Abstract
Objective
Fluctuations in response to levodopa in Parkinson’s disease (PD) are difficult to treat as tools to monitor temporal patterns of symptoms are hampered by several challenges. The objective was to use wearable sensors to quantify the dose response of tremor, bradykinesia, and dyskinesia in individuals with PD.
Methods
Thirteen individuals with PD and fluctuating motor benefit were instrumented with wrist and ankle motion sensors and recorded by video. Kinematic data were recorded as subjects completed a series of activities in a simulated home environment through transition from OFF to ON medication. Subjects were evaluated using the Unified Parkinson Disease Rating Scale Motor Exam (UPDRS-III) at the start and end of data collection. Algorithms were applied to the kinematic data to score tremor, bradykinesia, and dyskinesia. A blinded clinician rated severity observed on video. Accuracy of algorithms was evaluated by comparing scores with clinician ratings using a receiver operating characteristic (ROC) analysis.
Results
Algorithm scores for tremor, bradykinesia, and dyskinesia agreed with clinician ratings of video recordings (ROC area>0.8). Summary metrics extracted from time intervals before and after taking medication provided quantitative measures of therapeutic response (p<0.01). Radar charts provided intuitive visualization, with graphical features correlated with UPDRS-III scores (R=0.81).
Conclusion
A system with wrist and ankle motion sensors can provide accurate measures of tremor, bradykinesia, and dyskinesia as patients complete routine activities. Significance: This technology could provide insight on motor fluctuations in the context of daily life to guide clinical management and aid in development of new therapies.
Index Terms: Parkinson’s disease, levodopa, dyskinesia, technology, wearble sensors
I. Introduction
Long -term treatment with levodopa for Parkinson’s disease (PD) results in the development of motor complications, including wearing off of medication benefit, fluctuations throughout the day, and abnormal involuntary movements (dyskinesias) [1]. Treatment strategies to reduce the negative consequences of motor complications are diverse and depend on the timeline and presentation of symptoms [2]. Tools to monitor these temporal patterns in symptom fluctuation are hampered by several challenges. Validated rating scales such as the Unified Parkinson’s Disease Rating Scale (UPDRS) [3] and the newer Movement Disorders Society (MDS)-UPDRS [4] include both historical information, which can be subject to recall bias, and a physical examination that provides a valuable but temporally limited snapshot of symptom severity present when a patient is in the clinic and may not reflect the patient’s typical experience at home. Moreover, many of the tasks performed for the motor examination are not ecologically valid in that these movements are not representative of movements performed in everyday life.
The current standard for tracking changes outside of the clinic is the motor diary [5], [6], which requires a patient to choose a category that best matches his/her perceived motor state every half hour throughout the day. Diaries suffer from poor compliance, are prone to subjective errors due to patients’ difficulty differentiating between symptoms, and may lack the sensitivity to detect changes over time [6]. These limitations can make clinical decisions about medication adjustments particularly challenging and also impact clinical drug trials where observing the full dose cycle in the clinic is extremely cost-inefficient and the reduced sensitivity of patient dairies may necessitate a larger sample size [6]. Thus, a reliable method for detecting and monitoring motor signs of PD in an ambulatory setting with minimal patient burden would be highly valuable both for aiding clinicians in optimizing medication regimens and monitoring motor symptoms in clinical trials for new treatments of PD.
Wearable motion sensors and mobile devices such as smartphones have the potential to provide an objective, large-scale measurement of symptom fluctuation with minimal patient burden. Researchers have shown good acceptance of wearable sensors by individuals with PD [7] and high daily use compliance over a 12-week period [8]. A number of research and commercial systems have been developed to obtain accurate and objective measures of PD symptoms, including tremor, dyskinesia, gait, and bradykinesia [9] [23]. These assessments are often completed at discrete points in time and require patients to sit still [9], [23] or complete a predefined movement such as finger-tapping [14] or repetitive tapping of a touch screen [17]. While this approach leverages the cued motor intent to provide highly sensitive measures of symptom severity, it imposes a burden on patients by interrupting their normal lives. Alternative approaches monitor symptoms on a continuous basis during routine activities [21], [22], [24]–[26]. In previous work, we demonstrated accurate quantification of tremor [18], [19] and dyskinesia [10] during activities of daily living. This paper describes the validation of algorithms for continuous simultaneous monitoring of tremor, bradykinesia, and dyskinesia in the context of quantifying levodopa dose response and development of tools for quickly visualizing individualized response patterns. This technology could aid clinicians in maximizing patient outcomes and assist with the evaluation of treatments.
II. Methods
This work was approved by the institutional review boards of Great Lakes NeuroTechnologies, the University of Rochester, and Johns Hopkins University, and performed in accordance with the Declaration of Helsinki. All subjects gave prior informed consent. Thirteen individuals affected by PD with a history of levodopa-induced dyskinesia were recruited to participate in the study (Table 1).
Table I.
Participant Characteristics
| Gender | 7 male, 6 female |
| Age (years) | 59.8 (7.5) |
| Disease Duration (years) | 10.2 (3.2) |
| Levodopa equivalent dose (mg) | 1367 (768) |
| OFF Hoehn & Yahr | 2.6 (0.6) |
| Off State UPDRS Part 3 | 28.4 (12.1) |
All values are mean (standard deviation) unless otherwise noted.
A. Data Collection
Subjects were scheduled to begin the study in the relative OFF medication state either in the morning before the first dose of levodopa or, for patients with predictable end-of-dose wearing off, later in the day, prior to a scheduled dose. At the start of the study session, subjects were instrumented with Kinesia motion sensor units (Great Lakes NeuroTechnologies, Cleveland, OH), each containing a tri-axial gyroscope and triaxial accelerometer, on each wrist and each ankle.
Six activities of daily living stations were setup throughout a testing room. Each station was associated with the following specific activities of daily living: 1) hygiene: brushing hair and brushing teeth, 2) dressing: putting on and taking off a jacket and shoes, 3) eating: setting a table, eating a snack, and/or drinking a beverage, 4) desk work: writing on paper and using a computer, 5) entertainment: reading and/or watching television, and 6) laundry: folding towels and clothes from a basket. Some tasks were performed seated while others were performed standing. Data were recorded as subjects cycled through the stations for a total of two hours. Subjects were instructed to take their usual prescribed dose of dopaminergic medication after completing all stations at least once, enabling capture of kinematic data in the OFF state and during transition from OFF to ON. Once the fully ON state was achieved (based on subjects’ subjective reports and verified by clinical exam), subjects then completed tasks at all stations at least one more time.
B. Clinical Assessment
The Unified Parkinson’s Disease Rating Scale Motor Exam (UPDRS-III) was performed by a clinician not blinded to medication status at the beginning and end of the two-hour period for nine of the subjects and only at the beginning for four of the subjects. Four subjects did not achieve the ON state during the two-hour recording period. Sessions were video recorded for subsequent clinical scoring. Across all subjects, the two-hour long video files were segmented into 288 12-second clips such that a wide range of activities and symptom severities were represented. For each clip, the severity of tremor, dyskinesia, and global bradykinesia were evaluated by a neurologist with extensive experience in PD management (MAB) on 0––4 scales corresponding to 0-none, 1-minimal/subtle, 2-mild, 3-moderate, and 4-severe. This rater was not directly involved in analysis of the motion data and was blinded to the processed motion data.
C. Data Processing and Analysis
For each video clip, corresponding motion data from the wrist and ankle sensors on the more parkinsonian side of the body were processed into tremor and dyskinesia severity scores using previously validated algorithms [10], [18], [20]. In general, the algorithms extract spectral power in frequencies typical of tremor (e.g., 3–8Hz) and dyskinesia (e.g., 0.3–3Hz) and use them in multiple regression models based on expert clinician ratings of individuals performing standardized tasks and scripted activities of daily living. We limited analysis to two sensors as we previously demonstrated that data from two sensors on the more parkinsonian side (one on the upper extremity and one on the lower extremity) can generate accurate predictions and keeping the required number of sensors to a minimum reduces the burden on patients [10].
In addition to leveraging these previously validated algorithms, a bradykinesia quantification algorithm was developed to give a bradykinesia score for every 12-second timespan during which the sensors detected arm movement and/or gait [20]. That is, if the patient was active, the algorithm calculated how active. Specifically, for each 12-second timespan, data from the gyroscopes in both the wrist and ankle sensors were low pass filtered with a 3 Hz cutoff frequency. The magnitude of the angular velocities from each sensor was then calculated, followed by the root-mean-square (RMS) amplitude to give single amplitude values for the wrist and ankle sensors. These two amplitudes were multiplied together, log transformed, and mapped to a 0–4 range linearly based on all data collected in this study such that 90% of scores fall within the 0–4 range where higher values indicate less/slower movement. Values outside of the 0–4 range were set to 0 or 4. This processing was chosen empirically based on its sensitivity and specificity in detecting bradykinesia (see Results) and the 0–4 scaling was chosen to provide outputs on a range clinicians are accustomed to using.
Receiver operating characteristic (ROC) [27] curves were used to determine thresholds for maximizing accuracy (Youden index [28]) in detecting tremor, bradykinesia, and dyskinesia using the clinician scores from the 288 video clips as a ground truth. Clinician scores greater than or equal to one were used to define video clips in which tremor, bradykinesia, and/or dyskinesia were present. The area under the curve (AUC), as well as the sensitivity and specificity achieved using the empirically-determined thresholds were also calculated.
To characterize the dose response over the entire two-hour monitoring period for each subject, the motion sensor data was scored in 12-second epochs with the tremor, dyskinesia, and bradykinesia quantification algorithms. Post-processing with time windowing was applied to reduce noise and identify clear temporal trends as rendering two hours of data as scores every 12 seconds creates relatively noisy outputs over time. Specifically, the median of the individual scores for tremor were calculated across 10-minutes with overlapping segments such that a median score was calculated for every 2-minute period. A median score was also calculated from the individual bradykinesia and dyskinesia scores for every 2-minute period. Thresholds determined from the ROC analysis were then applied to these 2-minute severity scores to detect the presence of tremor and dyskinesia. Basic kinematic parameters used by the algorithms to characterize dyskinesia (e.g., movement velocity, frequency) can be similar to those observed during the completion of some purposeful movements; however, dyskinesias are typically more persistent for longer time periods compared to most purposeful movements [29], [30]. Thus, an additional time threshold was applied for dyskinesia classification such that a minimum duration of 10 minutes was required for an interval to be labeled as dyskinesia being present. For visualization, the algorithm outputs for tremor (severity score) and dyskinesia (binary detection as a yes or no) were viewed versus time. For bradykinesia, histograms were generated showing the distribution of severity scores for specific time ranges (e.g., before and after taking medication).
To examine the 13-subject study sample as a whole, data for each subject was partitioned based on the time at which medication was taken, and several summary metrics (e.g., median severity score, percentage of time detected) were calculated separately for the before and after medication periods. Before taking medication includes the period from the start of data collection to when medication was taken, while after taking medication includes the period from when medication was taken to the end of data collection. These metrics were then compared using paired one-sided Wilcoxon signed rank tests to evaluate their ability to capture the effects of medication.
III. Results
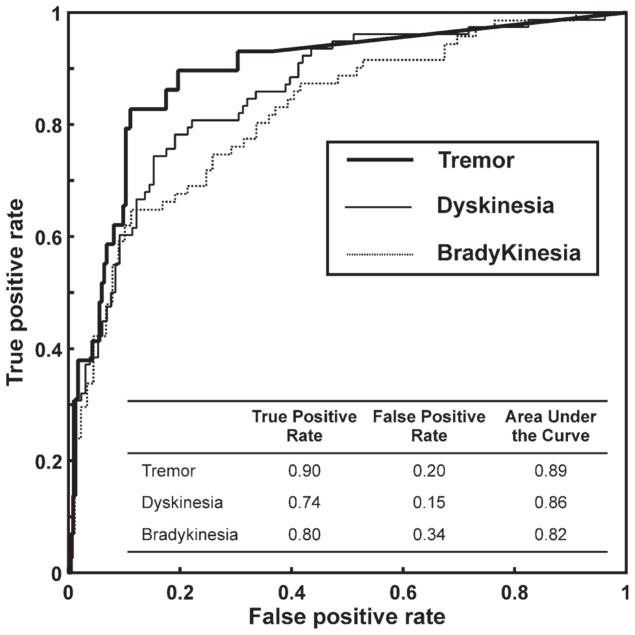
Tremor, bradykinesia, and dyskinesia quantification algorithm outputs were compared to clinician video assessments to calculate the true positive rate (TPR) and false positive rate (FPR) for detection of their respective symptom or side effect at specific threshold levels. ROC curves were used to define thresholds which optimized sensitivity (TPR) and specificity (1-FPR), with results summarized in Fig. 1.
Fig 1.
ROC curves for tremor, dyskinesia, and bradykinesia detection with the results of the analysis summarized in the inset table.
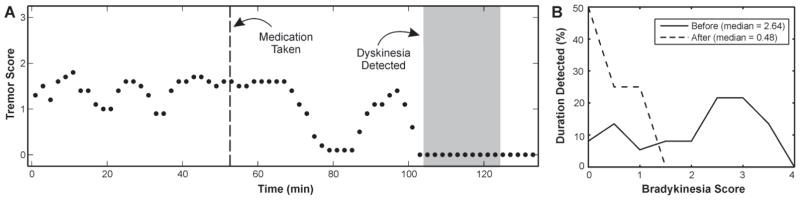
The continuous response for a representative subject is shown in Fig. 2. Tremor scores are higher during the first portion of the monitoring period before the subject took his dose of levodopa. Tremor scores then decrease after the medication dose and eventually, peak dose dyskinesia is detected. The histograms of bradykinesia scores from the periods before and after taking medication illustrate a decrease in severity after the dose.
Fig. 2.
Changes before and after taking medication are shown for a representative subject. A) Algorithms generated tremor severity scores and detected the presence/absence of dyskinesia every 2-minutes before and after medication was taken. Tremor scores are indicated by black dots and time when dyskinesia was detected is shaded gray. The time medication was taken is indicated by a vertical dotted line. B) Histograms of bradykinesia scores are shown for the intervals including all data collected before and after medication was taken.
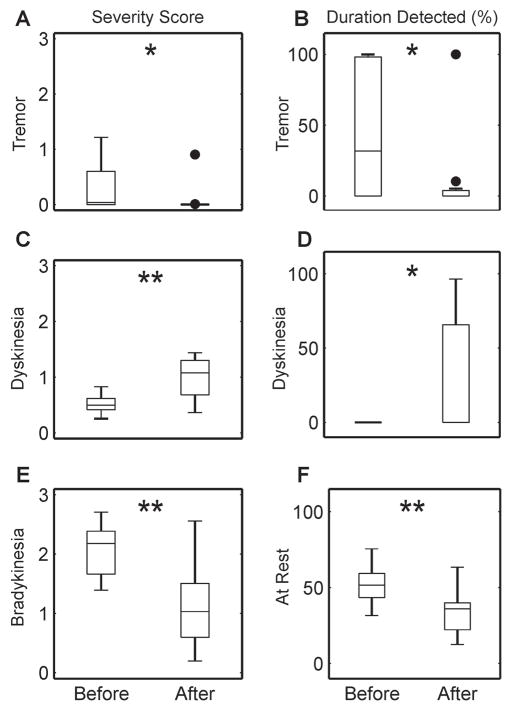
Fig. 3 shows changes in the summary metrics extracted from the time periods before and after taking medication across all subjects. Average algorithm severity scores for tremor, dyskinesia, and bradykinesia exhibited statistically significant changes after taking medication. The duration tremor and dyskinesia were detected as well as the duration spent at rest (no walking or arm activity detected) also changed significantly after taking medication.
Fig. 3.
Boxplots of the objective metrics generated by the symptom quantification algorithms for periods before and after taking medication are shown in each panel. Average severity scores for tremor, dyskinesia, and bradykinesia are shown in Panels A, C, and E, while duration detected of tremor, dyskinesia, and time at rest are shown in Panels B, D, and F. Filled circles indicate outliers. Asterisks indicate statistically significant differences using a one-sided Wilcoxon signed rank test (*p < 0.01, **p < 0.001).
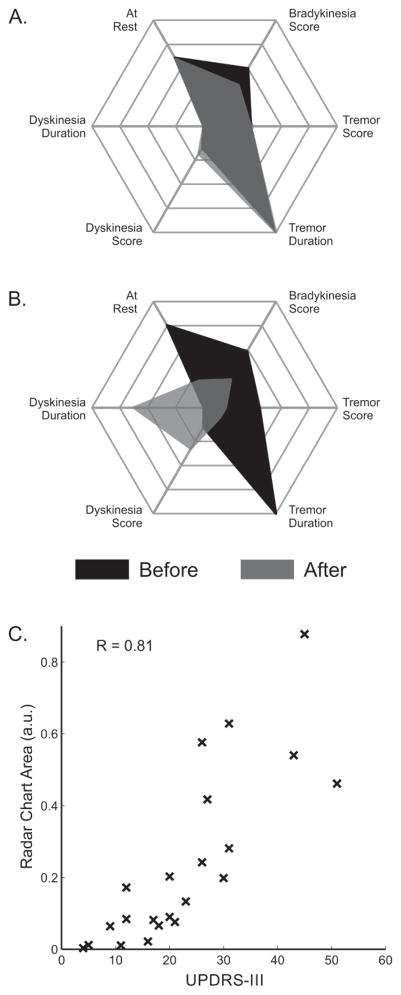
Radar charts were used to visualize the individualized dose response for each subject across all metrics. Examples from two subjects with varied therapeutic responses are shown in Fig. 4. Fig. 4A shows a subject with minimal response to medication (5-point decrease in UPDRS-III), while Fig. 4B shows a subject with a strong response to medication (36-point decrease in UPDRS-III) who also exhibited peak-dose dyskinesia. Across all subjects, areas circumscribed by the radar chart are correlated with the total UPDRS-III completed in that portion of the monitoring period (i.e., before or after medication was taken), with a correlation coefficient of 0.81 (Fig. 4C).
Fig. 4.
Panels A and B show radar charts of the objective metrics from the intervals before and after taking medication illustrating the responses of two subjects. Each axis of the chart represents one of the objective metrics, which range from 0 to 4 for the severity scores and 0 to 100 percent for the duration detected, with larger values indicative of more impairment. The two shaded areas correspond to metrics extracted from the period before (black) and after (grey) taking medication. Panel C compares the areas of the radar charts to the total UPDRS-III scores for all subjects from assessments completed during the corresponding time intervals (before and after medication for the 9 subjects who underwent two UPDRS-III assessments and before medication for the 4 subjects who only underwent a single UPDRS-III assessment).
IV. Discussion
Motion sensors were used to objectively quantify PD motor symptoms on several time scales, clearly distinguishing transitions in medication state. At the smallest resolution (every 12 seconds), the algorithms showed high sensitivity and specificity for detection of tremor, dyskinesia, and bradykinesia (Fig. 1). The dyskinesia algorithm was the least sensitive, which may be expected since dyskinesia can be difficult to discriminate from voluntary motion, unlike tremor (using frequency analyses [9]) or bradykinesia (using differences in the speed and duration of movement). The bradykinesia algorithm has the lowest specificity, which may be a limitation of our approach. When the algorithm detects movement (i.e., gait or arm activity) it calculates the intensity of activity as a metric of bradykinesia. While some actual bradykinesia is likely missed, this excludes volitional slowness/rest from being erroneously classified as bradykinesia to ensure sufficient sensitivity. All summary metrics demonstrated statistically significant changes before and after medication (Fig. 3), supporting their ability to capture the effects of dopaminergic medication. Moreover, quantitative metrics such as those described here may provide an improvement over patient diaries, which typically do not evaluate the severity of OFF time or dyskinesias on a continuous scale [6].
While wearable devices have great potential to revolutionize health care, the amount of information generated presents a growing challenge. This is especially difficult for management of complex conditions like PD where symptom severity and treatment side effects must be carefully balanced to optimize patient outcomes. Presenting the data in an easily understandable fashion is a critical consideration, particularly for clinical care applications where providers have limited time to interpret complex datasets. In this study, subjects’ medication responses were visualized using continuous plots (Fig. 2A), histograms (Fig. 2B), and radar charts (Fig. 4A and 4B). These provide intuitive methods to interpret each subject’s response across multiple domains. The areas of the multivariate radar charts were shown to correlate strongly with total UPDRS-III scores (Fig. 4C). This high correlation was achieved despite the fact that the sensor-based algorithms did not capture a number of items of the UPDRS-III such as speech, facial expression, rigidity, and posture.
This study does include several limitations that should be noted. The activities of daily living in this study were performed at stations in a room set up to simulate a home environment; however, subjects may have moved differently than they would have when interacting with their own familiar objects or in their habitual behaviors. Additionally, subjects were monitored for two hours over the course of a single levodopa dose cycle and UPDRS-III scores were compared to metrics processed from approximately one hour of recorded motion data. As UPDRS-III scores provide a brief snapshot of the patient at a specific time, the high correlations between total UPDRS-III scores and radar plot areas observed in this study may not hold for longer monitoring periods. Future work will include monitoring patients in during their actual daily activities in their homes and communities over extended periods across multiple dose cycles.
V. Conclusion
Our findings suggest that a system with wrist and ankle motion sensors can provide accurate measures of overall tremor, bradykinesia, and dyskinesia that correlate with the UPDRS-III during activities of daily living. Such a system could provide valuable insight on motor fluctuations in the context of daily life and aid in the development and evaluation of new therapies. Future studies are planned to evaluate the impact of this technology on clinical care.
Acknowledgments
This work was supported by the National Institute on Aging of the National Institutes of Health, 5R44AG044293. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NIA. The authors would like to thank Charlotte MacGowan, Arita McCoy, Nicole Bonsavage, and Faisal Alerwy for their assistance with data collection.
This work was supported by the National Institute on Aging of the National Institutes of Health, 5R44AG044293. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NIA.
Footnotes
Conflict of interest
Dr. Pulliam, Dr. Heldman, Dr. Brokaw, and Mr. Mera have received compensation from Great Lakes NeuroTechnologies Inc. for employment. Dr. Mari received research funding from Allergan, Great Lakes NeuroTechnologies Inc. and Merz Pharmaceuticals; served as a principal investigator for Allergan, Ipsen, and Merz; and served as a consultant for Merz. Dr. Burack received research funding from Great Lakes NeuroTechnologies Inc. and owned stock in Novartis.
Contributor Information
Christopher L. Pulliam, Great Lakes NeuroTechnologies Inc, Cleveland, OH, USA
Dustin A. Heldman, Great Lakes NeuroTechnologies Inc, Cleveland, OH, USA.
Elizabeth B. Brokaw, Great Lakes NeuroTechnologies Inc, Cleveland, OH, USA
Thomas O. Mera, Great Lakes NeuroTechnologies Inc, Cleveland, OH, USA
Zoltan K. Mari, Johns Hopkins University, Baltimore, MD, USA
Michelle A. Burack, University of Rochester Medical Center
References
- 1.Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson’s disease. CNS Drugs. 2007;21(8):677–692. doi: 10.2165/00023210-200721080-00005. [DOI] [PubMed] [Google Scholar]
- 2.DeMaagd G, Philip A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. P T. 2015 Aug;40(8):504–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz CC. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord. 2003 Jul;18(7):738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 4.Goetz CG, et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale CMDS-UPDRS): Process, Format, and Clinimetric Testing Plan. Mov Disord. 2007;22(1):41–7. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 5.Hauser R, et al. A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol. 2000;23(2):75–81. doi: 10.1097/00002826-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Papapetropoulos SS. Patient Diaries As a Clinical Endpoint in Parkinson’s Disease Clinical Trials. CNS Neurosci Ther. 2012;18(5):380–387. doi: 10.1111/j.1755-5949.2011.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher JM, et al. Body-Worn Sensors in Parkinson’s Disease: Evaluating Their Acceptability to Patients. Telemed J E Health. 2015 Jul;22(1):1–7. doi: 10.1089/tmj.2015.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira JJ, et al. Quantitative home-based assessment of Parkinson’s symptoms: The SENSE-PARK feasibility and usability study. BMC Neurol. 2015 Jan;15:89. doi: 10.1186/s12883-015-0343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mera TO, Burack MA, Giuffrida JP. Objective motion sensor assessment highly correlated with scores of global levodopa-induced dyskinesia in Parkinson’s disease. J Parkinsons Dis. 2013;3(3):399–407. doi: 10.3233/JPD-120166. [DOI] [PubMed] [Google Scholar]
- 10.Pulliam CL, et al. Motion sensor dyskinesia assessment during activities of daily living. J Parkinsons Dis. 2014;4(4):609–615. doi: 10.3233/JPD-140348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuffrida JP, et al. Clinically deployable Kinesia technology for automated tremor assessment. Mov Disord. 2009;24(5):723–730. doi: 10.1002/mds.22445. [DOI] [PubMed] [Google Scholar]
- 12.Salarian A, et al. Gait assessment in Parkinson’s disease: Toward an ambulatory system for long-term monitoring. IEEE Trans Biomed Eng. 2004;51(8):1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 13.Mera TO, et al. Quantitative analysis of gait and balance response to deep brain stimulation in Parkinson’s disease. Gait Posture. 2013;38(1):109–14. doi: 10.1016/j.gaitpost.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espay AJ, et al. Differential response of speed, amplitude, and rhythm to dopaminergic medications in Parkinson’s disease. Mov Disord. 2011;26(14):2504–2508. doi: 10.1002/mds.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldman DA, et al. The modified bradykinesia rating scale for Parkinson’s disease: Reliability and comparison with kinematic measures. Mov Disord. 2011;26(10):1859–1863. doi: 10.1002/mds.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keijsers N, Horstink M, Gielen S. Ambulatory motor assessment in Parkinson’s disease. Mov Disord. 2006 Jan;21(1):34–44. doi: 10.1002/mds.20633. [DOI] [PubMed] [Google Scholar]
- 17.Memedi M, et al. Automatic and objective assessment of alternating tapping performance in Parkinson’s disease. Sensors (Basel) 2013 Jan;13(12):16965–16984. doi: 10.3390/s131216965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldman DA, et al. Essential tremor quantification during activities of daily living. Park Relat Disord. 2011;17(7):537–542. doi: 10.1016/j.parkreldis.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulliam CL, et al. Continuous in-home monitoring of essential tremor. Park Relat Disord. 2014;20(1):37–40. doi: 10.1016/j.parkreldis.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldman DA, et al. Wearable sensors for quantifying deep brain stimulation washout effects on gait in Parkinson’s disease. Mov Disord. 2015 Apr;30(1):S221. [Google Scholar]
- 21.Griffiths RI, et al. Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J Parkinsons Dis. 2012 Jan;2(1):47–55. doi: 10.3233/JPD-2012-11071. [DOI] [PubMed] [Google Scholar]
- 22.Horne MK, McGregor S, Bergquist F. An objective fluctuation score for Parkinson’s disease. PLoS One. 2015;10(4):e0124522. doi: 10.1371/journal.pone.0124522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopane G, et al. Dyskinesia detection and monitoring by a single sensor in patients with Parkinson’s disease. Mov Disord. 2015 Aug;30(9):1267–1271. doi: 10.1002/mds.26313. [DOI] [PubMed] [Google Scholar]
- 24.Tsipouras MG, et al. An automated methodology for levodopa-induced dyskinesia: Assessment based on gyroscope and accelerometer signals. Artif Intell Med. 2012 Jun;55(2):127–135. doi: 10.1016/j.artmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Tsipouras MG, et al. Automated Levodopa-induced dyskinesia assessment. 2010 Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBC’10; August; 2010. pp. 2411–2414. [DOI] [PubMed] [Google Scholar]
- 26.Zwartjes DGM, et al. Ambulatory monitoring of activities and motor symptoms in Parkinsons disease. IEEE Trans Biomed Eng. 2010 Nov;57(11):2778–2786. doi: 10.1109/TBME.2010.2049573. [DOI] [PubMed] [Google Scholar]
- 27.Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin Biochem Rev. 2008 Aug;29(Suppl 1):S83–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Gour J, et al. Movement patterns of peak-dose levodopa-induced dyskinesias in patients with Parkinson’s disease. Brain Res Bull. 2007 Sep;74(1–3):66–74. doi: 10.1016/j.brainresbull.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Thanvi B, Lo N, Robinson T. Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J. 2007 Jun;83(980):384–388. doi: 10.1136/pgmj.2006.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]