
- 12.Hoeg T.B., Ellervik C., Buch H. Danish Rural Eye Study: epidemiology of adult visual impairment. Ophthalmic Epidemiol. 2016;23(1):53–62. doi: 10.3109/09286586.2015.1066396. [DOI] [PubMed] [Google Scholar]
- 13.Prokofyeva E., Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47(4):171–188. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- 14.Claessen H., Genz J., Bertram B. Evidence for a considerable decrease in total and cause-specific incidences of blindness in Germany. Eur J Epidemiol. 2012;27(7):519–524. doi: 10.1007/s10654-012-9705-7. [DOI] [PubMed] [Google Scholar]
- 15.Skaat A., Chetrit A., Belkin M. Time trends in the incidence and causes of blindness in Israel. Am J Ophthalmol. 2012;153(2):214–221.e1. doi: 10.1016/j.ajo.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Delcourt C., Korobelnik J.F., Buitendijk G.H. Ophthalmic epidemiology in Europe: the “European Eye Epidemiology” (E3) consortium. Eur J Epidemiol. 2016;31(2):197–210. doi: 10.1007/s10654-015-0098-2. [DOI] [PubMed] [Google Scholar]
- 17.Gragoudas E.S., Adamis A.P., Cunningham E.T., Jr. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 18.Wolf S. Current status of anti-vascular endothelial growth factor therapy in Europe. Jpn J Ophthalmol. 2008;52(6):433–439. doi: 10.1007/s10384-008-0580-4. [DOI] [PubMed] [Google Scholar]
- 19.Cachulo Mda L., Lains I., Lobo C. Age-related macular degeneration in Portugal: prevalence and risk factors in a coastal and an inland town. The Coimbra Eye Study - Report 2. Acta Ophthalmol. 2016;94(6):e442–e453. doi: 10.1111/aos.12950. [DOI] [PubMed] [Google Scholar]
- 20.Cachulo Mda L., Lobo C., Figueira J. Prevalence of Age-Related Macular Degeneration in Portugal: The Coimbra Eye Study - Report 1. Ophthalmologica. 2015;233(3–4):119–127. doi: 10.1159/000371584. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen R., Chakravarthy U., Vingerling J.R. Grading of age-related maculopathy for epidemiological studies: is digital imaging as good as 35-mm film? Ophthalmology. 2003;110(8):1540–1544. doi: 10.1016/S0161-6420(03)00501-3. [DOI] [PubMed] [Google Scholar]
- 22.Eurostatv3.1.15-20160425-5608-PROD_EUROBASE. http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=proj_15npms&lang=en Accessed February 8, 2016.
- 23.Haldane J.B. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet. 1956;20(4):309–311. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 24.Division UNSComposition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. Available at: https://unstats.un.org/unsd/methodology/m49; Accessed March 18, 2015
- 25.Klein R., Chou C.F., Klein B.E. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 26.Borger P.H., van Leeuwen R., Hulsman C.A. Is there a direct association between age-related eye diseases and mortality? The Rotterdam Study. Ophthalmology. 2003;110(7):1292–1296. doi: 10.1016/S0161-6420(03)00450-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Xue Y., Thapa S. Relation between age-related macular degeneration and cardiovascular events and mortality: a systematic review and meta-analysis. Biomed Res Int. 2016;2016:8212063. doi: 10.1155/2016/8212063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuinness M.B., Karahalios A., Kasza J. Survival bias when assessing risk factors for age-related macular degeneration: a tutorial with application to the exposure of smoking. Ophthalmic Epidemiol. 2017:1–10. doi: 10.1080/09286586.2016.1276934. [DOI] [PubMed] [Google Scholar]
- 29.Klein R., Knudtson M.D., Lee K.E. Age-period-cohort effect on the incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2008;115(9):1460–1467. doi: 10.1016/j.ophtha.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crimmins E.M., Hayward M.D., Hagedorn A. Change in disability-free life expectancy for Americans 70-years-old and older. Demography. 2009;46(3):627–646. doi: 10.1353/dem.0.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch M.B., Davidsen M., Andersen L.V. Increasing prevalence despite decreasing incidence of ischaemic heart disease and myocardial infarction. A national register based perspective in Denmark, 1980-2009. Eur J Prev Cardiol. 2015;22(2):189–195. doi: 10.1177/2047487313509495. [DOI] [PubMed] [Google Scholar]
- 32.Davies A.R., Smeeth L., Grundy E.M. Contribution of changes in incidence and mortality to trends in the prevalence of coronary heart disease in the UK: 1996 2005. Eur Heart J. 2007;28(17):2142–2147. doi: 10.1093/eurheartj/ehm272. [DOI] [PubMed] [Google Scholar]
- 33.Satizabal C.L., Beiser A.S., Chouraki V. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plessz M., Gueguen A., Goldberg M. Ageing, retirement and changes in vegetable consumption in France: findings from the prospective GAZEL cohort. Br J Nutr. 2015;114(6):979–987. doi: 10.1017/S0007114515002615. [DOI] [PubMed] [Google Scholar]
- 35.Pot G.K., Prynne C.J., Almoosawi S. Trends in food consumption over 30 years: evidence from a British birth cohort. Eur J Clin Nutr. 2015;69(7):817–823. doi: 10.1038/ejcn.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jungjohann S.M., Luhrmann P.M., Bender R. Eight-year trends in food, energy and macronutrient intake in a sample of elderly German subjects. Br J Nutr. 2005;93(3):361–378. doi: 10.1079/bjn20041333. [DOI] [PubMed] [Google Scholar]
- 37.OECD (2013), Change in smoking rates: Percentage change over the period 1990-2010 or latest available period, in OECD Factbook 2013, OECD Publishing, Paris. DOI: http://dx.doi.org/10.1787/factbook-2013-graph252-en.
- 38.Huang G.H., Klein R., Klein B.E., Tomany S.C. Birth cohort effect on prevalence of age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 2003;157(8):721–729. doi: 10.1093/aje/kwg011. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell P., Smith W., Attebo K., Wang J.J. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102(10):1450–1460. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 40.Erke M.G., Bertelsen G., Peto T. Prevalence of age-related macular degeneration in elderly Caucasians: the Tromso Eye Study. Ophthalmology. 2012;119(9):1737–1743. doi: 10.1016/j.ophtha.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Augood C.A., Vingerling J.R., de Jong P.T. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE) Arch Ophthalmol. 2006;124(4):529–535. doi: 10.1001/archopht.124.4.529. [DOI] [PubMed] [Google Scholar]
- 42.Jonasson F., Arnarsson A., Sasaki H. The prevalence of age-related maculopathy in Iceland: Reykjavik eye study. Arch Ophthalmol. 2003;121(3):379–385. doi: 10.1001/archopht.121.3.379. [DOI] [PubMed] [Google Scholar]
- 43.Bjornsson O.M., Syrdalen P., Bird A.C. The prevalence of age-related maculopathy (ARM) in an urban Norwegian population: the Oslo Macular study. Acta Ophthalmol Scand. 2006;84(5):636–641. doi: 10.1111/j.1600-0420.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 44.Group C.R., Martin D.F., Maguire M.G. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyer D.S., Heier J.S., Brown D.M. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731–1739. doi: 10.1016/j.ophtha.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Bloch S.B., Larsen M., Munch I.C. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153(2):209–213.e2. doi: 10.1016/j.ajo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Granstam E., Westborg I., Barkander A. Reduced occurrence of severe visual impairment after introduction of anti-Vascular Endothelial Growth Factor in wet age-related macular degeneration - a population- and register-based study from northern Sweden. Acta Ophthalmol. 2016;94(7):646–651. doi: 10.1111/aos.13187. [DOI] [PubMed] [Google Scholar]
- 48.Heraghty J., Cummins R. A layered approach to raising public awareness of macular degeneration in Australia. Am J Public Health. 2012;102(9):1655–1659. doi: 10.2105/AJPH.2012.300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertram B., Gante C., Hilgers R.D. Increase in examinations for cataracts, glaucoma, diabetic retinopathy and age-related macular degeneration: Comparative cross-sectional study between 2010 and 1997 in ophthalmological practices. Ophthalmologe. 2014;111(8):757–764. doi: 10.1007/s00347-013-2966-z. [in German]. [DOI] [PubMed] [Google Scholar]
- 50.Marques A.P., Macedo A.F., Perelman J. Diffusion of anti-VEGF injections in the Portuguese National Health System. BMJ Open. 2015;5(11):e009006. doi: 10.1136/bmjopen-2015-009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keenan T.D., Wotton C.J., Goldacre M.J. Trends over time and geographical variation in rates of intravitreal injections in England. Br J Ophthalmol. 2012;96(3):413–418. doi: 10.1136/bjophthalmol-2011-300338. [DOI] [PubMed] [Google Scholar]
- 52.Yehoshua Z., Gregori G., Sadda S.R. Comparison of drusen area detected by spectral domain optical coherence tomography and color fundus imaging. Invest Ophthalmol Vis Sci. 2013;54(4):2429–2434. doi: 10.1167/iovs.12-11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y.T., Tadarati M., Wolfson Y. Comparison of prevalence of diabetic macular edema based on monocular fundus photography vs optical coherence tomography. JAMA Ophthalmol. 2016;134(2):222–228. doi: 10.1001/jamaophthalmol.2015.5332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.