Abstract
Essential tremor (ET) is the most common type of tremor in adults. While ET does not result in decreased life expectancy, the disabilities associated with ET can have a significant impact on quality of life, mood, functional activities, and socialization. Patients suffering from ET not sufficiently treated with first line medications may be eligible for alternative strategies such as deep brain stimulation, radiofrequency ablation, and MRI guided focused ultrasound (MRgFUS). High-intensity MRgFUS is an emerging modality to treat ET, its attraction for patients being that it is noninvasive and associated with short recovery time, as patients are home the day after treatment. While MRgFUS centers are still limited, it will become important for clinicians to consider MRgFUS as a treatment alternative, particularly in the case of a patient for whom open surgery is contraindicated. This article outlines the steps of patient selection, equipment setup, sonication, and post-treatment follow-up, as well as critical steps to be aware of when performing a MRgFUS procedure.
Keywords: Medicine, Issue 130, Essential tremor, high-intensity focused ultrasound, MRI guided focused ultrasound, movement disorder, functional neurosurgery, thalamotomy, neuroablation
Introduction
Essential tremor (ET) is the most common movement disorder, with a prevalence of up to 4% among individuals 40 year of age or older1. It is characterized by a postural and intention tremor at a frequency of approximately 4 - 7 Hz. It typically affects the upper extremities as well as the head and voice but can also be seen in the legs. ET can be severely debilitating, affecting the ability to manage simple activities of daily living. 15 - 25% of patients are forced to retire prematurely as a result of ET, and 60% of patients do not apply for jobs or promotions due to uncontrollable shaking2. Despite the effectiveness of first line medical therapies, such as propranolol or primidone3, a significant proportion of patients cannot tolerate or are resistant to medical treatments. Patients who remain symptomatic and significantly disabled after trying at least two medications, or who develop intolerable side effects, are considered medication-refractory.
Medically-refractory cases of ET are eligible for surgical interventions. Targeting of the ventralis intermedius (Vim) nucleus of the thalamus, a key cerebello-motor relay structure, with deep brain stimulation (DBS)4 electrodes or radiofrequency ablation can help alleviate tremors5,6. However, both are open neurosurgical procedures, with potential complications including infection and hemorrhage. DBS further involves chronic implantation of an intracranial electrode and battery over the pectoralis major. Furthermore, patients require anesthetics, and in the case of DBS a general anesthetic, as well as hospital stays ranging from one to several days.
MR-guided Focused ultrasound (MRgFUS) is an emerging noninvasive technique to treat various neurologic and psychiatric disorders. The device consists of a helmet that focuses over 1000 ultrasound beams from independent transducers through the intact skull. High-intensity MRgFUS can generate heat at the target leading to coagulative necrosis. A multi-centered randomized sham-controlled trial of thalamotomy for essential tremor demonstrated 47% improvement in tremor score that was durable at 12 months7. This result has led to regulatory approval of MRgFUS as a treatment modality and the subsequent addition of MRgFUS to the clinician's armamentarium for tremor management. This protocol details the steps of patient selection, preparation, and sonication, as well as the important elements of patient follow-up.
Protocol
All human experiments were approved by the Institutional Research Ethics Board at Sunnybrook Health Sciences Centre.
1. Patient Identification
Administer informed consent with the patient or substitute decision maker. Explain potential adverse events related to the procedure, such as bleeding and neurological deficits, to the patient.
Assess the candidate patient for MRgFUS thalamotomy. A physician with expertise in movement disorder should perform the assessment. NOTE: It is important to rule out differential diagnoses such as dystonic tremor, Parkinson's Disease, and psychogenic tremor. Further, the neurologist should assess whether adequate trials of medical treatments have been attempted, and the patient deemed treatment-refractory.
Check for contraindications such as an inability to tolerate an MRI.
Assess the patient's medical fitness along with an anesthetist. NOTE: Active heart disease, impaired kidney function, and propensity for bleeding are all contraindications for MRgFUS.
Obtain bloodwork such as complete blood cell count, electrolytes, creatinine, and coagulation panel as part of the screening process.
Perform a full neurological exam (Table 1) and use the clinical rating scale for tremor (CRST) to document tremor and disability. It is useful to also administer a standard quality of life scale to capture this information at baseline and to follow it over time.
2. Imaging Setup
Obtain a CT scan prior to the day of treatment to assess for any intracranial calcifications and skull thickness. NOTE: The CT scan should have at least 512 by 512 resolution, 1 mm thickness with zero spacing, covering the entire skull from the very top to the skull-base.
Obtain an MR scan prior to the day of treatment to rule out contraindications such as previous intracranial hemorrhage or brain tumor.
Use a commercially available helmet transducer with 1,024 independent elements, each with central frequency 650 kHz and integrated with a 3 Tesla MR scanner8. MRI thermometry is available to provide real time feedback of the target temperature.
3. Patient Preparation
Advise the patient not to take their medication for essential tremor on the day of treatment and not to have anything by mouth beginning at midnight the night before surgery.
Check the patient into the hospital, typically through Same Day Surgery.
Ensure the entire team is present: neurosurgeon, movement disorder neurologist, physicist, imaging technician, and anesthetist. NOTE: An anesthetist should be available to support the patient throughout the procedure in case of discomfort, anxiety, and vertigo/vomiting, while keeping the patient conscious at all times.
Place suction equipment nearby for emergency airway maintenance and peripheral intravenous lines in case medications need to be administered urgently.
Attach non-invasive monitors of heart rate, electrocardiogram, blood pressure, and oxygen saturation.
Insert a Foley catheter, if necessary, for the patient to empty his/her bladder.
Put graduated compression stockings on the patient to help prevent deep venous thrombosis in the lower limbs.
Carefully shave the hair completely and check for scalp lesions.
Place the stereotactic frame with the help of a neurosurgeon and apply local anesthetic (e.g., lidocaine or bupivicaine) at the 4 pin sites.
Place the rubber diaphragm on the patient's head. NOTE: Because the space between the transducer and scalp is filled with degassed water, a rubber diaphragm encircles the patient's head to prevent the water from leaking. Cool water is circulated, carrying away any excess heat from the scalp.
Ensure the patient does not have any ferrous components before entering the procedure room.
Have the patient lie flat, headfirst into the ultrasound helmet.
Cover the patient with a warming blanket to prevent hypothermia. NOTE: The temperature in the treatment room is approximately 15 °C.
4. Target Selection of the Vim nucleus of the thalamus
Perform a preliminary 3D localizer MR scan and T2-weighted sequence with at least axial and sagittal planes immediately prior to sonication to register the patient to pre-treatment CT/MRI scans.
Fuse the new scans with the pre-treatment MRI and CT.
Contour any lesions on the scalp, calcifications in the brain, sinuses, and air volumes as no-pass zones so the transducers can avoid these specific areas.
- Choose the target on the axial T2 weighted image cutting through the anterior commissure (AC) and posterior commissure (PC). NOTE: There are different approaches to identifying and targeting the Vim nucleus of the thalamus. Given that the Vim is not visible even on 3T images, its location is deduced relative to known anatomic landmarks (i.e., indirect targeting).
- At the level of the AC-PC line, begin at 25% of the intercommissural distance, anterior to PC. This is typically approximately 6 mm. NOTE: The average AC-PC distance in humans is 24 - 28 mm, and can range from 20 - 30 mm. Dividing the distance into quarters, one begins by defining the point that is ¼ the distance of the anterior-posterior distance, anterior to PC.
- Laterally, select a point midway between the point 14 mm lateral to midline and the point 11.5 mm lateral to the lateral edge of the ventricle at the level of the AC-PC plane.
- Make small adjustments according to the actual length and width of the specific patient's third ventricle and regional anatomy. NOTE: Third ventricular width can vary widely, especially in elderly patients. It is important to be mindful of internal capsule fibers when determining the laterality of the proposed target. An individualized approach, taking into consideration the patient's specific anatomy, is critical.
5. Ultrasound Delivery
Provide a stop button for the patient so they can abort energy delivery at any time.
Perform a test sonication at low energy by raising the temperature in the target region to around 45 °C.
Verify the alignment of the heating volume to the target and correct in all three dimensions
Raise the temperature further to approximately 50 °C.
After each sonication, perform a screening exam of motor power and sensation to light touch along with a test for the patient's tremor. NOTE: At this point, the patient may experience transient dizziness, tingling and/or numbness.
Adjust the target (i.e., moving anterior if the patient reports parasthesias) according to the patient's responses before increasing the energy to make the permanent lesion around 55-60 oC.
Repeat the sonication until the tremor is reduced to a satisfactory level. NOTE: 12 - 29 sonications each with a duration of 10 - 25 s are typically administered in increments of 0, 1, or 2 °C in an awake patient.
6. Post-treatment
Immediately after treatment, perform a T2-weighted MRI to assess for lesion size and associated radiologic findings such as edema.
Once satisfactory, take the patient out of the scanner and remove the frame.
Check pin sites for bleeding. A few minutes of pressure will usually stop this.
Perform another neurologic exam (Table 1). NOTE: This exam is important to rule out any neurologic deficits such as paresthesia, weakness, speech or visual deficits.
Admit the patient to a post-surgical unit overnight for observation.
Perform an MRI on day one post-treatment to confirm the presence of the lesion and rule out any adverse events.
Request assessment by a neurosurgeon if an adverse event (e.g., mass effect on imaging, intracranial hemorrhage, or decreased level of consciousness) is detected.
Discharge the patient in the morning if they are doing well.
7. Follow-up
Schedule the patient for their first follow-up visit approximately 1 week after treatment.
Review the patient's tremor symptoms, side effects, medication changes, and neurologic exam.
Perform the CRST and quality of life measure to document and follow tremor and disability.
Document any adverse events including pin-site infection, motor deficits, sensory disturbances, and speech problems.
Request assessment of an adverse event by a physician, if necessary. Request a CT scan if the patient has a new neurological deficit to rule out intracranial etiology such as hemorrhage.
Representative Results
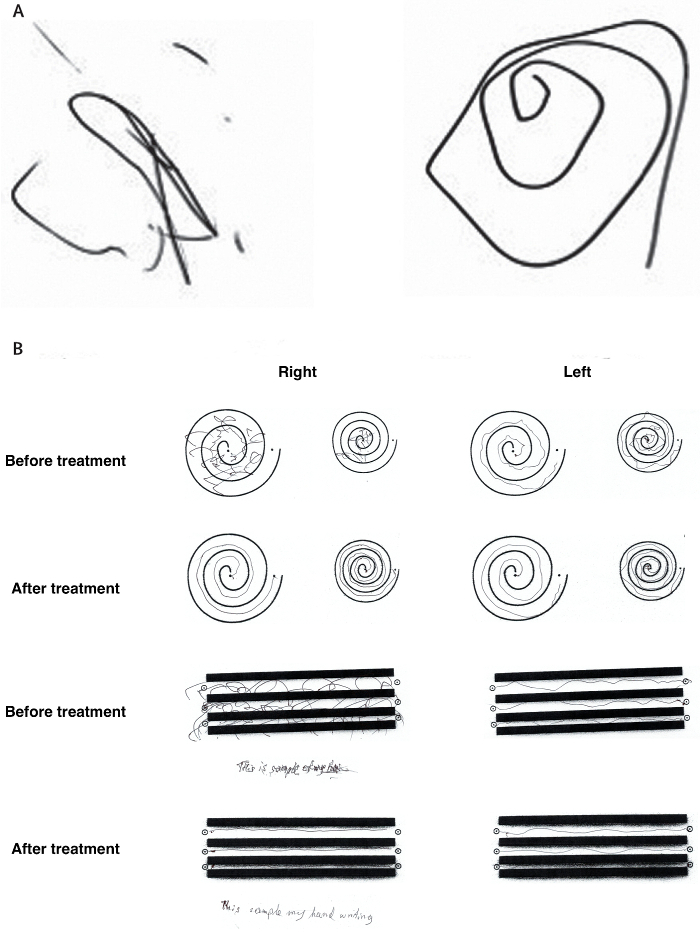
Long-term reduction in tremor in the treated extremity is on average 50% at 3 months and 40% at 12 months. Treatment success can be immediately evaluated after sonication through radiologic findings of a lesion at the Vim (Figure 1) and performance on clinical measures such as the hand-drawn spiral test (Figure 2). Additionally, intraoperative MR thermography provides real-time feedback to the target temperature. A permanent lesion is expected when the temperature reaches 55 - 60 °C.
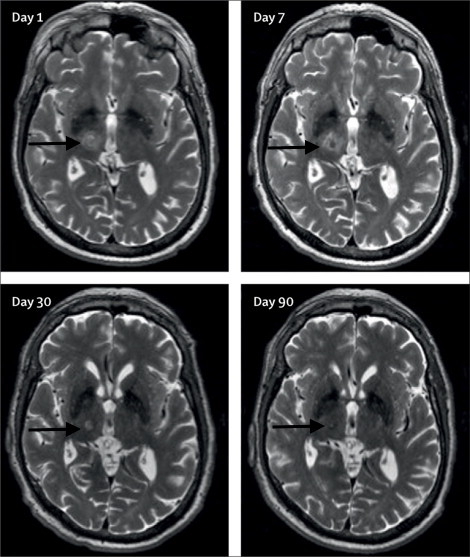
A patient should be assessed for adverse events radiologically and clinically. MRI should reveal any edema, hemorrhage, and mal-placement of the lesion. While edema is usually seen with the lesion, it is typically asymptomatic for the patient and does not warrant any clinical intervention. The pin sites should be examined for infection and scalp for any post-treatment lesions. Soft tissue infection at the pin sites (e.g., redness, tenderness, and swelling) can be treated with a short course of oral antibiotics. If a careful neurological exam reveals any new neurologic deficits, such as weakness, sensory problems, and ataxia, the patient should be assessed and treated by the appropriate physician.
Figure 1: Axial T2-weighted 3T-MRI. Images of the right unilateral thalamotomy for a patient with medically-refractory essential tremor at days 1, 7, 30, and 90 after treatment. Black arrows are pointing to the Vim. This figure has been modified from reference6.
Figure 2:Representative clinical improvement on spiral drawing test. (A) The Archimedean spiral is a clinical tool used to measure severity of essential tremor. The spiral also provides information about the tremor orientation axis. Freehand spiral drawings of a patient immediately before (left) and immediately after (right) thalamotomy demonstrate dramatic improvement. (B) During the clinical rating scale for tremor (CRST), the patient is asked to complete several line drawings. The drawings of the patient before treatment and 3 months after treatment again demonstrate significant improvement. This figure has been modified from reference6. Please click here to view a larger version of this figure.
| Alertness and orientation | |
| Cranial nerves | Cranial nerves II to XII |
| Sensory exam | Pain |
| Position | |
| Vibration | |
| Light touch | |
| Motor exam | Strength |
| Tone | |
| Reflexes | Deep tendon reflexes |
| Pathological reflexes | |
| Coordination and balance | Finger to nose testing |
| Heel to shin testing | |
| Rapid alternating movements | |
| Heel-to-toe walking | |
| Romberg’s test |
Table 1: Checklist for a full neurological exam.
Discussion
MRgFUS at high-intensity can noninvasively create an intracranial lesion. Current high-intensity MRgFUS with continuous wave mode at 650 kHz has been optimized for thermal ablation of deep brain structures, such as the Vim thalamus. The use of MRgFUS has some advantages over existing techniques such as DBS, gamma knife radiosurgery, or radiofrequency ablation for treatment-refractory ET. DBS is an open surgical procedure that can be associated with potential device-related complications, including hardware malfunctions, breakage, and battery depletion. RF ablation is also an open procedure, and associated with trans-cortical passage of brain electrodes. Although gamma knife is a non-invasive alternative, the effects of gamma knife thalamotomy are variable and delayed, often requiring weeks to months to become noticeable10. The coupling of MRI thermography and FUS enables surgeons to monitor lesion location, temperature, and size in real time, thereby reducing the risk of adverse events due to misplacement of lesions.
However, MRgFUS is not without risk. Heat injury to surrounding tissue, with associated pain or neurologic injury, can occur if lesions are too superficial, close to the skull, or adjacent to eloquent regions. Technological advances in imaging software, such as the integration of fiber tracking technology11, will allow for easier positioning and target localisation of the Vim thalamus, thereby possibly decreasing the duration of the procedure and the occurrence of adverse effects. Furthermore, several patient factors are important to consider to maximize the efficiency of the MRgFUS treatment. Skull density, thickness, and shape are critical elements correlated to the amount of acoustic energy needed for successful ablation. From the acoustic perspective, thin and large skulls are in general easier to treat. For thick and dense skulls, particularly if the skull volume is also small, higher energies are required resulting in greater skull heating for patients. Skull and scalp heating can be a source of discomfort and may lead to long-term sequelae. Circulating cool water at low temperatures (10 - 15 °C) between sonications is helpful to minimize heating.
During the sonication process, adjusting the target location based on patient response is key. To avoid permanent sensory deficits if patients report transient sensory symptoms, we typically move our target 1 mm anterior, away from the sensory relay and re-sonicate to check for both adverse events and clinical response. The treatment team must be keenly aware of the anatomy surrounding the Vim, such as its adjacency to the internal capsule on the lateral, inferior dimension. For instance, edema may extend to white matter internal capsule if the size of the lesion is large or the shape is oblique. Therefore, a particularly lateral target warrants extra caution.
High-intensity MRgFUS is an effective option for essential tremor, and is currently being investigated in other disorders, such as OCD and Parkinson's Disease, where a focal lesion may be beneficial to treat medically refractory symptoms. The results of these trials will help determine what role, if any, MRgFUS might play in the management of these disorders, as a less invasive surgical intervention for pathologic brain circuits.
Disclosures
KH is an inventor on patents and patent applications related to neuroablation with focused ultrasound. NL and KH have served as paid consultants for Focused Ultrasound Foundation (FUSF). FUSF is an independent, non-profit organization whose objective is the advancement of research in focused ultrasound technology and its applications. YM, YH, BS, MLS, and NS have no conflicts to declare.
Acknowledgments
We have no source of funding for this article to acknowledge.
References
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- Louis ED. Treatment of essential tremor: are there issues we are overlooking? Front Neurol. 2011;2:91. doi: 10.3389/fneur.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zesiewicz TA, Encarnacion E, Hauser RA. Management of essential tremor. Curr Neurol Neurosci Rep. 2002;2(4):324–330. doi: 10.1007/s11910-002-0008-3. [DOI] [PubMed] [Google Scholar]
- Yu H, Neimat JS. The treatment of movement disorders by deep brain stimulation. Neurotherapeutics. 2008;5(1):26–36. doi: 10.1016/j.nurt.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan A, Raju SS, Kooshkabadi A, Monaco E, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for essential tremor: Retrospective analysis of a 19-year experience. Movement Disorders: Mov Disord. 2017. [DOI] [PubMed]
- Lipsman N, Schwartz ML, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. The Lancet. Neurology. 2013;12(5):462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- Elias WJ, Lipsman N, et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2016;375(8):730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol. 1998;24(2):275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: a review of the published literature. Stereotact Funct Neurosurg. 2006;84:248–251. doi: 10.1159/000096499. [DOI] [PubMed] [Google Scholar]
- Lim SY, Hodaie M, Fallis M, Poon YY, Mazzella F, Moro E. Gamma knife thalamotomy for disabling tremor: a blinded evaluation. Arch Neurol. 2010;67:584–588. doi: 10.1001/archneurol.2010.69. [DOI] [PubMed] [Google Scholar]
- King NKK, Krishna V, Basha D, Elias G, Sammartino F, Hodaie M, Lozano AM, Hutchison WD. Microelectrode recording findings within the tractography-defined ventral intermediate nucleus. J Neurosurg. 2017;126:1669–1675. doi: 10.3171/2016.3.JNS151992. [DOI] [PubMed] [Google Scholar]


