Abstract
Urinary tract infections (UTI) are extremely common worldwide, incurring significant morbidity and healthcare-associated expenses. Small animal models, which accurately reflect disease establishment and progression, permit dissection of host-pathogen interactions and generation of immunity to infection. In mice, intravesical instillation of uropathogenic E. coli, the causative agent in more than 85% of community acquired UTI, recapitulates many of the stages of infection observed in humans. Until recently, however, UTI could only be modeled in female animals. This limitation has hindered the study of sex-related differences in UTI, as well as other bladder pathologies, such as cancer. Here, we describe a method to instill male mice that allows direct comparison between female and male animals and provide a detailed protocol to assess bladder tissue by flow cytometry as a means to better understand host responses to infection. Together, these approaches will aid in the identification of host factors that contribute to sex biases observed in UTI and other bladder-associated diseases.
Keywords: Infection, Issue 130, urinary tract infection, UTI, catheterization, intravesical instillation, male infection, uropathogenic E. coli, UPEC, bladder, mouse, flow cytometry, immune response, sex bias
Introduction
Urinary tract infections (UTI) are one of the most common infections in developed countries1. Infection rates are similar between females and males among neonates and the elderly2. Premenopausal adult women, however, have a greatly increased incidence of community-acquired UTI compared to men2,3. Given that this disease primarily impacts women, fundamental and clinical research has overwhelmingly focused on UTI in females. However, UTI in men is a significant and understudied health care challenge4. Indeed, because UTI in men are associated with higher morbidity, these infections are routinely defined as complicated4,5.
As our understanding of the central role of sex biases in physiology and pathology evolves, new methods are required to explore this previously neglected aspect of disease. Sex differences play an essential role in immunity and infection; females have higher incidence of autoimmune disease, while males are more susceptible to certain infections, such as tuberculosis, malaria, and HIV6,7. Bladder cancer, another urologic pathology, is significantly more prevalent in men than in women, and several studies have shown a role for androgens in the development of malignancy8,9,10,11,12. Notably, however, investigation of intravesical therapies for bladder cancer is performed exclusively in female animals, due to the inability to repeatedly catheterize male mice13.
The study of UTI pathogenesis relies heavily on rodent models of infection (e.g., mice and rats). Murine models of UTI may employ uropathogens originally isolated from human infections, such as uropathogenic E. coli (UPEC), Klebsiella, Enterococcus, Staphylococcus, or Proteus14. Typically, bacteria are introduced into the bladder via catheterization of the urethra. Following infection, bladders and kidneys can be removed to assess specific parameters of infection, such as bacterial colonization, tissue damage, or host immune response15,16. Universally, however, only female animals are used for UTI research. Indeed, many studies have noted that, due to anatomical reasons, catheterization of male mice is not possible13,17,18,19. More recently, a surgical approach to male instillation has been described, in which the abdomen is opened, the bladder is externally displaced by applying gentle pressure to the opening in the abdomen, and bacteria are injected into the bladder20. This approach enables male infection, at the cost of surgical intervention. Thus, a major caveat of this approach includes the influence of inflammation, on the outcome of infection such as the potential to induce an anti-inflammatory wound-healing response to the incision21. As our interests include understanding sex bias in response to disease, we developed a method of bacterial intravesical instillation in male mice that more closely matches the long-established non-surgical transurethral approach used in female rodents22.
Our model builds upon an established methodology and provides the ability to directly compare the host immune response to UTI in female and male animals. This method will permit dissection of sex-based differences in infection, and potentially offer molecular and cellular clues to the pronounced differences in susceptibility and response to infection between the sexes. Additionally, this model has value beyond UTI studies, allowing the establishment of models to investigate other bladder-associated diseases, such as bladder cancer, prostatitis, under- or over-active bladder syndrome, and interstitial cystitis.
Protocol
Mouse experiments were conducted in accordance with approval of protocol number 2012-0024 by the Comité d'éthique en expérimentation animale Paris Centre et Sud (the ethics committee for animal experimentation), in application of the European Directive 2010/63 EU.
1. Preparation of Catheters
Prepare one pediatric intravenous-access cannula for each group of mice to be infected. Using the inbuilt spring mechanism, divest each cannula of its needle, as instructed by the manufacturer. Discard the needles, preserving only the plastic intravenous cannula.
Sterilize catheters in a laminar flow hood for one ultraviolet (U.V.) cycle, typically 25 - 30 min. Note: Catheters can be used for more than one mouse, however a new catheter should be used between experimental groups, sexes, or bacterial strains.
2. Preparation of Uropathogenic E. coli for Infection
At least two days prior to infection, using a sterile inoculation loop, streak UPEC from a frozen bacterial stock onto a Luria Broth (LB) agar plate, containing antibiotics if appropriate. Incubate at 37 °C overnight. Plates can be stored at 4 °C for up to one week.
In a 100 ml sterile Erlenmeyer flask, inoculate 10 ml LB, containing antibiotics if appropriate, with a single colony from the LB plate and incubate standing at 37 °C overnight (approximately 16 - 18 hr). Note: Standing cultures permit the expression of type 1 pili, which are necessary for infection23. Cultures grown shaking will have less efficient pili expression, and therefore, inconsistent rates of infection.
Measure the optical density (OD)600 of the overnight culture and calculate the number of bacteria per ml using a pre-determined growth curve. As an example, for UPEC strain UTI89, OD600 = 0.35 is equivalent to 2 x 108 CFU per ml, thus, an overnight culture with an OD600 = 2.4 would be equivalent to 13.7 x 108 CFU per ml. Empirically determine the relationship between the OD600 and viable bacteria with every new strain employed for infection.
- Determine the amount of bacteria needed by considering the number of animals to be infected and that each mouse should receive approximately 107 colony-forming units (CFU) in 50 μl PBS. Include in the calculation the ~130 µl dead volume of the catheter and syringe nub and 100 µl needed to determine the inoculum.
- Spin the bacterial suspension in a tabletop microcentrifuge at 17,000 x g for 1 min and resuspend the resulting bacterial pellet at 2 x 108 CFU per ml in PBS. Serially dilute an aliquot of this suspension and plate on LB agar, with antibiotics if appropriate, to determine the exact inoculum for each infection.
Draw the bacterial inoculum into a 1 ml syringe and attach the catheter to the end of the syringe. Tap the syringe to remove any air and depress the plunger to fill the dead air space in the catheter before beginning the instillation.
3. Preparation of Mice
Anesthetize mice via intraperitoneal injection of 100 mg/kg ketamine and 5 mg/kg xylazine. Supplemental heat can be provided.
Ensure that each mouse is fully sedated by squeezing the footpad with medium pressure. Mice are fully sedated when they do not react and require 3 - 5 min to reach this state.
Place mice supine and apply medium pressure to the lower abdomen to empty the bladder of urine. Full bladders feel like a pea under the skin between the ileac crests. Note: Inhaled isoflurane has been used to catheterize female mice15,22, however, it has not been tested whether male mice are sufficiently anesthetized by inhaled isoflurane for this procedure.
4. Transurethral Instillation of Female Mice
Using the non-dominant hand, place the thumb on the tail and a finger of the same hand on the abdomen of the mouse and apply gentle pressure in opposing directions to hold the mouse firmly in place.
- Place the tip of the catheter perpendicular to the mouse at the urethral orifice. With gentle pressure, slide the catheter into the urethra, until the hub meets the urethral orifice, while simultaneously lowering the syringe so that it is parallel to the working surface. The catheter requires no lubrication. Do not push or force the catheter into the urethra. The catheter should slide smoothly and easily into the urethra, with little to no resistance.
- Before instilling the inoculum (step 4.3), once the catheter is in place, very gently pull the abdominal skin towards the head of the mouse with the finger from the non-dominant hand that is already on the abdomen of the mouse (step 4.1). If the catheter is in the urethra, the tissue composing the urethra orifice will not move, whereas if the catheter is incorrectly placed in the vagina, the tissue will move up and away from the catheter. This rapid test should be performed on each mouse.
When the hub of the catheter meets the urethral orifice, slowly dispense 50 µl of the bacterial inoculum. A slow instillation rate minimizes vesicoureteral reflux into the kidney. Slowly remove the catheter to prevent leakage, over a count of 5. Place mice in their cages in a supine position.
5. Transurethral Instillation of Male Mice
Place two thumb forceps cranially and caudally to the mouse's external genitalia. Retract the prepuce to fully expose the glans penis. Once the penis is externally positioned, release the thumb forceps.
Reposition the forceps to stabilize the protruding penis perpendicular to the animal, holding the organ gently but tautly. Visualize the urethral meatus and carefully introduce the catheter into the small opening at the tip of the organ. Gently guide the catheter into the penis, toward the body of the mouse, maintaining gentle tension with the forceps. The catheter requires no lubrication. Do not force the catheter into the penis; the catheter should slide smoothly into the urethra, indicating correct placement.
Once the hub of the catheter meets the tip of the penis, very slowly dispense 50 µl of the inoculum, while maintaining the position of the penis. The quality of the instillation can be noted at this time according to a predetermined instillation quality score, such as that shown in Figure 2.
Retract the catheter slowly, over a count of 5, to prevent leakage of the inoculum. Place mice in their cages in a supine position. Animals should begin to recover 30 - 45 min following administration of the anesthetic.
6. Determination of CFU in Infected Organs
At predetermined time-points, sacrifice mice using approved standard operating procedures (e.g., cervical dislocation or carbon dioxide overdose). Moisten the abdomen thoroughly with 70% ethanol to minimize contamination by fur.
Make an incision across the lower third of the mouse's abdomen of at least 2 cm using scissors and aseptically remove the bladder and any other desired organs, including but not limited to, kidneys, testis, seminal vesicles, or preputial glands, into 5 ml polypropylene snap cap tubes containing 1 ml sterile PBS. Keep all samples on ice.
Homogenize the organs with a handheld homogenizer until almost no solid tissue remains, approximately 15 - 60 sec depending on the organ. Fat does not homogenize well, and is easily recognizable as shiny beige-white tissue. Discard fat prior to or following homogenization. Wash the homogenizer in ethanol followed by PBS between each experimental or organ group. Note: Typically, use handheld homogenizers or bead mill homogenizers. While all of these systems have yielded similar results, as determined by direct comparison of bacterial CFU at 24 hr post-infection, the optimal conditions to homogenize host tissue while preserving bacterial viability should be determined empirically before performing this protocol routinely.
Perform serial dilutions of homogenized organ suspensions. Plate dilutions on LB agar plates, containing antibiotics when appropriate, and incubate overnight (~15 hr) at 37 °C. UPEC strains have very rapid doubling times and as such, care should be taken to not incubate the agar plates too long because this will hinder the ability to count individual colonies.
7. Flow Cytometric Analysis of Bladder Tissue
Prepare digestion buffer containing collagenase at 34 units/mL and DNase at 100 µg/mL, in PBS. Keep on ice. Prepare one 15 ml conical tube per animal to be analyzed and aliquot 1 ml digestion buffer per tube.
At predetermined timepoints, sacrifice mice using approved standard operating procedures (e.g., cervical dislocation or carbon dioxide overdose). Moisten the abdomen thoroughly with 70% ethanol to minimize contamination by fur.
- Make an incision across the lower third of the mouse's abdomen of at least 2 cm using scissors and remove the entire bladder. Mince well with scissors, cutting the bladder into 2 - 3 mm2 sized pieces, typically about 8 pieces.
- Add one minced bladder per tube containing digestion buffer. Shake to wash minced bladder tissue into the digestion buffer. Keep on ice until all bladders are dissected and minced.
Incubate minced bladders for 60 - 75 min at 37 °C, with vigorous shaking by hand for 5 s every 15 min. When the tissue has a glassy transparent appearance, resembling wet tissue paper, digestion is complete. Prolonged digestion will remove surface proteins from the cells needed for identification and should be avoided.
Inactivate the digestion enzymes by adding 2 - 3 mL of ice-cold flow cytometry buffer (PBS containing 2% fetal bovine serum (FBS) and 0.2 mM EDTA) and mix gently. Place tubes on ice.
To ensure a single cell suspension and to remove any connective tissue, pass the contents of the tube through a 100 µm filter placed in a 15 mL conical tube. Gently press any remaining tissue through the filter with the end of a syringe plunger. Wash the filter with an additional 2 mL flow cytometry buffer. Keep samples on ice.
Wash samples by centrifugation for 7 min at 200 x g, 4 °C. Resuspend pellets in 100 µL flow cytometry buffer containing Fc block, diluted to 5 µg/mL and transfer to a 96-well round bottom plate. After 10 - 15 min, add 100 µL desired antibody cocktail to each sample. Incubate samples for 30 - 45 min, on ice, protected from light. Note: Fc block is a reagent used to prevent the binding of the Fc portion of antibodies to cells expressing Fc receptors. Its purpose is to minimize nonspecific antibody binding. Determination of antibodies to use will be dependent upon the hypothesis being tested in the experiment and should be selected with input from the literature. In Figure 3, to measure the overall immune cell infiltration, an anti-CD45 antibody was employed, which is a pan-immune cell protein.
Wash samples by centrifugation for 7 min at 200 x g, 4 °C. Resuspend cell pellets in 200 µL flow cytometry buffer and pass samples through a 40 µm cell strainer on a 5 ml polystyrene tube just prior to acquisition on a flow cytometer.
Collect the entire sample on the flow cytometer. A good digestion will yield 200,000 - 400,000 cells, with 8,000 - 10,000 CD45+ immune cells from naïve mouse bladders, while infected organs will contain more cells16 (Figure 3). Note: Samples prepared for flow cytometric analysis cannot be used to evaluate CFU. It is not recommended to split the bladder into two parts as no evidence exists to demonstrate that UPEC colonization or immune cell infiltration is uniform throughout the tissue.
Representative Results
In the development of this protocol, cohorts of female and male C57Bl/6 mice aged 6 to 8 weeks were instilled, and bacterial burden evaluated in bladders at time points ranging from 1 hr to 30 days. The results from these studies are detailed elsewhere (Zychlinsky Scharff et al., pending). Here, we present representative data from 24 hr infections. Notably, bacterial burden was equivalent between male and female mice at 24 hr post-infection (Figure 1). Little variation in bacterial burden was observed within each group 24 hr post-infection, similar to what has been reported in female mice15,16.
If care is taken to sufficiently empty bladders of urine prior to infection, significant leakage of the inoculum is rarely observed in female mice. However, instillation into male mice resulted in significant amounts of bacterial inoculum leaking from the urethra, particularly during the development stage of this protocol. To determine whether loss of inoculum at the time of infection impacted colonization or the establishment of infection, we employed a rating system for each instillation. Each instillation was scored on a scale of 1 - 5, with 1 being the most optimal, and bacterial colonization was determined 24 hr post-infection (Figure 2, box). While this system was investigator dependent, and therefore, potentially subjective, the primary author of this study determined all scores immediately following instillation, prior to evaluation of bacterial burden in infected bladders. No statistically significant differences existed among the CFU obtained from animals with different instillation scores, as assessed by a nonparametric Kruskal-Wallis test comparing the mean rank of each column with the mean rank of every other column (p = 0.17) and correcting for multiple comparisons using a Dunn's test. From this analysis, it can be concluded that suboptimal instillations (score >3), resulting in substantial leakage of bacterial inoculum during infection, do not significantly impact bacterial colonization at 24 hr (Figure 2, graph). Notably, with experience, the frequency of leaking in male mice diminished significantly.
Finally, the objective in developing this model was to directly compare the immune response to UPEC in female and male animals. To test whether cellular infiltration is altered between the sexes in response to UTI, the number of CD45+ immune cells in naïve and infected cohorts of female and male mice were assessed by flow cytometry. While the number of immune cells present in naïve animals was not different between female and male mice (p = 0.95), there was a statistically significant increase in infiltration into the bladders of infected female mice (p = 0.0015) (Figure 3A-B).
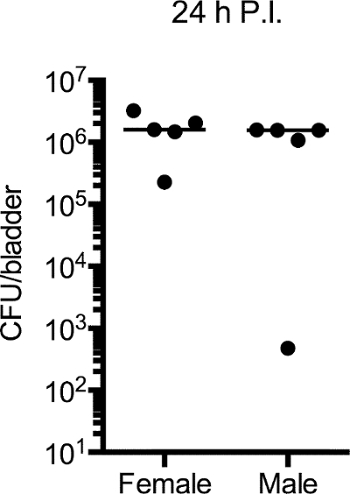 Figure 1:UPEC Colonizes Female and Male Bladders with Equal Efficiency. Six to 8 week old female and male C57Bl/6 mice were instilled with 107 CFU of UPEC. 24 hr post-infection, bladders were aseptically removed to enumerate bacterial burden. Plot depicts CFU/bladder. Each dot is one mouse, representative experiment of 5 shown. Please click here to view a larger version of this figure.
Figure 1:UPEC Colonizes Female and Male Bladders with Equal Efficiency. Six to 8 week old female and male C57Bl/6 mice were instilled with 107 CFU of UPEC. 24 hr post-infection, bladders were aseptically removed to enumerate bacterial burden. Plot depicts CFU/bladder. Each dot is one mouse, representative experiment of 5 shown. Please click here to view a larger version of this figure.
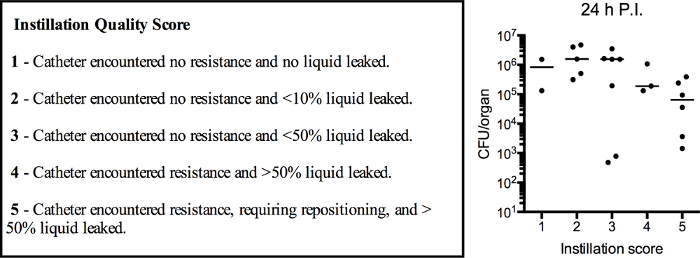 Figure 2:The Quality of Instillation does not Correlate with Bacterial Burden at 24 hr Post-infection. Six to 8 week old female and male C57Bl/6 mice were instilled with 107 CFU of UPEC. Immediately after each instillation, a single researcher assigned a quality score to the instillation, as defined by specific criteria (boxed text). 24 hr post-infection, bladders were aseptically removed to enumerate bacterial burden. Plot depicts the assigned quality score versus CFU/bladder. Each dot is one mouse, 5 pooled experiments are shown. p = 0.17, Kruskal-Wallis test comparing the mean rank of each column with the mean rank of every other column with post hoc Dunn's test for multiple comparisons. Please click here to view a larger version of this figure.
Figure 2:The Quality of Instillation does not Correlate with Bacterial Burden at 24 hr Post-infection. Six to 8 week old female and male C57Bl/6 mice were instilled with 107 CFU of UPEC. Immediately after each instillation, a single researcher assigned a quality score to the instillation, as defined by specific criteria (boxed text). 24 hr post-infection, bladders were aseptically removed to enumerate bacterial burden. Plot depicts the assigned quality score versus CFU/bladder. Each dot is one mouse, 5 pooled experiments are shown. p = 0.17, Kruskal-Wallis test comparing the mean rank of each column with the mean rank of every other column with post hoc Dunn's test for multiple comparisons. Please click here to view a larger version of this figure.
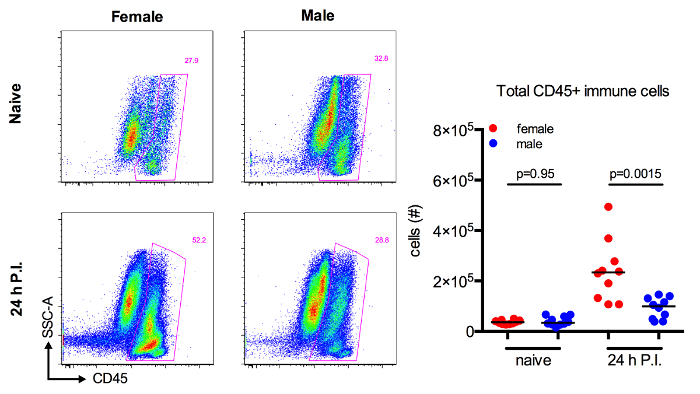 Figure 3:Immune Cell Infiltration is Increased in Female Mice in Response to UTI. Six to 8 week old female and male C57Bl/6 mice were instilled or not with 107 CFU of UPEC. Representative dot plots depict total bladder cells with CD45+ immune cells gated in pink from naïve or infected mice. Graph shows the absolute number of CD45+ immune cells in bladders from naïve or 24 hr infected mice. Each dot is one mouse, 2 pooled experiments are shown. p-values determined by Mann-Whitney test. Please click here to view a larger version of this figure.
Figure 3:Immune Cell Infiltration is Increased in Female Mice in Response to UTI. Six to 8 week old female and male C57Bl/6 mice were instilled or not with 107 CFU of UPEC. Representative dot plots depict total bladder cells with CD45+ immune cells gated in pink from naïve or infected mice. Graph shows the absolute number of CD45+ immune cells in bladders from naïve or 24 hr infected mice. Each dot is one mouse, 2 pooled experiments are shown. p-values determined by Mann-Whitney test. Please click here to view a larger version of this figure.
Discussion
Transurethral instillation of male mice offers many new research opportunities into the influence of sex on bladder mucosal disease, but also presents several challenges. Foremost, one limitation is that the instillation may initially prove to be technically difficult, resulting in excessive inflammation during catheter insertion. Improvement in technique can be achieved by the instillation of dead mice with a colored solution, such as Evans blue dye. To confirm that the instillation is successful, bladders should be visually inspected and the volume of instilled liquid can be evaluated by extraction with a tuberculin syringe. The importance of slow, gentle movements cannot be overstated: there should be no resistance and no force used to insert the catheter, as this will result in excess inflammation and tissue damage. When performed correctly, the catheter will slide effortlessly into the urethra. If resistance or obstruction is felt, it is best to remove the catheter and reattempt insertion.
By correlating the quality of the instillation to bacterial burden 24 hr post-infection, we demonstrated that an imperfect technique, in which a small amount of the bacterial inoculum is lost at the time of infection, still results in robust colonization of the bladder. In utilizing this protocol, an investigator should aim to achieve leak-free instillations. However, the robustness of the procedure ensures that imperfect instillations will still provide useful data and interpretable results.
The great advantage of our technique over the only published alternative method of male bladder infection is its non-invasive, non-surgical approach. Our approach using transurethral instillation follows the physiological route through the urethra to the bladder, without disrupting the structural integrity of the abdomen. The invasive technique, described by Olson and colleagues20, includes factors inherent to surgical procedures, including inflammation, delayed healing, and resultant scar tissue. Especially relevant with regard to studies of immune response to infection is the organism's response to surgical trauma. This includes the formation of a pro-inflammatory milieu and potential tissue granulation, as well as an anti-inflammatory wound healing program as part of the healing process. These factors represent undesirable influences within the experimental setting.
Finally, the objective of this study was to develop a method that allows direct comparison of male and female immune responses during the course of UTI, which can be applied to future studies addressing the influence of sex on bladder mucosal disease, such as infection and cancer biology. As a second distinct advantage, the method described here mirrors that used in female mice for decades19. Thus, experiments performed in male mice with this technique are comparable to a wide body of existing research. Our experiments have revealed that differences in response do exist and that these differences may provide clues to understanding sex-based disparities in response to mucosal infections, as well as other diseases of the bladder.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Dr Matthieu Rousseau for critical reading of the manuscript and the Laboratory of Dendritic Immunobiology for their helpful insights during the development of this protocol and project. This work was supported in part by funding from the European Union Seventh Framework Programme Marie Curie Action (PCIG11-GA- 2012-3221170, and the Immuno-Oncology LabEx (MAI).
References
- Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11(3):551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- Harper M, Fowlis G. Management of urinary tract infections in men. Trends in Urology, Gynaecology & Sexual Health. 2007;12(1):30–35. [Google Scholar]
- Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- Lipsky BA. Urinary tract infections in men. Epidemiology, pathophysiology, diagnosis, and treatment. Ann Intern Med. 1989;110(2):138–150. doi: 10.7326/0003-4819-110-2-138. [DOI] [PubMed] [Google Scholar]
- Conway LJ, Carter EJ, Larson EL. Risk Factors for Nosocomial Bacteremia Secondary to Urinary Catheter-Associated Bacteriuria: A Systematic Review. Urol Nurs. 2015;35(4):191–203. [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Yu CY, Whitacre CC. Sex, MHC and complement C4 in autoimmune diseases. Trends Immunol. 2004;25(12):694–699. doi: 10.1016/j.it.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Castelao JE, et al. Gender- and smoking-related bladder cancer risk. J Natl Cancer Inst. 2001;93(7):538–545. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- Donsky H, Coyle S, Scosyrev E, Messing EM. Sex differences in incidence and mortality of bladder and kidney cancers: national estimates from 49 countries. Urol Oncol. 2014;32(1):40, e23–e31. doi: 10.1016/j.urolonc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Garg T, et al. Gender Disparities in Hematuria Evaluation and Bladder Cancer Diagnosis: A Population-Based Analysis. J Urol. 2014. [DOI] [PMC free article] [PubMed]
- Dobruch J, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2015. [DOI] [PubMed]
- Hsu JW, et al. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am J Pathol. 2013;182(5):1811–1820. doi: 10.1016/j.ajpath.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Behi M, et al. An essential role for decorin in bladder cancer invasiveness. EMBO Mol Med. 2013;5(12):1835–1851. doi: 10.1002/emmm.201302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):05S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- Ingersoll MA, Kline KA, Nielsen HV, Hultgren SJ. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol. 2008;10(12):2568–2578. doi: 10.1111/j.1462-5822.2008.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Bau G, et al. Macrophages Subvert Adaptive Immunity to Urinary Tract Infection. PLoS Pathog. 2015;11(7):e1005044. doi: 10.1371/journal.ppat.1005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira PA, et al. Technical Report: Technique of Bladder Catheterization in Female Mice and Rats for Intravesical Instillation in Models of Bladder Cancer. (Conference Title)39th Scand-LAS and ICLAS Joint Meeting. 2009;36:5–9. [Google Scholar]
- Seager CM, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res (Phila) 2009;2(12):1008–1014. doi: 10.1158/1940-6207.CAPR-09-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L, et al. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin) Infect Immun. 1983;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson PD, Hruska KA, Hunstad DA. Androgens Enhance Male Urinary Tract Infection Severity in a New Model. J Am Soc Nephrol. 2015. [DOI] [PMC free article] [PubMed]
- Knipper JA, et al. Interleukin-4 Receptor alpha Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity. 2015;43(4):803–816. doi: 10.1016/j.immuni.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4(8):1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9(9):2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]


