Abstract
The present study examines the deleterious effect of biologically synthesized silver nanoparticles in adult zebrafish. Silver nanoparticles (AgNPs) used in the study were synthesized by treating AgNO3 with aqueous leaves extract of Malva crispa Linn., a medicinal herb as source of reductants. LC50 concentration of AgNPs at 96 h was observed as 142.2 μg/l. In order to explore the underlying toxicity mechanisms of AgNPs, half of the LC50 concentration (71.1 μg/l) was exposed to adult zebrafish for 14 days. Cytological changes and intrahepatic localization of AgNPs were observed in gills and liver tissues respectively, and the results concluded a possible sign for oxidative stress. In addition to oxidative stress the genotoxic effect was observed in peripheral blood cells like presence of micronuclei, nuclear abnormalities and also loss in cell contact with irregular shape was observed in liver parenchyma cells. Hence to confirm the oxidative stress and genotoxic effects the mRNA expression of stress related (MTF-1, HSP70) and immune response related (TLR4, NFKB, IL1B, CEBP, TRF, TLR22) genes were analyzed in liver tissues and the results clearly concluded that the plant extract mediated synthesis of AgNPs leads to oxidative stress and immunotoxicity in adult zebrafish.
Keywords: Zebrafish (Danio rerio), Plant extract, AgNPs, Intrahepatic localization, Gene expression
Graphical abstract
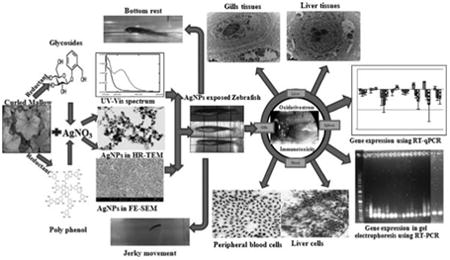
1. Introduction
Silver nanoparticles have been focused more in research because of the potential risk which they pose to humans and other biological organisms [1–3]. But the wide spread use of AgNPs in biomedical applications could be attributed for their potent antimicrobial [4], antioxidant [5] and anticancer activities [6]. Indeed, the increasing use of nanoparticles in consumer products results in leaching them into the environment and their interaction with ecological populations leads to pose a threat [7] to the environment. So the mode of interaction of nanoparticles in to the biotic components of ecosystems, their uptake, accumulation and impact at various levels is incapable of being avoided. Hence to device and act in such a way or implementing proper control measures will possibly avoid nano populations turning out to be a serious ecological concern [8]. Evidence to date indicated that most of the toxicological studies focused on chemically synthesized AgNPs against microbial communities [9] and animal cell lines [10] but only few studies were focused on biologically synthesized AgNPs against animal cells in particularly zebrafish [11,12].
The zebrafish (Danio rerio) were widely used in ecotoxicology experiments, particularly for the assessment of chemicals and nanoparticles toxicity [13,14]. Moreover, it is easy to handle, smaller size, controlled and visible embryological phase, genetics amenability and breeding potential, importantly similarity at the molecular and physiological levels with humans [15] renders these organisms as the most suited model organism for toxicological studies. In a study with zebrafish as model organism, Bilberg et al. [16] had shown the in vivo toxicity of chemically synthesized PVP coated AgNPs. On exposure at 48 h median lethal concentration (LC50) value of 84 μg/l increased the rate of operculum movement and surface respiration, confirming the respiratory toxicity. Very recently, Sarkar et al. [11] had shown the toxic evaluation of chemically synthesized and plant extract mediated synthesis of AgNPs on zebrafish and reported 96 h LC50 concentration of 100 μg/l for AgNO3, 80 μg/l for chemically synthesized AgNPs and 400 μg/l for plant extract mediated synthesis of AgNPs and their results concluded that chemically synthesized AgNPs and AgNO3 were more toxic to zebrafish compared to biologically synthesized AgNPs.
Malva crispa (Curled Mallow) is one of a phyto-therapeutic candidate belonging to the family Malvaceae and it has been used in folk medicine of Brazil and other countries for various treatments [17]. In South Korea, the leaves extract of the plant was commonly used as soup and ingredients in baby food. The powdered flowers and leaves were reported for stimulating the cellular regeneration, acting as cleansing and detoxification agents. In addition, an antitumor activity of M. crispa was also reported by Huang et al. [18]. Recently, in our previous study we have reported the presence of phyto-chemicals in the aqueous leaves extract of M. crispa was responsible for gold nanoparticles synthesis [19]. The present study details the AgNPs synthesis using the aqueous plant leaves extract (M. crispa) and evaluation of toxicological effects of biosynthesized AgNPs on adult zebrafish, to determine its environmental safety and viability for use in consumer products and in aquaculture. Hence the overall results in the study concluded that the plant mediated synthesis of AgNPs leads to deleterious effect on adult zebrafish but the results are not compared with the chemically synthesized AgNPs.
2. Materials and methods
Fresh and healthy leaves of M. crispa was purchased from Jeonju local market, South Korea. All the chemicals used in the study were reagent grade or higher purchased from Sigma–Aldrich (Korea).
2.1. Synthesis and characterization of AgNPs
Ten grams of leaves were surface cleaned with tap water, followed by distilled water (D.H2O) at several times and were boiled in 100 ml of D.H2O for 5 min in microwave oven, the resulting extract was filtered through Whatman filter paper. The reaction mixture containing 6 ml of extract and 44 ml of D.H2O was added to 1 mM AgNO3 to a final reaction volume of 50 ml and kept in dark at room temperature. A control setup was also maintained throughout the experiment with leaves extract alone. Initially, synthesis was confirmed by sampling the reaction mixture at regular time intervals and the absorption maxima was monitored at the wavelength of 200–800 nm in Shimadzu UV-1800 spectrophotometer. The reaction mixtures containing suspended AgNPs were concentrated by centrifugation at 15,000 rpm for 20 min and the resulting pellet was washed with D.H2O several times and subsequently lyophilized as described in our earlier report [19]. Further a known quantity of lyophilized powder was dissolved in D.H2O (1 mg/ml) using ultra-sonicator (SONICS Vibra cell, USA) and an aliquot was used for HR-TEM (JEM-2010) and FE-SEM (S-4800, Hitachi, Japan) analyses. EDS and XRD (XPERT-MRD, Phillips, Netherland) analysis were performed to ascertain the nature of metallic nanoparticles. Zeta potential and particle size distribution of AgNPs were analyzed in Nanoplus zeta/nano particle analyzer (ELSZ 1000, Photal Otsuka electronics, Japan). The concentration of silver in the reaction mixture was confirmed through ICP-MS spectrometry model 7500a, Agilent technologies, USA.
2.2. Zebrafish experimental study
Adult zebrafishes of both sexes with an average weight of 0.3 ± 0.02 g and average length of 24.57 ± 0.2 mm were selected for the study. Fishes were fed daily with the commercially purchased artemia and maintained in aquaria at a temperature of 28 ± 1 °C with a 14 h:10 h light-dark cycle for three weeks and later the fishes were removed from recirculating tanks, placed in static tanks, and fasted for 24 h prior to experimentation.
2.3. Toxicity tests
For achieving the sub lethal concentrations (LC50), followed the Organization for Economic Cooperation and Development (OECD) guidelines for testing chemicals (OECD, 1992). Six different concentration of AgNPs suspensions (23.7, 47.4, 142.2, 237, 284.4, 331.8 μg/l) were prepared and dispersed prior to use, using ultrasonicator for 10 min at 30 s pulse on and 10 s pulse off with 20% Amp (500 watt, 20 KHz) without addition of salts or stabilizing agents. The fishes were randomly assigned to six groups of 7 and in turn exposed to each concentration for 96 h in a 2 L tank containing 1 L of test solution. Seventh group of 7 fishes were acted as control. Each treatment was run in triplicates under the same conditions with a natural light/dark cycle. For maintaining the quality of water the fishes were not fed for 24 h prior to experiment and during experiments for reducing the absorption of NPs in food. The water temperature and pH were maintained at 28 ± 1 °C and 6.8–7.3 respectively. In addition the dissolved oxygen content (DO) of the water at 5.4 mg/l was maintained using DO meter. The number of dead fishes was recorded at every 12 h of intervals and they were removed from treatment tank immediately to avoid contamination. At the end of experiment the remaining survived fishes were anaesthetized, dissected and observed for any changes.
After the conclusion of LC50 concentration of 142.2 μg/l, half of the LC50 concentration of 71.1 μg/l was fixed and exposed once in every day to 10 fresh zebrafishes in a 2 L semi-static test solution tank for 14 days. In order to maintain the constant concentration the fresh water was changed at 24 h intervals, fed artemia and accurate concentration of NPs was exposed.
2.4. Intracellular localization of AgNPs
After 14 days of AgNPs treatment, the fish was anaesthetized and subsequently the gills and liver tissues were dissected (control and treated zebrafish). Tissues were fixed in modified Karnovsky's fixative (2% paraformaldehyde and 2% glutaraldehyde in 0.05 M sodium carcodylate buffer pH 7.2), washed thrice in carcodylate buffer at 4°C for 10min and post fixed in 1% osmium tetroxide at 4°C for 90min. Then the tissues were washed twice at room temperature using D.H2O and enbloc staining was done using 0.5% uranyl acetate and left overnight. Further the tissues were dehydrated using graded ethanol series 30, 40, 50, 60, 70, 80, 90 % and three changes of 100% ethanol for 10 min each and embedded in propylene oxide: Embed 812 resin mixture and allowed for polymerization at 60 °C for 48 h, sectioned using ultramicrotome (Leica Mikrosysteme GmbH, Austria), collected on copper grid and stained with 2% uranyl acetate for 7 min followed by Reynolds lead citrate for 7 min and examined under Bio-TEM (Hitachi H7650, Japan) [20].
2.5. Liver enzyme marker assay
Alanine aminotransferase (ALT or SGPT) and aspartate aminotransferase (AST or SGOT) levels from liver tissues of both control and treated zebrafishes were assayed using standard colorimetric kit manufacturers protocol (Biovision, Mountain View, CA) [21].
2.6. Micronuclei (MN) and nuclear abnormality (NA) test usingperiodic acid Schiffs (PAS) staining
Due to smaller size of fishes, the peripheral blood samples of 3 μl were obtained by caudal vein puncture using 0.2% EDTA as anticoagulant in syringe and pooled together (n = 3). Blood was immediately smeared on clean grease free microscope slides, air dried and fixed in methanol for 5 min and gently rinsed the slides in running tap water for 1 min followed by immersing in periodic acid solution for 5 min at room temperature. Then the slides were rinsed using D.H2O and immersed in Schiff's reagent for 15 min at room temperature and gently washed using running tap water for 5 min. Finally the counter staining was done using Hematoxyline solution for 90 s and rinsed in running tap water for 30 s. Then the slides were allowed to air dry and examined under light microscope using mineral oil (100×) [22,23].
2.7. Histopathology of gills and liver tissues
Gills and liver tissues of both control and treated zebrafishes were dissected and placed into vials containing Carnoy's fixative (3:1 methanol: acetic acid). The gills tissues were transferred into vials containing 20% acetic acid for 15 min and the liver tissues were transferred to 45% acetic acid solution for 30 min. After the tissues maceration, they were gently minced and filtered to obtain a cell suspension [24]. Then the cells were smeared on a clean grease free microscope slide and allowed to air dry followed by PAS staining as per previously described protocol.
2.8. RNA extraction and cDNA synthesis
Liver tissues of both control and treated zebrafishes were dissected and homogenized in TRIzol reagent and the total RNA was extracted using RNAse minikit (Qiagen) following manufacturers protocol. Total RNA extracted was eluted in 20 μl of nuclease-free water and the final concentration was determined by NanoDrop (Epoch Microplate spectrophotometer). RNA integrity was checked by ethidium bromide staining of 28S and 18S ribosomal RNA bands on 1% agarose gel and the RNA sample was stored at –80 °C until use. One hundred (100 ng) of total RNA was used for cDNA synthesis for all the primers using Topscript™ One-step RT-PCR kit manufacturer's protocol (Enzynomics) and the reaction mixture was applied in RT-PCR (Corbett research, Korea). Finally the product was run in agarose gel electrophoresis to know the expression pattern.
2.9. Real-time polymerase chain reaction (RT-qPCR)
RT-qPCR was performed with SYBR Green in C1000 Touch™ thermal cycler (CFX 96, BioRad). Reaction was set on 96 well plates by mixing 1 μl of TOPreal™ One-step RT qPCR enzyme mixture and 10 μl of 2 × TOPreal™ One-step RT qPCR reaction mixtures, 1 μl of total RNA(100 ng), 1 μl of forward primers, 1 μl of reverse primers, 6 μl of D.H2O and all the reactions were assembled on ice. Beta-actin (ACTB/ACTB) was used as housekeeping gene and suitable negative controls were maintained for all the primers (absence of RNA template) (Table 1). Immediately after assembling the reaction mixture it was placed on RT-qPCR and the cycle was initially set at 50 °C for 30min in a hold condition and the initial denaturation was done at 95 °C for 10min followed by denaturation at 95 °C for 5 s, annealing and elongation was done at 60 °C for 30 s each and repeated the cycle for 40 times. At the end of the 40 cycles melting curve analysis was fixed.
Table 1.
Primer sequences of this study.
| Gene | Short | Forward primer (5′-3′) | Reverse primer (5′-3′) | References |
|---|---|---|---|---|
| Metal transcription factor 1 | MTF-1 | GATTGAGCTTCTCCGTCAGG | TCCTCGTCTTCCTCTTCTCG | [50] |
| Heat shock protein70 | HSP70 | GGAAAAGAGGGAAGCTTTGG | ACGTTCCATGTTTCCAGACC | [50] |
| Toll like receptor4 | TLR4 | GGGAAGTCAATCGCCTCCA | ACGGCTGCCCATTATTCCT | [51] |
| Nuclear factor kβ | NFKB | AGAGAGCGCTTGCGTCCTT | TTGCCTTTGGTTTTTCGGTAA | [49] |
| Interleukin 1β | IL1B | CATTTGCAGGCCGTCACA | GGACATGCTGAAGCGCACTT | [49] |
| CCAAT/enhancer binding protein (C/EBP) β | CEBP | GCGACGCGAGAGGAACA | TGCGCATTTTGGCTTTGTC | [51] |
| Transferrin | TRF | TGGACGGCAGCAGGAAAA | GCAGGCTCTCTGGCGAAGT | [49] |
| Toll like receptor22 | TLR22 | CCAGCTCTCGCCGTACCA | TTGGGCCAGCGGATGT | [49] |
| β-Actin | ACTB | TGCTGTTTTCCCCTCCATTG | TTCTGTCCCATGCCAACCA | [49] |
3. Results and discussion
The present study demonstrated a simple synthesis and characterization of AgNPs from medicinally important aqueous plant leaves extract of M. crispa Linn., a medicinal herb as source of reducing agents. In addition, in vivo toxicity of synthesized nanoparticles was examined in adult zebrafish and based on the results it was concluded that the plant extract mediated synthesis of AgNPs leads to oxidative stress and immunotoxicity in adult zebrafish.
3.1. AgNPs synthesis
A visible color change of the leaves extract from pale yellow to reddish brown on addition of AgNO3 observed within 2 h indicated the formation of AgNPs and the characteristic absorption peak for AgNPs was observed around 430 nm (Fig. 1a).
Fig. 1a.
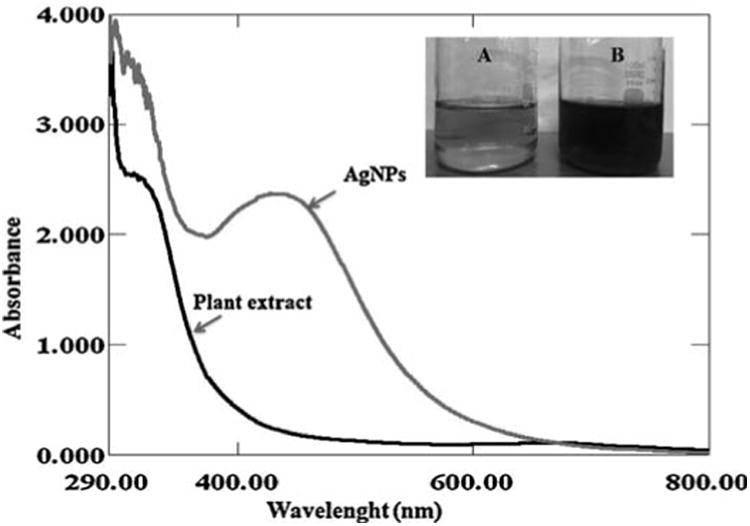
UV-vis spectra of biosynthesized AgNPs observed at 430 nm. Digital photographs showing the (A) leaves extract alone (B) reddish brown color formation of AgNPs after the addition of 1 mM AgNO3 into aqueous leaves extract.
3.2. Electron microscopy and XRD analysis
In HR-TEM and FE-SEM analyses, poly dispersed nanoparticles, spherical in shape and size ranging from 5 to 50 nm was observed (Fig. 1b). In addition, presence of silver in the reaction mixture was confirmed by EDS analysis (Fig. 2a). The XRD pattern of face-centered cubic nature of AgNPs illustrates well defined characteristic diffraction peaks indexed at 2θ = 38.16° (111), 44.11° (2 0 0), 64.1° (2 2 0) and 77.21° (3 1 1) of fcc Ag matched with the JCPDS file no. 04-0783, suggest the crystalline nature of AgNPs (Fig. 2b). Particle size distribution data showed the average size of AgNPs to range 24.1 nm and a charge of –16.10 mv suggested they were stable (Fig. 2c). Further the concentration of Ag in the reaction mixture was analyzed in ICP-MS and it was observed around 474 μg/mg of powder.
Fig. 1b.
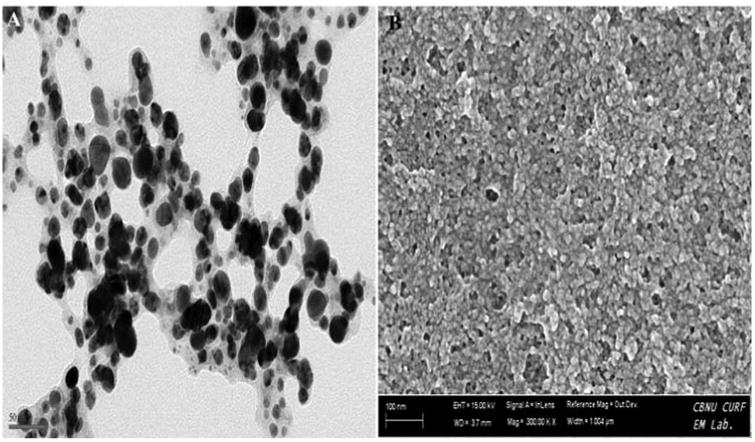
Formation of poly dispersed nanoparticles observed with the addition of 1 mM AgNO3 into aqueous leaves extract (A) HR-TEM analysis (B) FE-SEM analysis.
Fig. 2a.
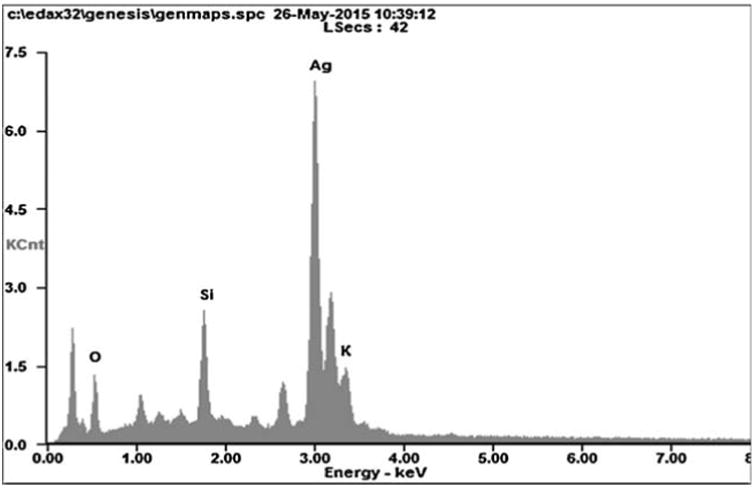
Energy-dispersive X-ray spectroscopy profile of biosynthesized AgNPs.
Fig. 2b.
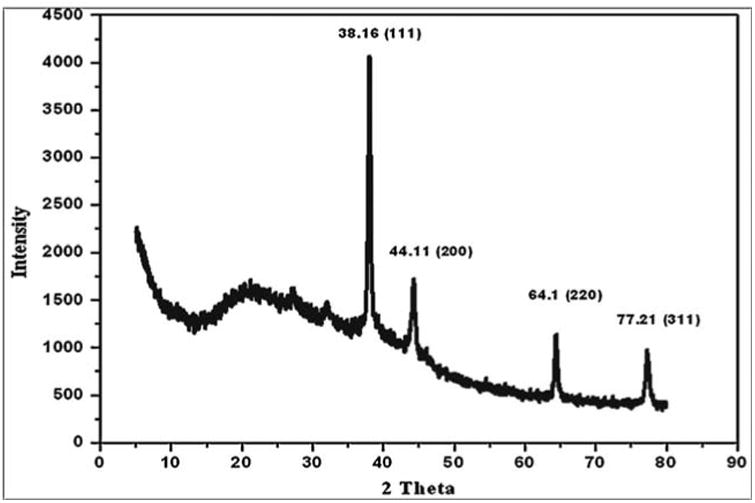
XRD spectrum of AgNPs indexed at (111), (20 0), (2 20) and (311) exhibits the facets of crystalline silver.
Fig. 2c.
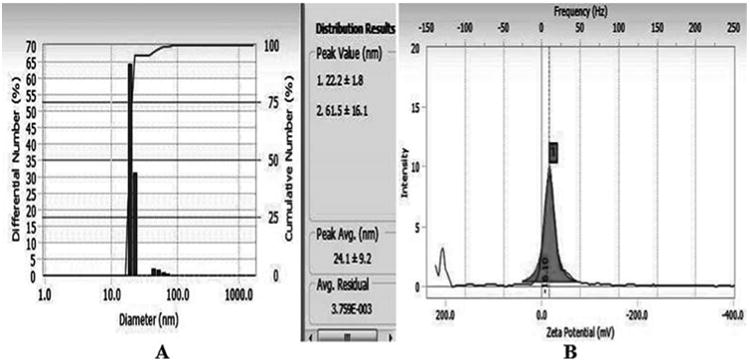
DLS analysis (A) and zeta potential (B) measurements of biosynthesized AgNPs.
3.3. Toxicity tests
Biologically synthesized AgNPs were acutely toxic to zebrafish with a static 96 h exposure at LC50 conc. of 142.2 μg/l (Fig. 3a). At the two lower concentrations (23.7 μg/l and 47.4 μg/l) tested, there were no fish mortality incidences and the fishes looked healthy over the entire treatment regime. Exposure to 331.8 μg/l of AgNPs (the maximum tested concentration) resulted in 100% mortality at 48 h, while the second highest concentration (284.4 μg/l) resulted in 100% mortality at 96 h. Aggressive behavior was observed within 6 h of treatment resulted in sign of toxicity stress emerged, starting with zebrafish lying on the bottom of the tank with increased respiratory rate. So the surface respiration took place and ultimately the fishes stood still in the middle of the water column where they lost equilibrium and sank on the bottom of the tank. Eventually the fishes started to show jerky movements few hours before the death. Extravasation of the blood was observed in the anterior ventral surface of the body just behind the head of the dead fish. But in case of lower concentrations no such sign of behavioral changes and extravasation of the blood were observed and pigment color was normal in all the fish, but only the jerky movement was observed few hours before the death. (Fig. 3b). Results obtained in the present study are consistent with [16], where in PVP stabilized AgNPs exhibited similar behavioral changes at a 48 h median lethal concentration value of 84 μg/l and the increased rate of operculum movement and surface respiration after nano silver exposure, suggested respiratory toxicity. It is also been reported that 24 h LC50 concentration of chemically synthesized AgNPs was 250 mg/l and approximately one half of the determined LC50 was used as the highest exposure concentration in the treatment of zebrafish [25]. Also, number of recent studies had shown the presence of Ag+ ion in NPs exhibit higher toxicity than the corresponding AgNPs in various toxicity models [26–28]. These statements are true in case of biologically synthesized AgNPs especially in plant extract mediated synthesis in which Sarkar et al. [11] had reported the 96 h LD50 value of 400 μg/l but in our study the values obtained for the 96 h LC50 was 142.2 μg/l. This striking variation may depend on the type of plant components used for synthesis in which phytochemicals (biological molecule) carried on the surface of NPs may also be possible to play a key role on toxicity [29]. However, several studies had reported the higher toxic effect of chemically synthesized AgNPs than the biologically synthesized AgNPs [30,31].
Fig. 3a.
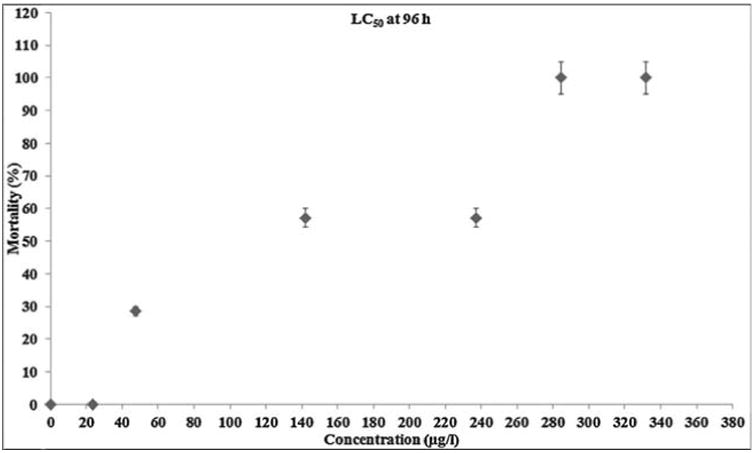
Zebrafish mortality during acute exposure to biogenic AgNPs. The fishes were exposed to 23.7, 47.4,142.2, 237, 284.4, 331.8 μg/l biogenic AgNPs for up to 96 h.
Fig. 3b.
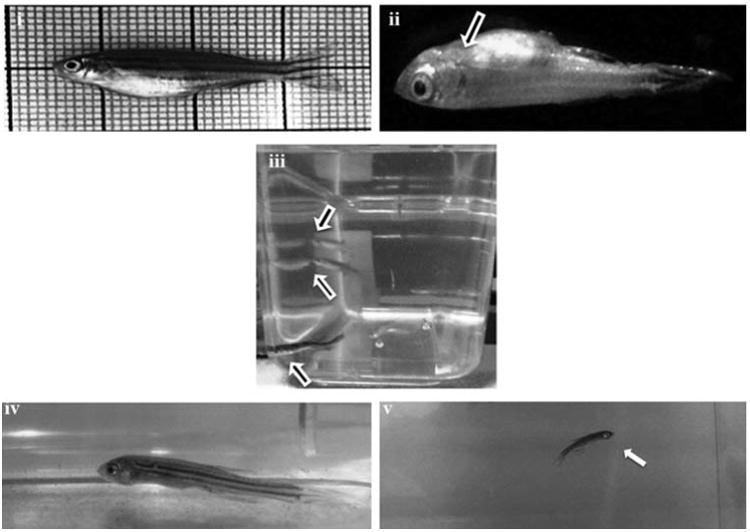
Pictographic representation of the behavioral and morphological changes of zebrafishes treated with AgNPs. (i) Normal zebrafish (ii) extravasation of the blood observed in the anterior ventral surface of the body just behind the head of the dead fish (iii) aggressive behavior(iv) bottom rest (v) jerky movement.
Following the LC50 determination, 14 days acute toxic exposure study was carried out to understand the possible penetration of nanoparticles in various organs of the tissues, histological alterations and eventually assessed the sign for oxidative stress as well as immune response related genes. Approximately when half of the determined LC50 conc (71.1 μg/l) was used as the highest concentration, no fish mortality was observed during treatment and no visible differences in behavior between control and treated group was observed.
3.4. Intracellular localization of AgNPs
Cytological changes in the gills tissue of both control and treated zebrafishes were observed under Bio-TEM. As shown in Fig. 4a gills cells of fish in the control group possessed normal cell structure with intact cell membranes, nuclei and cell organelles, normal appearance of the cytoplasm. However, gills cells exposed to AgNPs showed a number of morphological changes (Fig. 4b) including cell membrane damage, irregular cell outlines, pyknotic nuclei and complete disruption of gills cells. The trend of changing the morphology in AgNPs exposed zebrafish may be a sign for oxidative stress caused by ROS. Oxidative stress or stress on an organism due to increase in ROS such as hydroxyl radicals and peroxyl radicals could be caused by environmental toxicants [32]. Moreover, oxidative stress is considered to be an important toxic mechanism for AgNPs induced stress [25,32]. For supporting all the above statement, a recent study reported similar kind of morphological changes in gills of TiO2 and ZnO nanoparticles exposed to zebrafish and they also concluded the changes was due to generation of ROS such as .OH [33].
Fig. 4a.
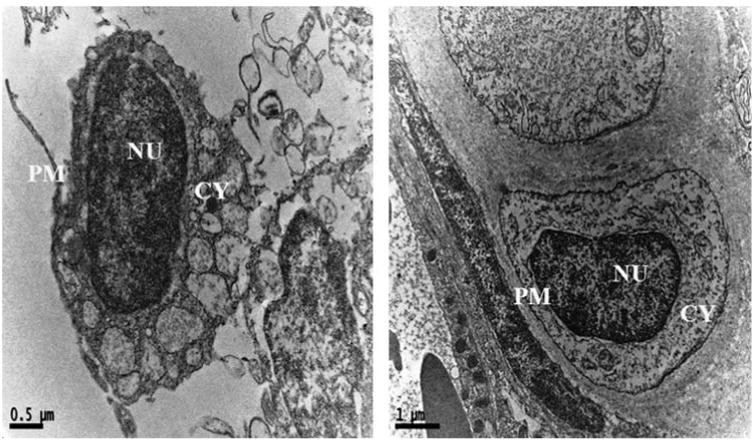
Ultrastructural images of control zebrafish gills tissues in Bio-TEM showing the intact cell membranes, nuclei and cell organelles, normal appearance of the cytoplasm PM-plasma membrane; CY-cytoplasm; NU-nucleus.
Fig. 4b.
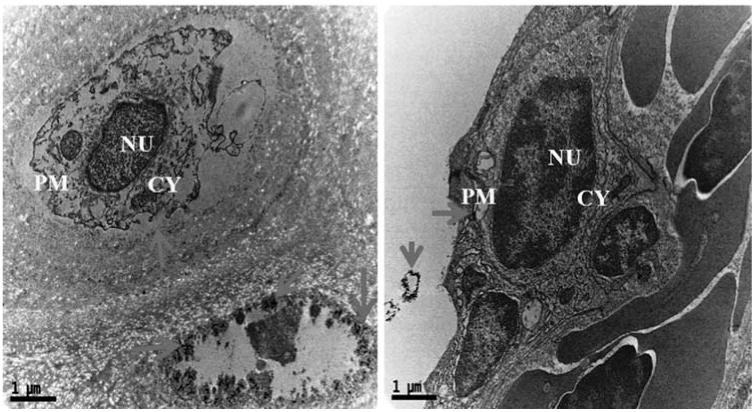
Ultrastructural images of AgNPs exposed zebrafish gills tissues in Bio-TEM showing morphological variations like cell membrane damage, irregular cell outlines, pyknotic nuclei and complete disruption of gills cells.
In addition, intracellular localization of AgNPs in the liver tissues of both control and treated zebrafishes were observed under Bio-TEM (Figs. 5a and b). As shown in Fig. 5a presence of nanoparticle was observed in various parts of the cytoplasm in particular between nucleus and plasma membrane and these results were in good agreement with the previously published research work done by Choi et al. [25] in which they had also reported the localization of AgNPs in liver tissues of adult zebrafish with the aim of studying the role of oxidative damage and apoptosis. In order to confirm the liver toxicity induced by AgNPs, SGOT and SGPT assays were performed in liver tissues in which an elevated level of both the enzymes observed in treated liver tissues compared to control, resulted in liver toxicity (Fig. 6).
Fig. 5a.
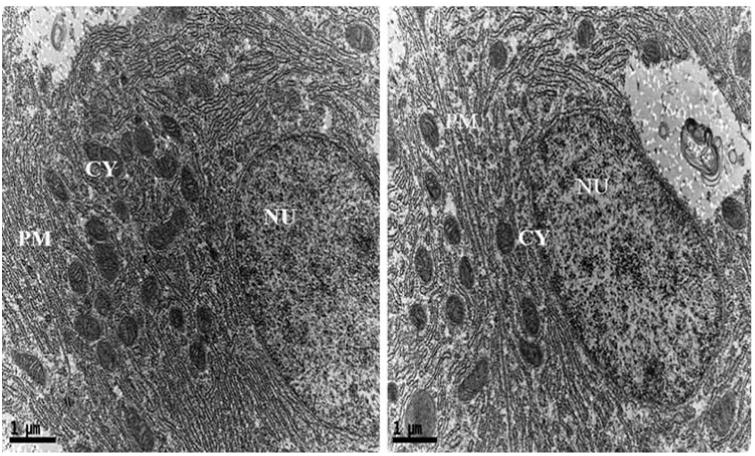
Ultrastructural images of control zebrafish liver tissues in Bio-TEM.
Fig. 5b.
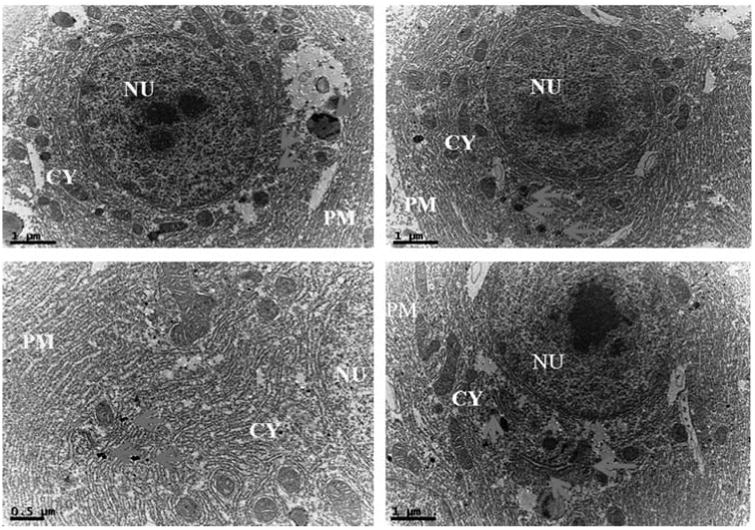
Ultrastructural images of AgNPs exposed zebrafish liver tissues in Bio-TEM showing localization of AgNPs
Fig. 6.
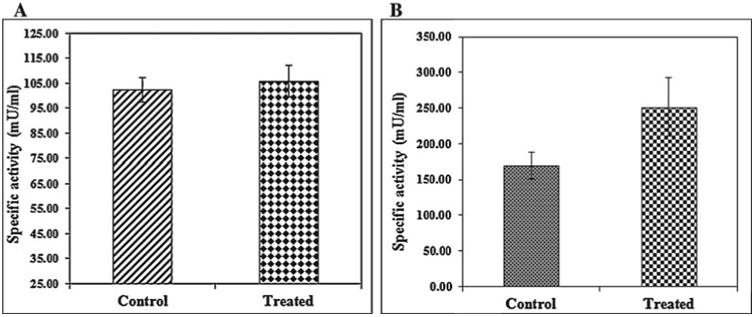
SGOT(A) and SGPT(B) analysis of control and treated zebrafish showing elevated level of both the enzymes in AgNPs exposed zebrafish.
3.5. MN and NA tests
In addition to oxidative stress, a sign of genotoxic activity was also observed in various parts of AgNPs exposed zebrafish. To step up the genotoxic activity, MN test was conducted in peripheral blood cells in which the presence of MN and NA like blebbed nuclei, lobed nuclei, and notched nuclei were observed in peripheral blood erythrocytes of AgNPs treated zebrafish (Fig. 7a). MN test is used to measure the subcellular process of chromosomal breaks (clastogenesis) or cell spindle malfunction (aneugenesis) [34] and the formation of micronucleus in fish cells, as an indicator of chromosomal damage [24]. Moreover, micronuclei is a cytoplasmic chromatin masses with the appearance of small nuclei that arise from chromosome fragments and generally this technique is performed in enucleated peripheral blood erythrocytes and reported as sensitive biomarker for genotoxicity [34,35]. It is also been reported by several studies that engineered nanomaterial induced chromosomal damage which was assayed in vitro MN test [36,37] and recently suggested as an appropriate method for screening of nanoparticles for potential genotoxicity [38]. In the present study also presence of MN and NA were observed in peripheral blood erythrocyte which clearly shows the genotoxicity of AgNPs exposed zebrafish.
Fig. 7a.
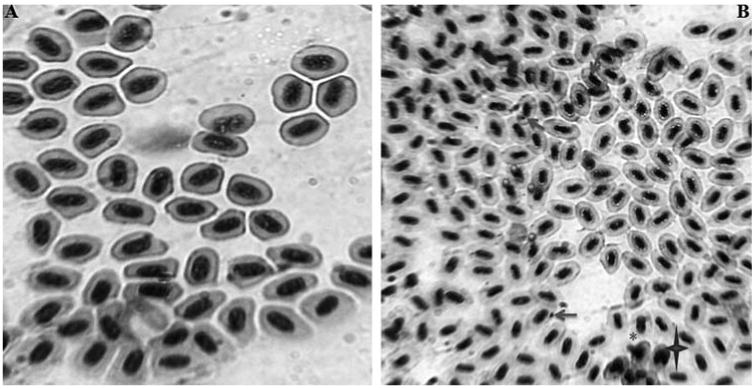
(A) Photomicrographs of erythrocytes with normal nucleus in peripheral blood cells of control zebrafish (B) Photographs of peripheral blood erythrocytes in zebrafish exposed with AgNPs. Micronuclei indicated by nuclear abnormalities like blebbed nuclei indicated by (*); lobed nuclei marked by notched nuclei marked by
3.6. Histopathology of gills and liver tissues
Histopathological changes of the gill tissues in response to biologically synthesized AgNPs were carried out using PAS staining (Fig. 7b). Exposure of AgNPs caused damage to gill lamella resulted in edema of the gill filaments. In addition, the qualitative histological examination of liver tissues stained with PAS staining had revealed that hepatic parenchyma cells appeared to be less homogeneous, loose in cell contact and the cells were dissociated with irregular in shape while control liver was filled with well-delineated polygonal cells (Fig. 7b). Similar to our findings, other research group has also reported the histological alterations in liver section of AgNPs exposed zebrafish including disruption of hepatic cell cords and apoptotic changes such as chromatin condensation and pyknosis while stained with counter staining of hematoxylin and eosin (H&E) [25] and they concluded the possible histological alteration was due to oxidative stress and apoptosis induced by AgNPs in liver of adult zebrafish. In addition, recently Wu et al. [39] reported on AgNPs cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days exposure in which they also observed the adverse histological changes in liver tissues of treated fish like hemocyte overfilling in blood vessels, hepatocyte enlargement, global basophilia, ballooning degeneration, and loosened liver parenchyma. From the results of acute toxicity, micronuclei, nuclear abnormalities and histological alterations in liver sections confirmed the possible oxidative stress and genotoxic effect of AgNPs induced in zebrafish.
Fig. 7b.
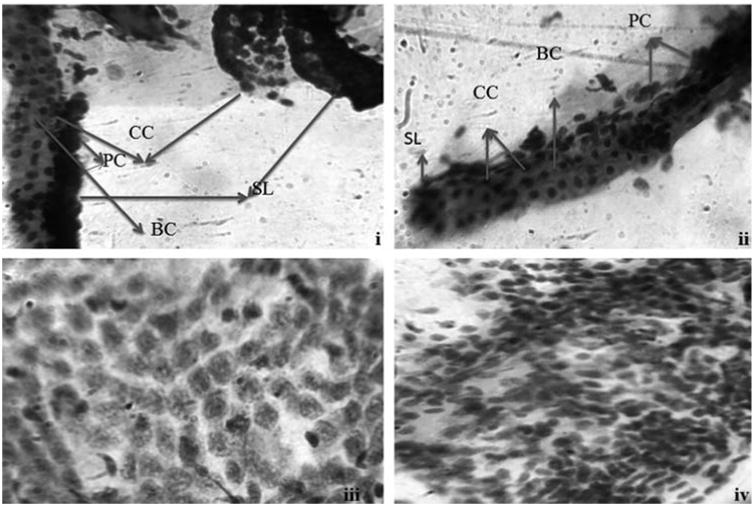
Photomicrographs of normal gills (i) and liver (iii) tissues stained with PAS staining. Photomicrographs of AgNPs exposed gills (ii) and liver tissues (iv). CC-chloride cells; PC-pavement cells; BC-blood channel; SL-secondary lamella.
3.7. Expression of oxidative stress and Immune response related genes
The mRNA expression of oxidative stress and immune response related genes of MTF-1, HSP70, TLR4, NFKB, IL1B, CEBP, TRF, TLR22 were analyzed in liver tissues of both control and AgNPs treated zebrafish and ACTB was used as housekeeping gene for normalization. Expression levels of all the genes were surveyed in which stress related gene of MTF-1 and immune response related genes of TLR4, IL1B, CEBP, TRF and TLR22 were down regulated (fold increase 0.68,0.44,0.78,0.25,0.18,0.34) respectively. The other genes like HSP70 (1.12) and NFKB (1.38) were up regulated in AgNPs treated zebrafish (Fig. 8a). Metal response element-binding transcription factor-1 (MTF-1) regulates the transcription of genes in response to a wide variety of stimuli, including hypoxia and oxidative stress leading to increase in labile cellular zinc, nuclear translocation, DNA binding and transcriptional activation of metallothionein genes (MT genes) by MTF-1 [40–42]. MTF-1 is also essential for the development and differentiation of embryonic hepatocytes [43]. Expression level of this gene in response to AgNPs exposed zebrafish liver tissue was down regulated with 0.68 fold and this shows the sign for oxidative stress. In addition, the biological processes affected by exposure to AgNP, reported across a variety of organs, including induction of oxidative damage, alterations to the regulation of enzymes responsible for free radical scavenging, altered regulation of gene expression pathways involved in apoptosis, and disrupted regulation of the cellular machinery involved in storing, detoxification and metabolism of metals (such as metallothionein 2) [44–46].The expression of heatshock proteins associated with a general shock response, which is universally conserved throughout the animal kingdom. Measurement of heat-shock protein induction, in particular hsp70, has been proposed as a useful technique in toxicological screening and environmental monitoring by for a variety of stressors including heavy metals, teratogens, anoxia [47,48]. Expression level of this gene in response to AgNPs exposed zebrafish liver tissue was up regulated with 1.12 fold. In addition, six key genes directly related to innate immune cascade were investigated in AgNPs exposed liver tissues of zebrafish. Interestingly, the gene NFKB involved in cellular responses to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation, oxidized LDL and bacterial or viral antigens showed up regulation with 1.38 fold by which may be due to the generation of stress. But in case of all other genes a sign of down regulations were observed including IL1B a marker of inflammation in zebrafish [49] resulted in response to stress. These changes in up and down regulation in response to AgNPs exposed liver tissues resulted in various kinds of stressors. Further to confirm the gene expression pattern, the cDNA was synthesized using RT-PCR for all the primers and the product was applied in agarose gel in which clear expressions were observed for all the tested genes in treated liver tissues compared to control (Fig. 8b).
Fig. 8a.
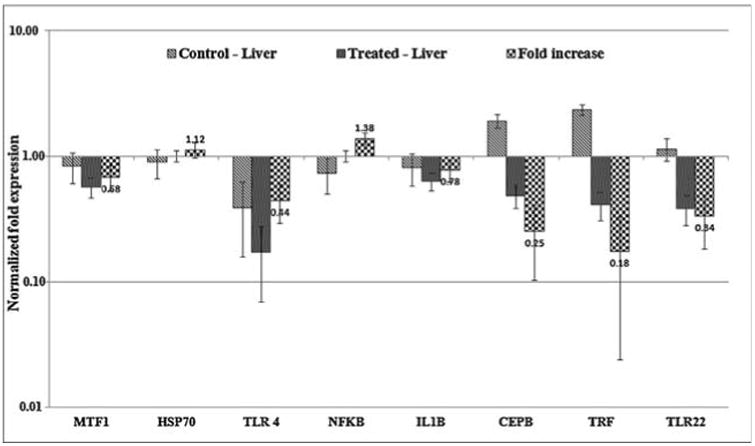
Expression of oxidative stress and immunotoxicity related genes in AgNPs exposed liver cells using RT-qPCR. Normalized fold expression of mRNA from treated zebrafish.
Fig. 8b.
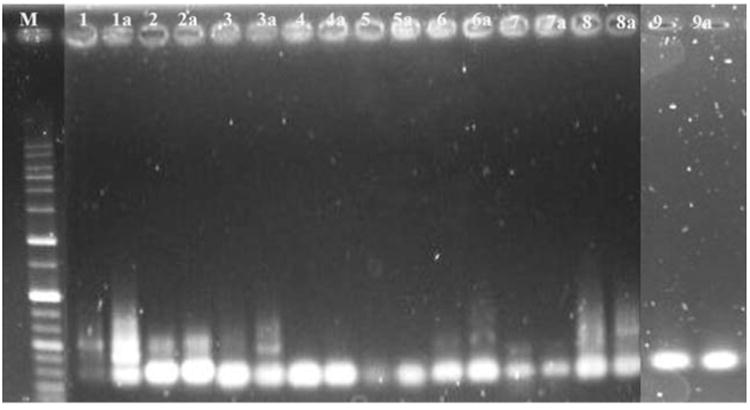
Gene expression pattern using RT-PCR in agarose gel electrophoresis (M) Marker (1) control-MTF1 (1a) treated-MTF1 (2) control-HSP70 (2a) treated-HSP70 (3) control-TLR4 (3a) treated-TLR4 (4) control-NFKB (4a) treated NFKB (5) control-IL1B (5a) treated-IL1B (6) control-CEBP (6a) treated-CEBP (7) control-TRF (7a) treated-TRF (8) control-TLR22 (8a) treated-TLR22 (9) control-ACTB (9a) treated-ACTB.
4. Conclusion
The present study revealed the in vivo toxicity of biologically synthesized AgNPs in adult Zebrafish (D. rerio). From the results it can be concluded that: (1) AgNPs in any form of synthesis either chemical or biological mediated could pose threat to zebrafish but based on the acute toxicity study plant mediated synthesis shows least toxic effect than the chemically synthesized (2) Gills is the primary organs directly in contact with the aquatic contaminants and hence in order to confirm the effect of AgNPs on gills, Bio-TEM analysis was carried in which a clear morphological changes like cell membrane damage, irregular cell outlines, pyknotic nuclei and complete disruption of cells were observed resulting from oxidative stress (3) Similarly localization of nanoparticle was observed in liver tissues in various parts of the cytoplasm in particular between the nucleus and plasma membrane (4) In addition to oxidative stress, a sign of genotoxic effect also observed in peripheral blood cells in which a presence of micronuclei and nuclear abnormalities like blebbed nuclei, lobed nuclei, and notched nuclei were observed (5) Qualitative histological examination of liver tissues stained with PAS staining had revealed that the hepatic parenchyma cells appeared to be less homogeneous, loose in cell contact and the cells dissociated with irregular in shape while control liver is filled with well-delineated polygonal cells and these changes were possibly due to related stress (6) In order to document the possible chances of oxidative stress and immunotoxicity induced by AgNPs in zebrafish, a gene expression study was carried out in liver tissues in which MTF-1, TLR4, IL1B, CEBP, TRF, TLR22 genes were down regulated, HSP70 and NFKB genes were up regulated and all these data were clearly confirmed the plant extract mediated synthesis of AgNPs leads to oxidative stress and immunotoxicity in adult zebrafish.
Highlights.
Synthesis of AgNPs achieved using Malva crispa Linn., leaves extract.
96 h LC50 concentration of AgNPs was observed at 142.2 μg/l in adult zebrafish.
Cytological changes and intrahepatic localization of AgNPs were demonstrated in tissues.
Presence of micronuclei and nuclear abnormalities were observed.
The mRNA expression of stress and immune response related genes were analyzed.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2007953) and also funds from Chonbuk National University, Republic of Korea.
References
- 1.Levard C, Hotze EM, Lowry GV, Brown GE. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol. 2012;46:6900–6914. doi: 10.1021/es2037405. [DOI] [PubMed] [Google Scholar]
- 2.Panyala NR, Pena-Mendez EM, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed. 2008;6:117–129. [Google Scholar]
- 3.Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR. Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int. 2011;37:517–531. doi: 10.1016/j.envint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against waterborne pathogens. Colloids Surf B Biointerfaces. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Dipankar C, Murugan S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B Biointerfaces. 2012;98:112–119. doi: 10.1016/j.colsurfb.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Jeyaraj M, Rajesh M, Arun R, Mubarak Ali D, Sathishkumar G, Sivanandhan G, Kapildev G, Manickavasagam M, Premkumar K, Thajuddin N, Ganapathi A. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf B Biointerfaces. 2013;102:708–717. doi: 10.1016/j.colsurfb.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 7.Impellitteri CA, Tolaymat TM, Scheckel KG. The speciation of silver nanoparticles in antimicrobial fabric before and after exposure to a hypochlorite/detergent solution. J Environ Qual. 2009;38:1528–1530. doi: 10.2134/jeq2008.0390. [DOI] [PubMed] [Google Scholar]
- 8.Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A, Quigg A, Santschi PH, Sigg L. Environmental behaviour and ecotoxicity of engineered nanoparticles to algae plants and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 9.Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B Biointerfaces. 2013;108:255–259. doi: 10.1016/j.colsurfb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Sukirtha R, Priyanka KM, Antony JJ, Kamalakkannan S, Thangam R, Gunasekaran P. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012;47:273–279. [Google Scholar]
- 11.Sarkar B, Netam SP, Mahanty A, Saha A, Bosu R, Krishnani KK. Toxicity evaluation of chemically and plant derived silver nanoparticles on zebrafish (Danio rerio) Proc Natl Acad Sci India Sect B Biol Sci. 2014;84:885–892. [Google Scholar]
- 12.Daniel SCGK, Kumar R, Sathish V, Sivakumar M, Sunitha S, Sironmani TA. Green Synthesis (Ocimum tenuiflorum) of silver nanoparticles and toxicity studies in zebrafish (Danio rerio) Model. Int J NanoSci Nanotechnol. 2011;2:103–117. [Google Scholar]
- 13.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 14.Illan OB, Albrecht RM, Fako VE, Furgeson DY. Toxicity assesments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;16:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- 15.Westerfield M. A Guide for the Laboratory Use of Zebra Fish (Danio rerio) 4th. University of Oregon Press; Eugene: 2000. The Zebrafish Book. [Google Scholar]
- 16.Bilberg K, Hovgaard MB, Besenbacher F, Baatrup E. In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio) J Toxicol. 2012:293784. doi: 10.1155/2012/293784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteves PF, Sato A, Esquibel MA, Buzzi FC, Meira AV, Filho VC. Antinociceptive activity of Malva sylvestris L. Lat Am J Pharm. 2009;28:454–456. [Google Scholar]
- 18.Huang CY, Zeng LF, He T, Wang CJ, Hong JR, Zhang XQ, Hou YH, Peng SS. In vivo and in vitro studies on the antitumor activities of MCP (Malva crispa L. Powder) Biomed Environ Sci. 1998;11:297–306. [PubMed] [Google Scholar]
- 19.Chandran K, Song S, Yun SI. Effect of size and shape controlled biogenic synthesis of gold nanoparticles and their mode of interactions against food borne bacterial pathogens. Arabian J Chem. 2014 http://dx.doi.org/10.1016/j.arabjc.2014.11.041.
- 20.Sathishkumar Y, Velmurugan N, Lee HM, Rajagopal K, Im CK, Lee YS. Effect of low shear modeled microgravity on phenotypic and central chitin metabolism in the filamentous fungi Aspergillus niger and Penicillium chrysogenum. Antonie Van Leeuwenhoek. 2014;106:197–209. doi: 10.1007/s10482-014-0181-9. [DOI] [PubMed] [Google Scholar]
- 21.Marcolin E, Miguel BS, Vallejo D, Tieppo J, Marroni N, Gallego JG, Tunon MJ. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis 1-3. J Nutr. 2012:30. doi: 10.3945/jn.112.165274. http://dx.doi.org/10.3945/jn.112.165274. [DOI] [PubMed]
- 22.Hotchkiss RD. A microchemical reaction resulting in the staining of polysaccharide structure in fixed tissue preparations. Arch Biochem. 1948;16:131–141. [PubMed] [Google Scholar]
- 23.Kiernan JA. Histological and Histochemical Methods: Theory and Practice. 3rd. Butterworth-Heinemann; Oxford: 1999. [Google Scholar]
- 24.Jiraungkoorskul W, Sahaphong S, Kosai P, Kim MH. Micronucleus test: the effect of ascorbic acid on cadmium exposure in fish (Puntius altus) Res J Environ Toxicol. 2007;1:27–36. [Google Scholar]
- 25.Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu DY. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol. 2010;100:151–159. doi: 10.1016/j.aquatox.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Asghari S, Johari SA, Lee JH, Kim YS, Jeon YB, Choi HJ, Moon MC, Yu IJ. Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J Nanobiotechnol. 2012;2 doi: 10.1186/1477-3155-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H. Toxicity of silver nanoparticles-nanoparticle or silver ion? Toxicol Lett. 2012;208:286–292. doi: 10.1016/j.toxlet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Massarsky A, Dupuis L, Taylor J, Beygi SE, Strek L, Trudeau VL, Moon TW. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere. 2013;92:59–66. doi: 10.1016/j.chemosphere.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 29.Moore M. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Pavagadhi S, Sathishkumar M, Balasubramanian R. Uptake of Ag and TiO2 nanoparticles by zebrafish embryos in the presence of other contaminants in the aquatic environment. Water Res. 2014;55:280–291. doi: 10.1016/j.watres.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Kovriznych JA, Sotnikova R, Zeljenkova D, Rollerova E, Szabova E, Wimmerova S. Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages—comparative study. Interdiscip Toxicol. 2013;6:67–73. doi: 10.2478/intox-2013-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saquib Q, Al-Khedhairy AA, Siddiqui MA, Abou Tarboush FM, Azam A, Musarrat J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol In Vitro. 2012;26:351–361. doi: 10.1016/j.tiv.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, and ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ. 2011;409:1444–1452. doi: 10.1016/j.scitotenv.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, Tucker JD, Vanparys P, MacGregor JT. Micronuclei as an index of cytogenetic damage: past, present and future. Environ Mol Mutagen. 1991;18:277–291. doi: 10.1002/em.2850180414. [DOI] [PubMed] [Google Scholar]
- 35.Ayllon F, Garcia-Vazquez E. Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and Mollie Poecilia latipinna: an assessment of the fish micronucleus test. Mutat Res. 2000;467:177–186. doi: 10.1016/s1383-5718(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 36.Doak SH, Manshian B, Jenkins GJS, Singh N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat Res. 2012;745:104–111. doi: 10.1016/j.mrgentox.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M. Mechanisms of genotoxicity. a review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8:233–278. doi: 10.3109/17435390.2013.773464. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez L, Sanderson BJS, Kirsch-Volders M. Adaptations of the in vitro MN assay for the genotoxicity assessment of nanomaterials. Mutagenesis. 2011;26:185–191. doi: 10.1093/mutage/geq088. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Zhou Q. Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environ Toxicol Chem. 2013;32:165–173. doi: 10.1002/etc.2038. [DOI] [PubMed] [Google Scholar]
- 40.Lichtlen P, Schaffner W. Putting its fingers on stressful situations: the heavy metal-regulatory transcription factor MTF-1. Bioessays. 2011;23:1010–1017. doi: 10.1002/bies.1146. [DOI] [PubMed] [Google Scholar]
- 41.Murphy BJ, Andrews GK, Bittel D, Discher DJ, McCue J, Green CJ, Yanovsky M, Giaccia A, Sutherland RM, Laderoute KR, Webster KA. Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor-1. Cancer Res. 1999;59:1315–1322. [PubMed] [Google Scholar]
- 42.Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK. Zinc and cadmium can promote the rapid nuclear translocation of MTF-1. J Biol Chem. 2000;275:9377–9384. doi: 10.1074/jbc.275.13.9377. [DOI] [PubMed] [Google Scholar]
- 43.Gunes C, Heuchel R, Georgiev O, Muller KH, Lichtlen P, Bluthmann H, Marino S, Aguzzi A, Schaffner W. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 1998;17:2846–2854. doi: 10.1093/emboj/17.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aerle RV, Johnston BD, Lange A, Bastos ED, Moorhouse A, Booth T, Paszkiewicz K, Tyler CR, Ball K, Santos EM. Molecular mechanisms of toxicity of silver nanoparticles in zebrafish embryos. Environ Sci Technol. 2013;47:8005–8014. doi: 10.1021/es401758d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roh JY, Sim SJ, Yi J, Park K, Chung KH, Ryu DY, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- 46.Nair PM, Choi J. Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquat Toxicol. 2011;101:550–560. doi: 10.1016/j.aquatox.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Scown TM, Santos EM, Johnston BD, Gaiser B, Tyler CR. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci. 2010;115:521–534. doi: 10.1093/toxsci/kfq076. [DOI] [PubMed] [Google Scholar]
- 48.Williams JH, Farag AM, Stansbury MA, Young PA, Bergman HL, Petersen NS. Accumulation of hsp70 in juvenile and adult rainbow trout gill exposed to metal-contaminated water and/or diet. Environ Toxicol Chem. 1996;15:1324–1328. [Google Scholar]
- 49.Oyarbide U, Iturria I, Raineiri S, Pardo MA. Use of gnotobiotic zebrafish to study Vibrio anguillarum pathogenicity. Zebrafish. 2015;12:71–80. doi: 10.1089/zeb.2014.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio) Environ Sci Technol. 2007;41:8178–8186. doi: 10.1021/es071235e. [DOI] [PubMed] [Google Scholar]
- 51.Rojo I, Ilarduya OMD, Estonba A, Pardo MA. Innate immune gene expression in individual zebrafish after Listonella anguillarum inoculation. Fish Shellfish Immunol. 2007;23:1285–1293. doi: 10.1016/j.fsi.2007.07.002. [DOI] [PubMed] [Google Scholar]


