Abstract
Abstract
The signaling molecules TNF-α, AP-1, and NF-κB act to integrate multiple stress signals into a series of diverse antiproliferative responses. Disruption of these processes can promote tumor progression and chemoresistance. Naturally occurring plant derived compounds are considered as attractive candidates for cancer treatment and prevention. Phytoconstituents can control and modify various biological activities by interacting with molecules involved in concerned signaling pathways. The aim of this study was to find binding conformations between phytoconstituents and these signaling molecules responsible for multiple stress signals of UVB induced photodamage. Induced fit docking was carried out for understanding the binding interactions of pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid) with TNF-α, AP-1, and NF-κB. Favorable binding conformations between these signaling molecules and the four phytoconstituents were observed. A number of poses were generated to evaluate the binding conformations and common interacting residues between the ligands and proteins. Among them, the best ligands against TNF-α, AP-1, and NF-κB are reported. The present investigation strongly suggests the probable use of these flavonoids for the amelioration of UVB induced photodamage.
Graphical abstract
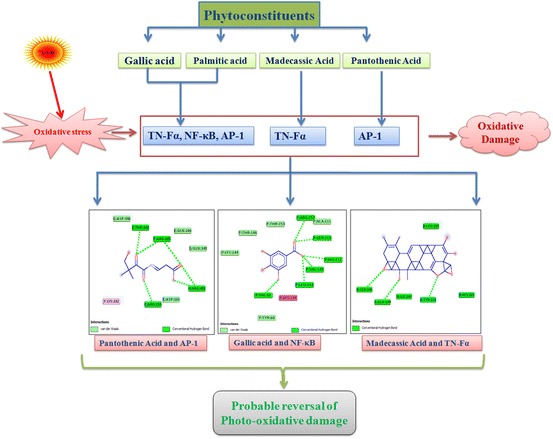
Keywords: Oxidative damage, Docking, Flavonoids, TNF-α, AP-1, NF-κB
Background
Ultraviolet-B (UVB) radiation is the most damaging component of the solar radiation reaching the Earth. UVB is implicated in the generation of free radicals and oxidative damage. It reportedly causes numerous skin disorders in humans and induces a number of harmful responses including inflammation, photoaging and skin cancer (Halliday 2005; Afaq et al. 2005). UVB induced ROS generation can oxidize and damage cellular lipids, proteins and DNA, leading to unfavorable changes including cytological impairments in the structure of skin that could result in inhibition of their regular functions (Sklar et al. 2013). Direct absorption of UVB leads to the formation of cyclobutane–pyrimidine dimers and pyrimidine-pyrimidone (6-4) photoproducts. UVB rays can also alter protein function by directly interacting with aromatic amino acids in proteins (Verschooten et al. 2006).
UVB exposure is known to stimulate inflammatory responses in skin via up regulation of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1, IL-6, and IL-8 (Ishida and Sakaguchi 2007). Enhanced expression of TNF-α under UVB-irradiation is mainly responsible for the activation of multifunctional cytokines such as interleukin-6 (IL-6), COX-2 and nuclear factor kappa-B (NF-κB) expressions (Balupillai et al. 2015). Activation of NF-κB by various pro-inflammatory proteins including TNF-α, COX-2 and IL-6 lead to skin inflammation has also been reported by Young et al. (2008). It is also known that UVB-induced damage to the keratinocytes frequently promotes hot spot mutations in the p53 gene that ultimately may lead to a faulty trigger to apoptosis and there by promote the non-melanoma skin cancers.
The MAPK family includes c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinases (ERK) and p38 kinase. All these three members play important roles in the regulation of cellular functions, such as differentiation, apoptosis, proliferation, inflammation and photo-aging (Sun et al. 2016). UVB-irradiation induced ROS significantly disturb the signaling pathways leading to altered gene expression and activation of mitogen-activated protein kinases (MAPKs) that play a vital role in UVB induced skin damage.
Phytochemicals possess antioxidant properties and their consumptions could effectively salvage damages mediated by free radicals. Therefore phytochemicals are considered as valuable tools for maintaining human health. Attempt have been made in this study to derive support to the ameliorative effects of selected flavanoids by analyzing their molecular docking behavior with some signalling molecules involved in photo-oxidative damages.
Materials and methods
Molecular docking
Molecular docking was performed on Centos 6 Linux workstation using Maestro (Schrodinger LLC 2009, USA) by following Parasuraman et al. 2012. Grid based Ligand Docking with Energetics (GLIDE-6.0) searches were performed for understanding docking interactions of compounds viz; pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid) with TNF-α, NF-kB and AP-1. All molecular modeling was carried out using optimized potential liquid simulation for all atoms (OPLS-AA) force field. Ligprep 2.3 module (Schrodinger) was employed for all compounds preparation. The three-dimensional crystal structures of TNF-α (PDB: 2E7A), NF-kB (PDB: 1SVC) and AP-1 (PDB: 1FOS), were downloaded from the protein data bank (PDB) (http://www.rcsb.org). Protein preparation wizard of Schrodinger was used for TNF-α, NF-kB and AP-1 preparation. No hydrogen atoms were minimized until the average root mean square deviation reached a default value of 0.3 Å. Sitemap 2.3 was used to understand binding site in the ligand-binding domain (LBD) of the above mentioned proteins. Induced fit docking was performed to predict compound binding modes and structural movements in the LBD region of proteins using Glide and Prime modules. The prepared proteins were loaded on the workstation and the grid values were calculated about 20 Å to cover the entire active site amino acids. About 20 conformational images were created and analyzed for the best conformation pose based on the docking score and glide energy.
Molecular modeling calculations
All computational works were performed on Red Hat Enterprise Linux EL-5 workstation using the molecular modeling software Maestro (Schrodinger LLC 2009, USA). GLIDE-6.0 searches were made for favorable docking interactions between one or more ligand molecules with the proteins macromolecules. All the molecular modeling simulations were carried out using OPLS-AA force field (Glide 6.0) (Friesner et al. 2004). Hydrogen bond interactions and hydrophobic contacts were observed between protein and ligand using Ligplot software (Wallace 1995).
Ligand preparation
Compounds like pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid) were built using builder panel in Maestro. The four compounds were taken for ligand preparation by Ligprep 2.3 module (Schrödinger, USA), which performs addition of hydrogen as well as 2D to 3D conversions, measurement of realistic bond lengths and bond angles, assessing low energy structure with correct chiralities, ionization states, tautomers, stereochemistries and ring conformations.
Protein preparation and active site prediction
For the docking study, modifications were carried out in TNF-α (PDB: 2E7A), NF-kB (PDB: 1SVC) and AP-1 (PDB: 1FOS). Missing hydrogen atoms were added and correct bond orders were assigned, and then formal charges and orientation of various groups were fixed. Following this, optimization of the amino acid orientation of hydroxyl groups and amide groups were carried out. All amino acid flips were assigned and H-bonds were optimized. Non hydrogen atoms were minimized until the average root mean square deviation reached default value of 0.3 Å. Sitemap 2.3 was used to explore binding site in the docking studies (Halgren 2007).
Induced fit docking
The prepared protein was loaded in the workspace and the sitemap predicted active site was specified for IFD. Grid was calculated about 20 Å to cover all the active site residues defined by the sitemap. The van der waal’s radii of non polar receptor and ligand atoms were scaled by a default factor of 0.50. IFD calculations were carried out for the pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid) with the TNF-α (PDB: 2E7A), NF-kB (PDB: 1SVC) and AP-1 (PDB: 1FOS) receptors. Following this, 20 conformational poses were calculated and the best conformational pose was selected based on the docking score, glide energy, hydrogen bonding and hydrophobic bonding interactions.
Results
Induced fit docking studies using pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid) with the TNF-α (PDB: 2E7A), NF-kB (PDB: 1SVC) and AP-1 (PDB: 1FOS) (Fig. 1) have revealed that the interactions with flavonoids have significant levels of docking score and glide energies (Table 1). The glide energy scores against TNF-α (PDB: 2E7A) were found to be − 6.72943, − 9.85743, and − 7.6138 kcal/mol for 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid), respectively (Table 1 and Fig. 2). The glide energy scores against AP-1 (PDB: 1FOS) were found to be − 7.31009, − 6.13536 and − 6.43068 kcal/mol for pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid) and hexadecanoic acid, ethyl ester (palmitic acid), respectively (Table 1 and Fig. 3). The glide energy scores against NF-kB (PDB: 1SVC) were found to be − 7.01477 and − 8.72394 kcal/mol for 3,4,5-trihydroxy benzoic acid (gallic acid) and hexadecanoic acid, ethyl ester (palmitic acid), respectively (Table 1 and Fig. 4). All the flavonoids used in this study, showed very good glide energy scores, docking scores and hydrogen bond interaction with all the tested proteins.
Fig. 1.
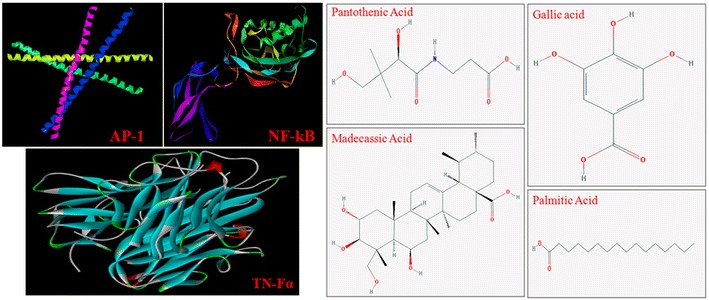
Structure of proteins and compounds used for docking analysis
Table 1.
Induced fit docking of selected flavonoids
| Proteins | Flavonoids | Docking score/energy (kcal/mol) | Hydrogen bond interactions | Distance between donor and acceptor (Å) | No. of hydrogen bond |
|---|---|---|---|---|---|
| 2E7A (TNF-α) | 3,4,5-Trihydroxy benzoic acid (Gallic acid) | − 6.72943 | TYR119 (O–H….O) | 2.80 | 3 |
| TYR261 (O–H….O) | 2.76 | ||||
| ILE58 (O….O) | 2.35 | ||||
| Madecassic acid | − 9.85743 | TYR115 (O–H….O) | 2.51 | 3 | |
| SER245 (O….O) | 2.90 | ||||
| GLN149 (O….O) | 3.02 | ||||
| Hexadecanoic acid, ethyl ester (Palmitic acid) | − 7.6138 | ILE208 (O….N) | 3.30 | 1 | |
| 1FOS (AP-1) | Pantothenic acid (vitamin B5) | − 7.31009 | ARG159 (N….O) | 2.22 | 6 |
| THR162 (O….O) | 2.18 | ||||
| ARG285 (N….O) | 2.61 | ||||
| ARG285 (N….O) | 2.68 | ||||
| ARG401 (N….O) | 3.00 | ||||
| ARG401 (N….O) | 2.57 | ||||
| 3,4,5-Trihydroxy benzoic acid (Gallic acid) | − 6.13536 | GLN166 (N….O) | 3.00 | 7 | |
| THR352 (O….O) | 2.36 | ||||
| ARG401 (N….O) | 2.98 | ||||
| ARG404 (N….O) | 2.80 | ||||
| GLU356 (O….O) | 2.53 | ||||
| ASP170 (O….O) | 3.02 | ||||
| GLN349 (O….O) | 2.76 | ||||
| Hexadecanoic acid, ethyl ester (Palmitic acid) | − 6.43068 | THR306 (O….Cl) | 2.98 | 1 | |
| 1SVC (NF-κB) | 3,4,5-Trihydroxy benzoic acid (Gallic acid) | − 7.01477 | VAL61 (N….O) | 2.86 | 6 |
| HIS112 (N….O) | 3.05 | ||||
| SER113 (O….O) | 2.71 | ||||
| VAL145 (N….O) | 2.77 | ||||
| ARG157 (N….O) | 2.61 | ||||
| LEU143 (O….O) | 2.76 | ||||
| Hexadecanoic acid, ethyl ester (Palmitic acid) | − 8.72394 | PRO51 (C….Cl) | 2.99 | 3 | |
| SER237 (C….Cl) | 3.59 | ||||
| VAL85 (N….Cl) | 3.16 |
Fig. 2.
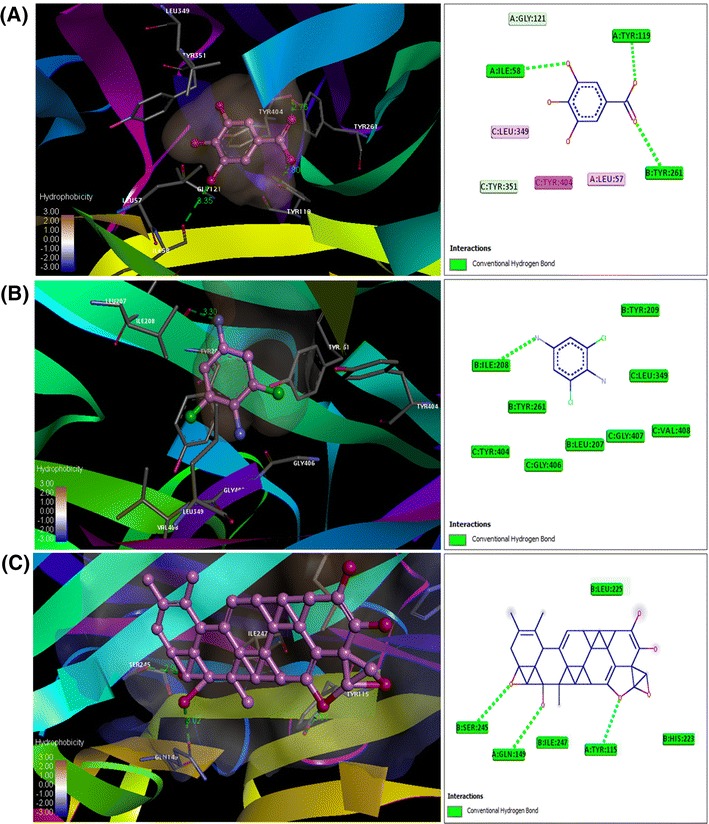
Induced-fit docking models of the flavonoids a 3,4,5-trihydroxy benzoic acid (Gallic acid); b Hexadecanoic acid, ethyl ester (Palmitic acid) and c Madecassic Acid, at drug-binding sites of TNF-α. Pymol on left side and Ligplot on right side. Hydrogen bonds are shown by green dotted lines
Fig. 3.
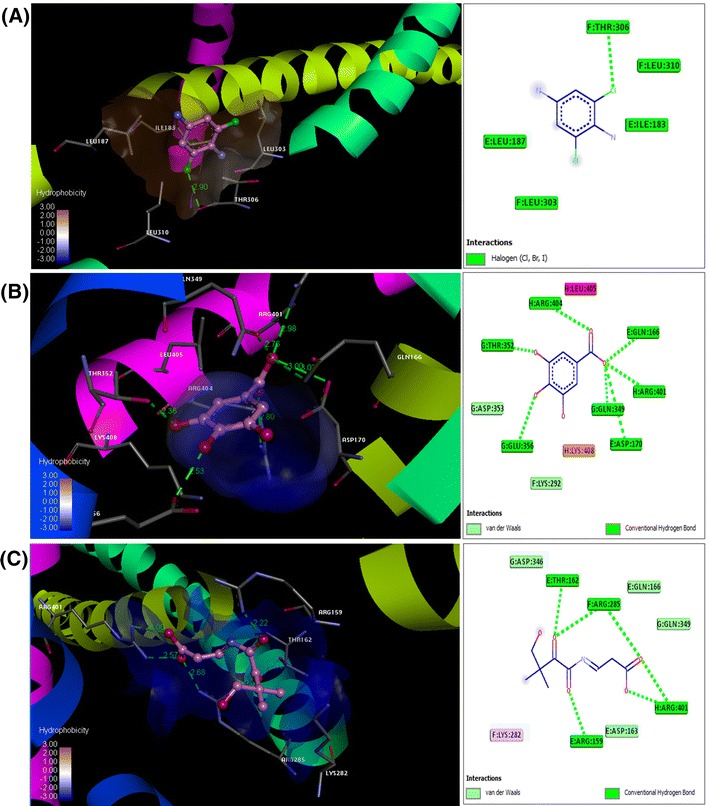
Induced-fit docking model of the flavonoids a hexadecanoic acid, ethyl ester (Palmitic acid); b 3,4,5-trihydroxy benzoic acid (Gallic acid) and c pantothenic acid (vitamin B5), at drug-binding sites of AP-1. Pymol on left side and Ligplot on right side. Hydrogen bonds are shown by green dotted lines
Fig. 4.
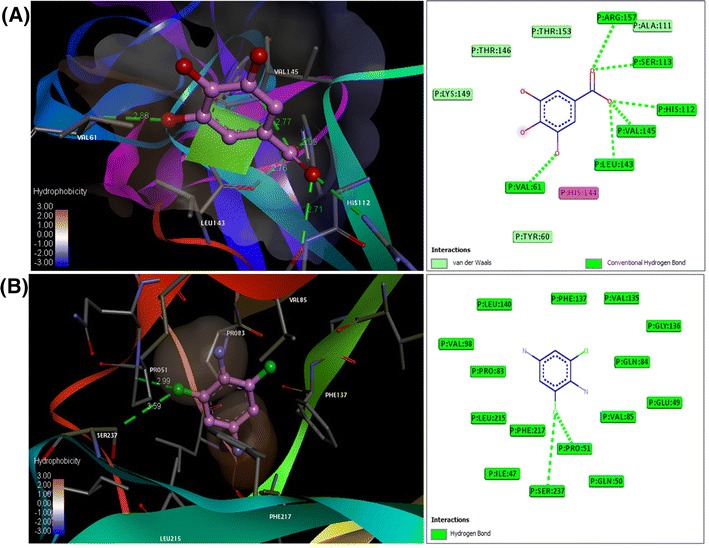
Induced-fit docking model of the flavonoids a 3,4,5-trihydroxy benzoic acid (Gallic acid) and b hexadecanoic acid, ethyl ester (Palmitic acid), at drug-binding sites of NF-kB. Pymol on left side and Ligplot on right side. Hydrogen bonds are shown by green dotted lines
Discussion
Environmental stressors like UVB-irradiation alter the production of some pro-inflammatory cytokines, apoptotic and photo-aging markers such as tumor necrosis factor-alpha (TNF-α), NF-kB, interleukin IL-6, p53 and AP-1, leading to the process of inflammation, apoptosis and photo-aging in human epidermal keratinocytes. However flavonoids with significant antioxidant properties exert regulation of different signaling molecules in UVB-induced photo-damage (Mansuri et al. 2014).
NF-κB is found in almost all animal cell types and is involved in cellular responses to different stimuli such as cytokines, free radicals, heavy metals, ultraviolet irradiation, and bacterial or viral antigens (Gilmore 2006; Brasier 2006; Perkins 2007). NF-kB plays an important role in inflammatory pathways, mostly induced by oxidative stress. NF-κB transcription factors also regulate the expression of hundreds of genes that are involved in regulating cell growth, differentiation, development, and apoptosis (Oeckinghaus and Ghosh 2009). Hence methods of inhibiting NF-κB signalling could have potential therapeutic applications in cancer and inflammatory diseases. The discovery that oxidative stress mediated activation of NF-κB nuclear transcription factor could be kept under control by phytochemicals, gives a promising avenue for developing novel strategies for targeting NF-κB inhibition (Vlahopoulos et al. 1999). Drugs such as disulfiram, olmesartan and dithiocarbamates that are capable of inhibiting the nuclear factor-κB (NF-κB) signalling cascade are already available in market (Cvek and Dvorak 2007).
Induced fit docking (IFD) is one of the main complicating steps in docking studies, which predicts accurate ligand-binding modes and concomitant structural movements in the receptor using Glide and Prime modules. In IFD, when ligand binds to the receptor, it undergoes side chain or backbone conformational changes or both, in many proteins. These conformational changes allow the receptor for better binding according to the shape and binding mode of the ligand (Keskin 2007). The present investigation has revealed that the interactions of NF-kB (PDB: 1SVC) with 3,4,5-trihydroxy benzoic acid (gallic acid) and hexadecanoic acid ethyl ester (palmitic acid) produced the GLIDE energy scores of − 7.01477 and − 8.72394 kcal/mol, respectively. The results of the present study are significant in the light of the previous report by Ramachandra et al. (2008), that the interaction of daunorubicin and dexamethasone (well-known drugs for NF-kB inhibition) with NF-kB yielded the glide energy score of − 6.15 and − 7.6, respectively. The present study has also indicated better GLIDE scores than the ones previously reported for the interaction of NF-kB with Coclaurine (− 4.199), hirsutine (− 3.993) and haiderine (4.103), isolated from Cocculus hirsutus L. (Thavamani et al. 2016).
TNF-α is a critical cytokine involved in various autoimmune diseases as it plays an extensive role in inflammatory disorders by acting as a central regulator of inflammatory pathways (Esposito and Cuzzocrea 2009). The primary role of TNF-α is in the regulation of immune cells. TNF-α, being an endogenous pyrogen, is able to induce fever, inflammation, apoptotic cell death as well as inhibition of tumorigenesis and viral replication. Deregulation of TNF-α production has been implicated in a variety of human diseases including cancer (Locksley et al. 2001), psoriasis (Victor and Gottlieb 2002), inflammatory bowel disease (IBD) (Brynskov et al. 2002), Alzheimer’s disease (Swardfager et al. 2010) and major depression (Dowlati et al. 2010). Inhibition of TNF-α effect can be achieved with a number of drug formulations such as infliximab (Remicade), adalimumab (Humira), certolizumab pegol (Cimzia), and golimumab (Simponi), thalidomide (Immunoprin) and its derivatives (Haraoui and Bykerk 2007). However, most of these TNF-α inhibitors exhibit serious side effects including secondary infections (especially reactivation of latent tuberculosis), lymphomas, demyelinating disease, congestive heart failure, a lupus-like syndrome, injection site reactions, induction of auto-antibodies, and systemic side effects (Scheinfeld 2004).
The effects of TNF-α are inhibited by several natural compounds, including curcumin (a compound present in turmeric) (Gulcubuk et al. 2006), and catechins (in green tea) (Chen et al. 2011) without any apparent side effects. Activation of cannabinoid (CB1 or CB2) receptors by cannabis or Echinacea purpurea also seems to have anti-inflammatory properties through TNF inhibition (Raduner et al. 2006). In the present study the docking of 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid) with TNF-α shows significant levels of interactions (Table 1) compared to the previously reported interactions of isorhamnetin-3-o-rutinoside, Kaempferol and kaempferol-3-glucuronide with TNF-α having glide scores of − 9.1, − 4.8 and − 7.0, respectively (Shankaran et al. 2016).
Reactive oxygen species (ROS) are necessary participants in multiple MAPK (mitogen activated protein kinase) pathways that are responsible for the activation of Activator protein 1 (AP-1) which in turn, up regulates MMP expressions (Jung et al. 2014). AP-1 controls a number of cellular processes including differentiation, proliferation, apoptosis and photoaging (Mukherjee et al. 2006). Increased AP-1 levels lead to increased transactivation of target gene expression (Vesely et al. 2009). Regulation of AP-1 activity is therefore important for cell functions. The present study revealed the interaction of pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid) and hexadecanoic acid, ethyl ester (palmitic acid) with AP-1 (PDB: 1FOS) and the glide energy score were found to be − 7.31009, − 6.13536 and − 6.43068 kcal/mol, respectively, which is significantly higher than previously reported interaction of nutraceutical like 6-gingerol with AP-1, having a docking score of − 10.2340 (Teimouri et al. 2016). These interactions specificities against the transcription factors used in the present study give a theoretical entry to use these phytoconstituents as a potential inhibitor against the signalling molecules involved in different stress mediated responses.
Conclusion
In this study, the binding interactions of four phytoconstituents viz., pantothenic acid (vitamin B5); 3,4,5-trihydroxy benzoic acid (gallic acid); madecassic acid and hexadecanoic acid, ethyl ester (palmitic acid) were carried out against TNF-α, NF-kB and AP-1, the major signaling molecules involved in the UVB induced photo-oxidative pathway. All the constituents interact with these target proteins through hydrogen bonding, hydrophobic interactions etc., and show significant glide energies. Results of the present investigation strongly suggest the probable use of these flavonoids for the amelioration of UVB induced photodamage. Further studies need to be carried out to explore the pharmacological properties and inhibitory potentials of these flavonoids in experimental models.
Acknowledgements
Special thanks are expressed to Annamalai University for providing lab facilities.
Author contributions
All Authors have contributed substantially to the design, performance, analysis and reporting of the work (UM, VIP, NRP—designed study, performed study; UM, VIP, NRP—collected data, Dock data, wrote paper). All authors read and approved the final manuscript.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
All authors declare no actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations. Additionally, the Authors declare no prior interactions with the in Current Drug Safety regarding the submitted manuscript.
Funding
The Authors declare no sources of funding for the research.
References
- Afaq F, Malik A, Syed D, Maes D, Matsui MS, Mukhtar H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor κ B in normal human epidermal keratinocytes. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264.1. [DOI] [PubMed] [Google Scholar]
- Balupillai A, Prasad NR, Ramasamy K, Muthusamy G, Shanmugham M, Govindasamy K, Gunaseelan S. Caffeic acid iInhibits UVB-induced inflammation and photocarcinogenesis through activation of peroxisome proliferator-activated receptor-γ in mouse skin. Photochem Photobiol. 2015;91:1458–1468. doi: 10.1111/php.12522. [DOI] [PubMed] [Google Scholar]
- Brasier A. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/CT:6:2:111. [DOI] [PubMed] [Google Scholar]
- Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Saermark T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut. 2002;51:37–43. doi: 10.1136/gut.51.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv Clin Chem. 2011;53:155–177. doi: 10.1016/B978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvek B, Dvorak Z. Targeting of nuclear factor-kappaB and proteasome by dithiocarbamate complexes with metals. Curr Pharm Des. 2007;13:3155–3167. doi: 10.2174/138161207782110390. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16:3152–3167. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shaw DE, Shelley M, Perry JK, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Gulcubuk A, Altunatmaz K, Sonmez K, Haktanir-Yatkin D, Uzun H, Gurel A, Aydin S. “Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med Physiol Pathol Clin Med. 2006;53:49–54. doi: 10.1111/j.1439-0442.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- Halgren T. New method for fast and accurate binding-site identification and analysis. Chem Biol Drug Des. 2007;69:146–148. doi: 10.1111/j.1747-0285.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Haraoui B, Bykerk V. Etanercept in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2007;3:99–105. doi: 10.2147/tcrm.2007.3.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Sakaguchi I. Protection of human keratinocytes from UVB-induced inflammation using root extract of Lithospermum erythrorhizon. Biol Pharm Bull. 2007;30:928–934. doi: 10.1248/bpb.30.928. [DOI] [PubMed] [Google Scholar]
- Jung YR, Kim DH, Kim SR, An HJ, Lee EK. Anti-wrinkle effect of magnesium lithospermate B from Salvia miltiorrhiza BUNGE: inhibition of MMPs via NF-kB signaling. PLoS One. 2014;9:e102689. doi: 10.1371/journal.pone.0102689. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Keskin O. Binding induced conformational changes of proteins correlate with their intrinsic fluctuations: a case study of antibodies. BMC Struct Biol. 2007;7:31. doi: 10.1186/1472-6807-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/S0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Mansuri ML, Parihar P, Solanki I, Parihar MS. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014;9:400. doi: 10.1007/s12263-014-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman S, Raveendran R, Vijayakumar B, Velmurugan D, Balamurugan S. Molecular docking and ex vivo pharmacological evaluation of constituents of the leaves of Cleistanthus collinus (Roxb.) (Euphorbiaceae) Indian J Pharmacol. 2012;44(2):197–203. doi: 10.4103/0253-7613.93848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, Altmann KH, Gertsch J. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J Biol Chem. 2006;281:14192–14206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Prasad NR, Pugalendi KV, Venugopal P. Modulation of UVB-induced oxidative stress by ursolic acid in human blood lymphocytes. Asian J Biochem. 2008;3:11–18. doi: 10.3923/ajb.2008.11.18. [DOI] [Google Scholar]
- Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor blockers etanercept, infliximab and adalimumab. J Dermatol Treat. 2004;15:280–294. doi: 10.1080/09546630410017275. [DOI] [PubMed] [Google Scholar]
- Shankaran KS, Ganai SA, Mahadevan V. In silico and In vitro evaluation of the anti-inflammatory potential of Centratherum punctatum Cass-A. J Biomol Struct Dyn. 2016;8:1–16. doi: 10.1080/07391102.2016.1160840. [DOI] [PubMed] [Google Scholar]
- Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci. 2013;12:54–64. doi: 10.1039/C2PP25152C. [DOI] [PubMed] [Google Scholar]
- Sun Z, Park SY, Hwang E, Zhang M, Seo SA, Lin P, Yi TH. Thymus vulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system. J Cell Mol Med. 2016 doi: 10.1111/jcmm.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Teimouri M, Muhammad J, Shoaib S, Abbas K, Arif A. In-vitro analysis of selective nutraceuticals binding to human transcription factors through computer aided molecular docking predictions. Bioinformation. 2016;12:354–358. doi: 10.6026/97320630012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavamani BS, Mathew M, Dhanabal SP. Cocculus hirsutus: molecular docking to identify suitable targets for hepatocellular carcinoma by in silico technique. Pharmacogn Mag. 2016;12:350–352. doi: 10.4103/0973-1296.185769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschooten L, Claerhout S, Van LA, Agostinis P, Garmyn M. New strategies of photoprotection. Photochem Photobiol. 2006;82:1016–1023. doi: 10.1562/2006-04-27-IR-884.1. [DOI] [PubMed] [Google Scholar]
- Vesely P, Willi S, Philipp B, Hoefler G, Kenner L. Translational regulation mechanisms of AP-1 proteins. Mutat Res Fund Mol Mech Mut. 2009;682:7–12. doi: 10.1016/j.mrrev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Victor FC, Gottlieb AB. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol. 2002;1:264–275. [PubMed] [Google Scholar]
- Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–1889. [PubMed] [Google Scholar]
- Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd SL, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]


