Abstract
Obesity and metabolic disorders are of great societal concern and generate significant human health care costs. Recently, attention has focused on the potential for environmental contaminants to act as metabolic disruptors. This study sought to evaluate the adipogenic activity of indoor house dust extracts and a suite of semi-volatile organic chemicals (SVOCs) that are often ubiquitously detected in indoor environments. 3T3-L1 cells were exposed to extracts of indoor dust or individual SVOCs and assessed for triglyceride accumulation and pre-adipocyte proliferation. Ten of 11 house dust extracts exhibited significant triglyceride accumulation and/or proliferation at environmentally relevant levels (<20 μg of dust/well), and significant adipogenic activity was also exhibited by 28 of the SVOCs. Notably, pyraclostrobin, dibutyl phthalate, tert-butyl-phenyl diphenyl phosphate, and the isopropylated triaryl phosphates (ITPs) exhibited near maximal or supra-maximal triglyceride accumulation relative to the rosiglitazone-induced maximum. The adipogenic activity in house dust occurred at concentrations below EPA estimated child exposure levels, and raises concerns for human health impacts, particularly in children. Our results delineate a novel potential health threat and identify putative causative SVOCs that are likely contributing to this activity.
Key Terms: Endocrine Disrupting Chemicals, Obesogen, Adipogenesis, 3T3-L1, House dust
Introduction
Endocrine disrupting chemicals (EDCs) include 1,000 or more synthetic or naturally occurring chemicals or mixtures that can interfere with any aspect of hormone action 1; some of these, termed “obesogens”, have been demonstrated to directly increase weight gain in animal models and/or triglyceride accumulation in vitro 2, 3. The global prevalence of metabolic disorders, such as obesity, is currently of great societal concern. Obesity contributes to an estimated $215 billion in annual US health care costs, and in addition drives increased risks of type II diabetes, cardiovascular disease, hypertension, and other adverse health effects 4. Legler et al. previously estimated the economic costs of obesity, diabetes, and associated costs reasonably attributable to EDCs in the European Union at €18–29 billion 5. Importantly, this study likely significantly underestimates the cost as it only assessed effects from five chemicals with the strongest epidemiological evidence, whereas putative obesogens are being identified via in vitro and in vivo studies at an ever-increasing rate 2. Despite increased attention and attempted interventions, rates of occurrence remain high: 8.9% of infants and toddlers, 17.0% of 2–19 year olds, and 36.3% of adults aged 20 and older are currently classified as obese in the US 6.
Due to the costs and time involved with confirming putative “obesogens” in vivo, utilizing appropriate in vitro models is crucial for screening large numbers of individual environmental chemicals and mixtures. The 3T3-L1 mouse pre-adipocyte cell line is commonly used for this purpose; following exposure to adipogenic chemicals, these cells differentiate into adipocytes, undergoing morphological changes, accumulating triglycerides, and eventually coming to resemble a mature human white fat cell 7, 8. This model has been rigorously applied to putative metabolic disruptors over the last forty years, with active chemicals in 3T3-L1 cells demonstrated in many cases to be active in vivo as well 9–12. Many nuclear receptor pathways can regulate adipogenesis, including the peroxisome proliferator-activated receptor-gamma (PPARγ), thyroid receptor-beta (TRβ), liver X receptor (LXR), farnesoid X receptor (FXR), glucocorticoid receptor (GR), estrogen receptor (ER), androgen receptor (AR), retinoid X receptor (RXR), insulin receptor, and others 13. EDCs that can impact these receptors are ubiquitous, commonly found in consumer products, and accumulating in the indoor environment 14–17. As such, humans are exposed to complex mixtures of contaminants throughout development. Newborn cord blood often contains hundreds of contaminants 18, 19, and exposure continues via oral routes such as breast-feeding 20, 21 and from inhalation and dermal contact in indoor environments 14, 22–24 in early life.
House dust is reportedly contaminated with several classes of EDCs (e.g. flame retardants, phthalates, pesticides, etc.) that can span several orders of magnitude in concentration. People, and particularly small children, are chronically exposed to dust, and thus receive exposure to EDCs present in the dust. Research from our laboratory and others has characterized the chemicals present in house dust and reported a number of semi-volatile organic contaminants (SVOCs) that are suspected of being hormonally active, including phthalates, flame-retardants, and perfluoroalkyl substances (PFAS) 23, 25, 26. Notably, the EPA estimates children ingest 50 mg of dust per day from indoor environments 22, contributing to chronic oral and inhalation exposures to EDCs 14, 23, 24. Previous research from our laboratory assessed the ligand binding and subsequent activation of PPARγ by house dust extracts, reporting that 21 of 24 samples exhibited significant PPARγ binding at 3 mg dust equivalence per mL (DEQ/mL; mass of extracted dust per volume of assay medium; or 120 μg dust per assay well) 27 and receptor activation in 15 of 25 samples at ≤50% of the maximal positive control response and at concentrations ≥100 μg DEQ/mL (4 μg dust per well) 28, 29. Other studies have reported PPARγ, GR, and ER agonism as well as AR and TR antagonism, notably at concentrations as low as 12 μg, 40 μg, and 38 μg for ER agonism, GR agonism, and AR antagonism, respectively 30–32. Given that these pathways, all known to regulate adipogenesis, are all activated or disrupted by house dust at lower concentrations than estimated child exposure levels, it raises questions about the potential adipogenic activity of house dust.
As such, the goals of this study were to address this knowledge gap via testing of a small subset of house dust samples to determine whether extracts of indoor house dust were sufficient to regulate adipogenesis at environmentally relevant exposure levels. We further assessed a wide range of chemical contaminants routinely measured in indoor environments, including: polybrominated diphenyl ethers (PBDEs) and other brominated flame retardants (BFRs), organophosphate flame retardants (PFRs), phenols, pesticides, parabens, phthalates, and perfluoroalkyl substances (PFASs). Specifically, we used 3T3-L1 cells (Zenbio, Inc.) to determine the ability of dust extracts and individual chemicals to promote triglyceride accumulation and/or pre-adipocyte proliferation. We hypothesized that many of these indoor contaminants, as well as house dust samples at environmentally relevant exposure levels, would exhibit significant adipogenic activity via triglyceride accumulation and/or pre-adipocyte proliferation.
Materials and Methods
Chemicals
Chemicals tested are described in further detail in Table 1. Forty-one chemicals were selected, including PBDEs, BFRs, PFRs, phenolics, pesticides, parabens, phthalates, and PFASs that our laboratory and others had commonly detected in indoor house dust and that therefore were considered to represent a chronic source of human exposure 23, 25, 26. Stock solutions were prepared in 100% DMSO (Sigma cat # D2650) and stored at −20 °C between uses.
Table 1.
Triglyceride Accumulation and Pre-Adipocyte Proliferation Results for Tested Compounds
| Chemical | Acronym | LOEL (μM) | % TG Max | % TG Max (μM) | EC20 (μM) | EC50 (μM) | % Prolif Max | % Prolif (μM) | EC20 (μM) | EC50 (μM) |
|---|---|---|---|---|---|---|---|---|---|---|
| Polybrominated Diphenyl Ethers (PBDEs)/Metabolites | ||||||||||
| 2,2′,4,4′-tetrabromodiphenyl ether | BDE-47 | 10 | 16.6% | 10 | 0.02 | 1.40 | 3.6% | 10 | N/A | N/A |
| 6-hydroxide-2,2′,4,4′-tetrabromodiphenyl ether | 6-OH BDE-47 | N/A | 0.0% | N/A | N/A | N/A | 0.0% | N/A | N/A | N/A |
| 2,2′,4,4′,5-pentabromodiphenyl ether | BDE-99 | N/A | 3.8% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Decabromodiphenyl ether | BDE-209 | N/A | 0.0% | N/A | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Brominated Flame Retardants (BFRs) | ||||||||||
| 2-ethyl hexyl-2,3,4,5-tetrabromobenzoate | TBB | N/A | 4.2% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Bis (2-ethyl hexyl)-2,3,4,5-tetrabromophthalate | TBPH | 0.1 | 66.9% | 10 | 0.01 | 1.00 | 38.9% | 1 | 0.001 | 0.004 |
| Tris (2,3-dibromopropyl) isocyanurate | TDBPIC | 10 | 10.2% | 10 | 0.13 | 1.40 | 0.0% | N/A | N/A | N/A |
| Organophosphate Flame Retardants (PFRs) | ||||||||||
| Tris (1-chloro-isopropyl) phosphate (mix of isomers) | TCIPP | N/A | N/A | 10 | N/A | N/A | 0.6% | N/A | N/A | N/A |
| Tris (2,4-dichloro-isopropyl) phosphate | TDCIPP | 10 | 1.5% | 10 | N/A | N/A | 2.9% | 10 | N/A | N/A |
| Triphenyl phosphate | TPHP | 1 | 24.6% | 10 | 1.40 | 3.05 | 16.4% | 10 | 1.60 | 3.25 |
| Tris (4-butyl-phenyl) phosphate | TBPP | N/A | 0.0% | N/A | N/A | N/A | 16.7% | 10 | 1.80 | 3.50 |
| Tri-iso-butyl-phosphate | TiBP | 10 | 2.4% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Tri-n-butyl-phosphate | TnBP | 10 | 10.1% | 10 | 1.40 | 3.05 | 0.0% | N/A | N/A | N/A |
| Tri-(2-butoxyethyl)-phosphate | TBOEP | 10 | 20.9% | 10 | 1.80 | 3.50 | 0.0% | N/A | N/A | N/A |
| Tert-buty-phenyl, diphenyl phosphate | TBPDP | 1 | 140.7% | 10 | 1.25 | 2.80 | 1.4% | 10 | N/A | N/A |
| Firemaster™ 550 (mixture) | FM550 | 1 | 100.8% | 10 | 1.50 | 3.20 | 19.7% | 10 | 1.10 | 1.66 |
| Isopropylated triaryl phosphates (mixture) | ITPs | 1 | 110.3% | 10 | 0.30 | 1.40 | 37.1% | 10 | 1.40 | 3.05 |
| Phthalates | ||||||||||
| Bis(2-ethylhexyl) phthalate | DEHP | 10 | 19.9% | 10 | 1.50 | 3.25 | 28.6% | 10 | 0.02 | 0.04 |
| Dibutyl phthalate | DBP | 1 | 149.0% | 10 | 1.75 | 3.33 | 9.0% | 10 | N/A | N/A |
| Di-isobutyl phthalate | DiBP | 10 | 8.1% | 10 | N/A | N/A | 1.2% | 10 | N/A | N/A |
| Benzyl butyl phthalate | BBP | 10 | 6.6% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Di-ethyl phthalate | DEP | 10 | 0.0% | N/A | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Bis (2-ethylhexyl) terephthalate | DEHT | 10 | 7.0% | 10 | N/A | N/A | 5.5% | 10 | N/A | N/A |
| Perfluoralkylated substances (PFASs) | ||||||||||
| 1H, 1H,2H,2H-Perfluorodecyl acrylate | 8:2 FTAcr | 1 | 35.6% | 10 | 0.02 | 0.60 | 0.0% | N/A | N/A | N/A |
| 2-Perfluorooctyl ethanol | 8:2 FTOH | 10 | 2.2% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A |
| 2-Perfluorohexyl ethanol | 6:2 FTOH | 10 | 0.0% | N/A | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Parabens | ||||||||||
| Butyl paraben | 10 | 0.9% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A | |
| Ethyl paraben | 10 | 1.8% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A | |
| Methyl paraben | 10 | 0.0% | N/A | N/A | N/A | 0.0% | N/A | N/A | N/A | |
| Propyl paraben | 10 | 0.4% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A | |
| Pesticides | ||||||||||
| Chlorpyrifos | 1 | 12.7% | 1 | 0.03 | 0.10 | 0.0% | N/A | N/A | N/A | |
| Permethrin (mix of isomers) | 1 | 81.1% | 10 | 0.17 | 1.50 | 0.0% | N/A | N/A | N/A | |
| Cypermethrin (mix of isomers) | 1 | 60.4% | 10 | 0.03 | 1.33 | 0.0% | N/A | N/A | N/A | |
| Chlorfenapyr | 10 | 2.0% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A | |
| Fipronil | 10 | 7.3% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A | |
| Pyraclostrobin | 1 | 122.3% | 1 | 0.18 | 0.30 | 19.2% | 10 | 0.13 | 0.25 | |
| Azoxystrobin | 10 | 18.7% | 10 | 0.75 | 3.30 | 31.2% | 10 | 0.55 | 1.15 | |
| Fluoxastrobin | 1 | 33.2% | 10 | 0.73 | 3.60 | 33.9% | 10 | 1.80 | 4.40 | |
| Trifloxystrobin | 10 | 19.3% | 10 | 0.70 | 3.20 | 10.9% | 10 | 2.12 | 5.33 | |
| Phenolics | ||||||||||
| 2,4,6-tribromophenol | 2,4,6 TBP | 10 | 3.9% | 10 | N/A | N/A | 0.0% | N/A | N/A | N/A |
| Triclosan | TCS | 10 | 0.0% | N/A | N/A | N/A | 3.8% | 10 | N/A | N/A |
Triglyceride accumulation (relative to rosiglitazone-induced maximum) and pre-adipocyte proliferation (relative to vehicle control) provided as maximum efficacies, and concentrations at which each chemical exhibits this activity. Potencies for each adipogenic mechanism provided as EC20 and EC50 values in μM (concentration at which the chemical exhibits 20% or 50% of its maximal activity, respectively). LOEL = lowest observed effect level, the lowest test concentration in μM that exhibited significant activity via either triglyceride accumulation (normalized to DNA content) or pre-adipocyte proliferation.
Dust sample collection and processing
House dust samples (n=11) were collected as part of another ongoing study and processed as described previously 33. Briefly, dust samples were collected using a vacuum cleaner with a crevice tool attachment and cellulose thimble, as previous 34, 35. Samples were collected from households in central North Carolina, USA, between May and October of 2014. Each sample was collected from a resident that had lived in their household for at least two years and who was instructed not to vacuum their home for at least two days prior to collection. During collection, the main living area of the home was vacuumed and dust collected, wrapped in foil, and frozen. After sieving to <500 μm, approximately 100 mg of dust was extracted with 50:50 dichloromethane:hexane (used to extract a wide range of chemical classes) via sonication extraction and concentrated under nitrogen gas. An aliquot of this fraction was further evaporated to dryness and reconstituted in DMSO for use in these bioassays. Three laboratory blanks were prepared using laboratory solvents and techniques in the absence of dust to ensure that lab procedures did not impart any active chemicals to our assays. None of these samples exhibited significant triglyceride accumulation or pre-adipocyte proliferation at any concentration tested.
Cell Care
3T3-L1 cells were obtained from Zenbio, Inc. at passage 8 (cat# SP-L1-F, lot# 3T3062104; Research Triangle Park, NC) and were maintained in Dulbecco’s Modified Eagle Medium – High Glucose (DMEM-HG; Gibco cat# 11995) supplemented with 10% bovine calf serum and 1% penicillin and streptomycin (Gibco cat# 15140) 36. Cells were maintained in a sub-confluent state until differentiation, and each thaw was differentiated within 8 passages (p8–15), with no significant changes in control chemical response observed in that time.
Differentiation Induction and Maintenance
Cells were induced to differentiate as described in detail previously 36. Briefly, cells were seeded into 96-well tissue culture plates (Greiner cat # 655090) at approximately 30,000 cells per well in pre-adipocyte media. Once confluent, cells were cultured for an additional 48 hours to undergo growth arrest and initiate clonal expansion. Following this window, media was replaced with test chemicals, dust samples, and/or controls using a 0.1% DMSO vehicle diluted in differentiation media (DMEM-HG with 10% fetal bovine serum, 1% penicillin/streptomycin, 1.0 μg/mL human insulin, and 0.5 mM 3-isobutyl-1-methylxanthine, IBMX). After 48 hours, media was replaced with test chemicals diluted in adipocyte maintenance media (differentiation media without IBMX) and was refreshed every 2–3 days until assay.
Lipid and DNA staining protocols
Plates were assayed for triglyceride accumulation, DNA content, and cell viability (ATP production) ten days after induction of differentiation, as described previously 36. Media was removed from wells of tissue culture plates and cells rinsed with phosphate-buffered saline before replacing with 200 μL of dye mixture (19 mL saline, 1 drop/mL NucBlue (Thermo# R37605), and 500 μL AdipoRed (Lonza# PT-7009)). Plates were wrapped to protect from light and incubated for approximately forty minutes, then read using a Molecular Devices SpectraMax M5 fluorimeter; excitation 485 nm/emission 572 nm for AdipoRed, excitation 360 nm/emission 460 nm for NucBlue. Cell Viability was assessed using the CellTiter-Glo assay (Promega# G7572). Briefly, following the lipid and DNA protocols, 170 μL of media was removed from each well, 30 μL of CellTiter reagent was added, and media was pipetted up and down several times to homogenize. Plates were incubated at room temperature for ten minutes prior to reading luminescence.
Efficacies (percent activities) across the full dose responses tested were calculated relative to the intra-assay average rosiglitazone-induced maximal fold induction (Figure S1; average of 3–4 complete dose responses from separate plates within each assay; EC50: ~35 nM; max activation: 1 μM; sensitivity: 10 nM) over intra-assay differentiated vehicle controls (0.1% DMSO; included in every plate), after correcting for background fluorescence by subtracting raw fluorescence units from cell-free wells (intra-assay Z′-factor: 0.81; signal/noise: 9.2-fold change). DNA content and cell viability were calculated as percent change from vehicle controls for each chemical at each concentration, and DNA content was then used to normalize total triglyceride values in each well to obtain triglyceride content per cell. As pre-adipocytes can be driven to accumulate intracellular lipids and/or proliferate in response to test chemicals, normalizing triglyceride accumulation to DNA content allowed us to tease apart these mechanisms for individual chemicals. Significant cell viability loss in the absence of cytotoxicity (decreased DNA content) was only observed for pyraclostrobin, azoxystrobin, trifloxystrobin, and fluoxastrobin; these results are discussed in light of the adipogenic responses within the Discussion. Potencies were determined using EC20’s and EC50’s (concentration of each chemical that exhibits 20% or 50% of its own maximal activity, respectively) values determined using GraphPad Prism 6.0.
Statistical Analysis
Data are presented as means ± SE from four technical replicates of three independent experiments. Linear mixed models were used to analyze the results from the three biological replicate assays, and incorporated random effects to account for dependence among quadruplicate technical replicates. Post-test comparison between treatment groups was performed between groups using least-square means to determine 95% confidence intervals and the Tukey-Kramer multiple comparison test with differences considered statistically significant at p < 0.05 to determine differences between treatment groups and from vehicle control. Cell proliferation results were log transformed for normal distributions and adjusted means back-transformed for presentation. Proc GLIMMIX in SAS 9.4 (SAS Inc.) was used for this analysis. EC20/50 values were estimated using curves generated from raw fluoresence data using a 4-parameter variable-slope Hill model in GraphPad Prism 6.0.
Results
Eleven indoor house dust extracts and forty-four SVOCs from seven structural groups were assessed for percent adipogenic activity utilizing 3T3-L1 cells. Cells were differentiated according to standard protocols and assessed after ten days for triglyceride accumulation (relative to maximal rosiglitazone response) and pre-adipocyte proliferation (relative to vehicle control) using fluorescent stains.
House dust extract adipogenic activity
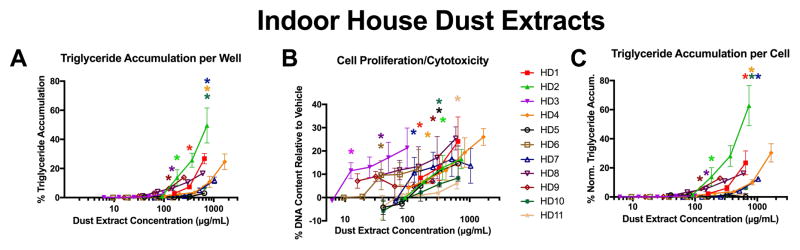
Eleven extracts from house dust samples were assessed for adipogenic activity (Figure 1). Seven of eleven dust extracts exhibited significant triglyceride accumulation (relative to DMSO vehicle control), all at concentrations below 1 mg/mL DEQ (200 μg dust per assay well, based on 200 μL assay volume; Figure 1A). Nine of eleven dust extracts exhibited significant cell proliferation (Figure 1B), with only one dust extract completely inactive (HD11). Three samples that were inactive for triglyceride accumulation stimulated significant cell proliferation (HD3, 5, 6), and one sample that was inactive for cell proliferation stimulated significant triglyceride accumulation (HD9). Comparing these different endpoints, cell proliferation appeared to provide more sensitive detection of adipogenic activity for dust extracts, with three samples exhibiting significant effects on cell proliferation below 100 μg/mL (20 μg dust per well), and no samples eliciting triglyceride accumulation at this concentration.
Figure 1. House Dust Extracts Induce Adipogenic Activity at Environmentally Relevant Concentrations.
Zenbio 3T3-L1 cells were differentiated as described in Methods and assessed for adipocyte differentiation (Nile Red staining of lipid accumulation) and cell proliferation (Hoechst staining) after ten days of differentiation while exposed to various house dust extracts at varying dust equivalent concentrations. Percent raw triglyceride accumulation per well relative to maximal response for rosiglitazone (A), increase (cell proliferation) or decrease (potential cytotoxicity) in DNA content relative to vehicle control (B), and percent normalized triglyceride accumulation per cell relative to maximal rosiglitazone response (normalized to DNA content) (C). Data presented as mean ± SEM from three independent experiments.
Dust extract concentrations of μg/well can be converted to μg/mL by multiplying concentrations by five, as treatments are diluted in 200 μL of total media per well.
HD = house dust sample extract, 1–11. Three laboratory blanks (all solvents and procedures without addition of dust matrix) were tested and exhibited no significant triglyceride accumulation or pre-adipocyte proliferation.
* indicates lowest concentration with significant increase in triglyceride over vehicle control or cell proliferation/cytotoxicity relative to vehicle control, p<0.05, as per linear mixed model in SAS 9.4.
BFR adipogenic activity
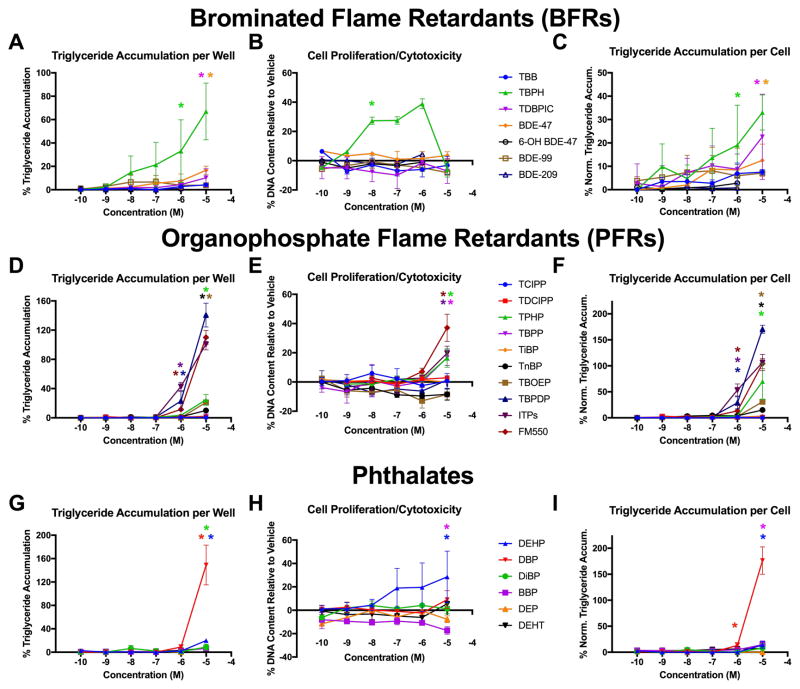
Seven BFRs were tested for adipogenic activity, including three legacy PBDEs: BDE-47, BDE-99, BDE-209, one PBDE metabolite: 6-OH BDE-47, and three current-use BFRs: TBB, TBPH, and TDBPIC (Figure 2, Table 1). TBPH, TDBPIC, and BDE-47 exhibited significant triglyceride accumulation and/or increased cell proliferation, while no adipogenic activity was exhibited by BDE-99, BDE-209, 6-OH BDE-47, or TBB. The most active BFR was TBPH, which exhibited approximately 67% triglyceride accumulation per well (relative to the rosiglitazone max), with an EC20 and EC50 of 0.01 and 1.0 μM, respectively (Figure 2A, C). BDE-47 and TDBPIC exhibited 17 and 10% triglyceride accumulation, with EC20’s of 0.02 and 1.20 μM, respectively (Figure 2A, C). TBPH also exhibited 39% increased cell proliferation, with an EC50 of 4.0 nM; no other BFRs exhibited any significant cell proliferation (Figure 2B). As a result, while TBPH induced less triglyceride accumulation on a per cell basis, corrected to DNA content, no other BFRs were affected.
Figure 2. Flame Retardants and Phthalates Induce Wide Range of Adipogenic Activity.
Zenbio 3T3-L1 cells were differentiated as described in Methods and assessed for adipocyte differentiation (Nile Red staining of lipid accumulation) and cell proliferation (Hoechst staining) after ten days of differentiation while exposed to various brominated flame retardants (BFRs; A–C), organophosphate flame retardants (PFRs; D–F), and phthalates (G–I) from 0.1 nM to 10 μM in concentration. Percent raw triglyceride accumulation per well relative to maximal response for rosiglitazone (A, D, G), increase (cell proliferation) or decrease (potential cytotoxicity) in DNA content relative to vehicle control (B, E, H), and percent normalized triglyceride accumulation per cell relative to maximal rosiglitazone response (normalized to DNA content) (C, F, I). Data presented as mean ± SEM from three independent experiments.
TBB = 2-ethylhexyl-2,3,4,5-tetrabromobenzoate, TBPH = bis (e-ethyl hexyl)-2,3,4,5-tetrabromophthalate, TDBPIC = tris (2,3-dibromopropyl) isocyanurate, BDE-47 = 2,2′4,4′-tetrabromodiphenyl ether, 6-OH BDE-47 = 6-hydroxide-BDE-47, BDE-99 = 2,2′,4,4′,5-pentabromodiphenyl ether, BDE-209 = decabromodiphenyl ether.
TCIPP = tris (1-chloro-isopropyl) phosphate, TDCIPP = tris (2,4-dichloro-isopropyl) phosphate, TPHP = triphenyl phosphate, TBPP = tris (4-butyl-phenyl) phosphate, TiBP = tri-iso-butyl phosphate, TnBP = tri-n-butyl phosphate, TBOEP = tri-(2-butoxyethyl)-phosphate, TBPDP = tert-butyl-phenyl, diphenyl phosphate, FM500 = Firemaster® 550, ITPs = isopropylated triaryl phosphates.
DEHP = bis (2-ethylhexyl) phthalate, DBP = dibutyl phthalate, DiBP = di-isobutyl phthalate, BBP = benzyl butyl phthalate, DEP = di-ethyl phthalate, DEHT = bis (2-ethylhexyl) terephthalate.
* indicates lowest concentration with significant increase in triglyceride over vehicle control or cell proliferation/cytotoxicity relative to vehicle control, p<0.05, as per linear mixed model in SAS 9.4.
PFRs and flame retardant mixtures adipogenic activity
Ten PFRs were assessed for adipogenic activity, including TPHP, TBPP, TnBP, TBOEP, TBPDP, TCIPP, TDCIPP, TBPP, TiBP, ITPs (isomer mixture), and the commercial Firemaster® 550 (FM550) mixture (Figure 2, Table 1). TBPDP, ITPs, FM550, TPHP, TnBP, and TBOEP exhibited significant triglyceride accumulation (Figure 2D, F), with EC20’s of 1.25, 0.30, 1.50, 1.40, 1.40, and 1.80 μM, respectively. In addition, FM550, ITPs, TPHP, and TBPP induced significant cell proliferation (Figure 2E). Correcting triglyceride accumulation for DNA content did not modify potencies, but did increase triglyceride accumulation per cell for TBPDP and decreased FM550, ITPs, TPHP, and TBPP. TCIPP, TDCIPP, and TiBP exhibited no adipogenic activity. Interestingly, TBPDP exhibited the greatest triglyceride accumulation of any PFR (141%), though no significant cell proliferation. In contrast, TBPP exhibited no triglyceride accumulation but exhibited 17% increased cell proliferation. Both the ITP and FM550 mixtures exhibited both increased triglyceride accumulation (110% and 101%, respectively) and increased cell proliferation (37% and 20%, respectively), making them the most active PFRs tested. It’s important to note that ITP is a large component of FM550, and thus the activity in FM550 may be partly or wholly reflective of this. In general, PFRs tended to exhibit much greater adipogenic activities than the BFRs tested above.
Phthalate adipogenic activity
Six phthalates were assessed for adipogenic activity, including DEHP, BDP, DiBP, BBP, DEP, and DEHT (Figure 2, Table 1). DBP and DEHP exhibited significant triglyceride accumulation (Figure 2G, I) and significant cell proliferation (Figure 2H), while DiBP, BBP, DEP, and DEHT exhibited no significant adipogenic activity. DBP was the most efficacious chemical tested, exhibiting 149% triglyceride accumulation and an EC20 of 1.75 μM, though only 9% increased cell proliferation. DEHP exhibited 20% increased triglyceride accumulation and an EC20 of 1.50 μM, but 29% increased cell proliferation, one of the most efficacious chemicals tested. BBP, which exhibited no significant triglyceride accumulation on a well basis, also appeared to exhibit slight cytotoxicity, resulting in significant triglyceride accumulation on a cell basis. Cell normalization increased the triglyceride accumulation of DBP on a per cell basis, and resulted in slight decreases for DEHP and DiBP. While phthalates were overall relatively inactive, DEHP and DBP were two of the most active chemicals tested.
Perfluoroalkylated substances (PFASs) adipogenic activity
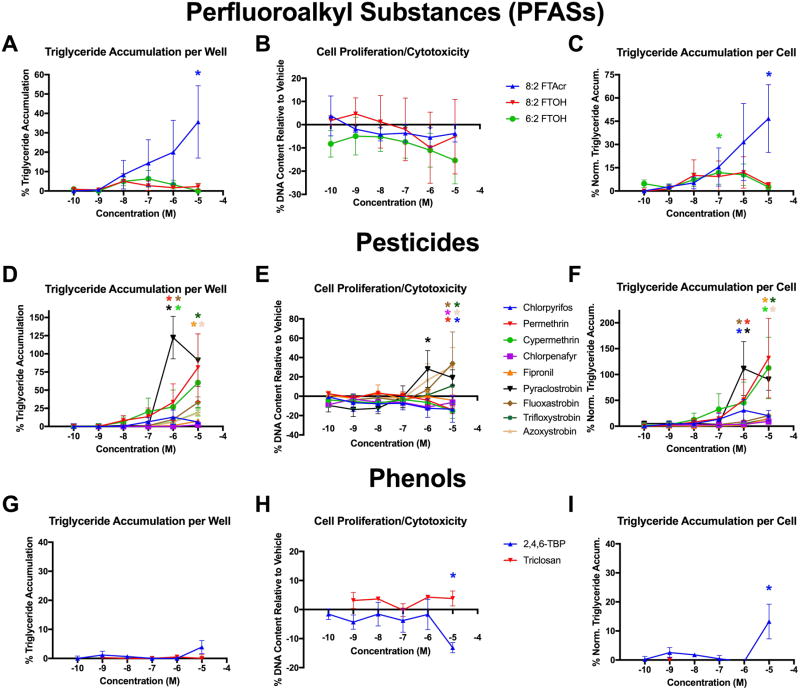
Three PFAS were assessed for adipogenic activity, including 8:2 FTAcr, 8:2 FTOH, and 6:2 FTOH (Figure 3, Table 1). 8:2 FTAcr exhibited significant triglyceride accumulation (36%; Figure 3A, C), though did not induce cell proliferation (Figure 3B). Both 6:2 and 8:2 FTOH exhibited no significant adipogenic activity. While the triglyceride accumulation for 8:2 FTAcr was not particularly efficacious, it was one of the more potent, with an EC20 and EC50 of 0.02 and 0.6 μM, respectively (Table 1). Due to slight apparent cytotoxicity, 6:2 FTOH appeared to exhibit significant triglyceride accumulation on a per cell basis.
Figure 3. Perfluorinated Chemicals, Pesticides, and Phenolics Induce Adipogenic Activity.
Zenbio 3T3-L1 cells were differentiated as described in Methods and assessed for adipocyte differentiation (Nile Red staining of lipid accumulation) and cell proliferation (Hoechst staining) after ten days of differentiation while exposed to various perfluoroalkyl substances (PFASs; A–C), pesticides (D–F), and phenolic compounds (G–I) from 0.1 nM to 10 sμM in concentration. Percent raw triglyceride accumulation per well relative to maximal response for rosiglitazone (A, D, G), increase (cell proliferation) or decrease (potential cytotoxicity) in DNA content relative to vehicle control (B, E, H), and percent normalized triglyceride accumulation per cell relative to maximal rosiglitazone response (normalized to DNA content) (C, F, I). Data presented as mean ± SEM from three independent experiments.
FTAcr = 1H, 1H, 2H, 2H-perfluorodecyl acrylate, 8:2 FTOH = 2-perfluorooctyl ethanol, 6:2 FTOH = 2-perfluorohexyl ethanol, 2,4,6-TBP = 2,4,6-tribromophenol
* indicates lowest concentration with significant increase in triglyceride over vehicle control or cell proliferation/cytotoxicity relative to vehicle control, p<0.05, as per linear mixed model in SAS 9.4.
Paraben adipogenic activity
Four parabens were assessed for adipogenic activity, including butyl, ethyl, methyl, and propyl paraben (Figure S2, Table 1). None of these chemicals exhibited any significant triglyceride accumulation or cell proliferation over the doses tested.
Pesticide adipogenic activity
Nine pesticides were assessed for adipogenic activity, including chlorpyrifos, permethrin, cypermethrin, chlorpenafyr, fipronil, pyraclostrobin, azoxystrobin, fluoxastrobin, and trifloxystrobin (Figure 3, Table 1). Pyraclostrobin, azoxystrobin, fluoxastrobin, trifloxystrobin, permethrin, cypermethrin, and chlorpyrifos exhibited significant triglyceride accumulation (Figure 3D, F), pyraclostrobin, azoxystrobin, fluoxastrobin, and trifloxystrobin exhibited increased cell proliferation (Figure 3E), and fipronil and chlorpenafyr exhibited no significant adipogenic activity. Pyraclostrobin was one of the most efficacious chemicals tested in this study, with triglyceride accumulation of 122% and an EC50 of 0.3 μM (Table 1). This pesticide also induced 19% increased DNA content; azoxystrobin and fluoxastrobin induced approximately 33% each, but much less triglyceride accumulation (19% and 33%, respectively). As a result, pyraclostrobin and the other strobins exhibited slightly less triglyceride accumulation on a per cell basis, while slight cytotoxicity for permethrin, cypermethrin, and chlorpyrifos resulted in increased triglyceride accumulation per cell. Permethrin and cypermethrin exhibited 81% and 60% triglyceride accumulation, respectively, with EC50’s of 1.50 and 1.33 μM, 5–10-fold less potent than pyraclostrobin. While chlorpyrifos exhibited a similar potency to pyraclostrobin, it was much less efficacious in triglyceride accumulation (13%). Interestingly, despite increased cell proliferation for each of the four strobins (Figure 3E), these compounds also resulted in a complete inhibition of ATP production in the cell viability assay (Figure S3).
Phenolic adipogenic activity
Two phenols were assessed for adipogenic activity, including 2,4,6-TBP and triclosan (Figure 3, Table 1). 2,4,6-TBP did not exhibit significant total triglyceride accumulation per well (Figure 3G), but due to apparent cytotoxicity (or decreased proliferation relative to differentiated DMSO control) at 10 μM (Figure 3H), did exhibit significant triglyceride accumulation on a per cell basis (Figure 3I). Triclosan exhibited no significant adipogenic activity, and neither exhibited any cell proliferation.
Discussion
This study is the first to report that house dust extracts can induce both triglyceride accumulation and pre-adipocyte proliferation; notably, this occurred at environmentally relevant exposure levels. The EPA estimates that children consume 50 mg of house dust each day 22, and while oral bioavailability of every chemical in these complex mixtures is not known, adipogenic effects from house dust extracts were observed at concentrations as low as 75 μg/mL (15 μg/well) for triglyceride accumulation and 15 μg/mL (3 μg/well) for cell proliferation (given 200 μL treated media per well). An exposure level of 50 mg of dust per day is >16,000 times greater than the dust mass exhibiting significant effects in this study, suggesting effects of environmentally-relevant exposure levels below the 100 μg/mL (4 μg/well) that was previously reported as the lowest concentration to exhibit PPARγ activity 27, 28 and the 12–40 μg/well previously reported for other bioactivities discussed above 30–32. Interestingly, while pre-adipocyte proliferation (assessed herein via DNA content) is not routinely assessed by most publications utilizing this model system, we report that this was a more potent exposure metric for the house dust extracts, with several samples eliciting effects at up to order of magnitude lower concentrations than for triglyceride accumulation. Further, two samples elicited effects via pre-adipocyte proliferation that did not exhibit significant triglyceride accumulation, and one sample elicited triglyceride accumulation without pre-adipocyte proliferation. These examples provide further support for incorporating both metrics into routine analyses using this system. Only one of eleven dust samples appeared completely inactive, suggesting that the causative chemical(s) are nearly ubiquitous in the indoor environment. As such, research is needed to determine whether there are impacts of these adipogenic mixtures on the metabolic health of residents, particularly children, and to identify the causative chemicals promoting this activity.
To begin to delineate the potential causative chemicals driving the observed adipogenicity of house dust, we further assessed the adipogenic activity of 41 SVOCs from diverse chemical classes that are known to be common indoor contaminants 23, 25, 26, 37 and that represent a chronic exposure source through oral, dermal, and inhalation routes 22, 38–41. Metabolic disruption has been previously demonstrated for some of these chemicals in vivo, including FM550 and several components 42, permethrin 43 and cypermethrin 44, and DEHP 45. Some of these chemicals have been previously assessed in 3T3-L1 cells, and agree with our results. BDE-47 has been previously reported to promote a low level of triglyceride accumulation 46–48, chlorpyrifos has previously been demonstrated to increase body weight in vivo but not promote (and perhaps inhibit) differentiation in vitro 49, 50, triclosan has been demonstrated to inhibit adipogenesis 51, and fipronil has been demonstrated to exhibit minimal triglyceride accumulation 52. Previous work has demonstrated a low level of triglyceride accumulation for several parabens 50, 53, in apparent contrast with our results, though this occurred only at concentrations greater than those we tested.
However, adipogenic activity via increased triglyceride accumulation and/or increased pre-adipocyte proliferation has not been demonstrated for the majority of the chemicals examined herein. Interestingly, despite a previous report of adipogenic activity for FM550 and component chemicals ITPs and TPHP 54, which we also report as active, no PPARγ activity was found for TBPH and it was thus not tested for adipogenesis. Despite this, we found TBPH was more efficacious than TPHP and more potent than the ITPs, suggesting this may contribute to an equal or greater degree of total FM550 adipogenicity and delineating a clear role for other receptors in adipogenesis. Notably, several high-volume production chemicals were also found to exhibit supra-maximal triglyceride accumulation relative to the rosiglitazone control, including pyraclostrobin (a fungicide), the flame-retardant TBPDP, and a commonly used plasticizer, DBP. Interestingly, triglyceride accumulation and pre-adipocyte proliferation did not appear to always co-occur, suggesting that these adipogenic phenotypes may be driven through overlapping but distinct receptor mechanisms. Metabolism of TBPH, DEHP, TBB, and others have been previously demonstrated to result in more bioactive metabolites that exhibit greater activation of PPARγ and may subsequently result in greater adipogenic activity 27, 28. As such, it is possible that we may be underestimating the degree of adipogenicity of these chemicals in vivo, given that relative degrees of metabolism in this cell line have not been definitively characterized.
As noted above, the four strobin compounds appeared to exhibit increased cell proliferation, increased triglyceride accumulation, and decreased cell viability (ATP production) at overlapping concentrations. Induction of oxidative stress and other mitochondrial toxicant mechanisms have been demonstrated to result in ATP depletion 55, 56, suggesting a potential mechanism. We suspect that these chemicals are acting to inhibit ATP production without resulting in cytotoxicity, hence the disparate findings between these assays. Interestingly, antimycin A, which inhibits complex III of the electron transport chain (important for ATP production), has been reported to induce triglyceride accumulation in 3T3-L1 cells via a differentiation-independent mechanism 57; these cells exhibit a phenotypically distinct phenotype (multi-vesicular lipid accumulation), exhibit reduced expression of standard differentiation markers such as fatty acid binding protein 4 (FABP4; aP2) and CCAAT/enhancer binding protein (C/EBP), and exhibit suppression of PPARγ and RXR activities. Importantly, the strobins have also been demonstrated to exhibit complex III inhibition 58, suggesting a potential mechanism for these effects. A recent publication assessed pyraclostrobin in a human adipose-derived stem cell model, reporting that it was also capable of promoting triglyceride accumulation, and while there appeared to be some borderline activation of C/EBPα and PPARγ, other standard markers of differentiation were absent 59, bolstering the case for a differentiation-independent mechanism. Further research should evaluate these potential mechanisms for the observed adipogenicity of these fungicides, given their high application to produce of >2 million pounds per year 60, 61.
Historically, there has been a strong focus on PPARγ, the only receptor considered necessary and sufficient to induce adipogenesis. However, as we have demonstrated previously, many other nuclear receptors can regulate and/or modulate adipogenesis and lipogenesis to drive increased triglyceride accumulation and/or cell proliferation 36, and some of our most active adipogenic chemicals have been reported to exhibit minimal or no activity for PPARγ. Previous work in our laboratory characterized the PPARγ activity and ligand binding of fifteen of the chemicals tested herein 27, 28; while we found no significant correlation between the potencies of PPARγ activation and triglyceride accumulation for these same chemicals (Figure S4A; p=0.564), efficacies were more highly (but not significantly) correlated (Figure S4B; p=0.060). It’s possible that the narrow range of potencies from this study inhibited our ability to detect a significant correlation, hence the more highly correlated efficacies, although it must be noted that maximal activation and potency are distinct metrics; while maximal activation of PPARγ may be more associated with maximal triglyceride accumulation, it may not explain results observed at low concentrations. Notable disparities in responses between these assays were also observed; BBP and the ITPs both exhibited 30% PPARγ activity 28, yet the ITPs exhibited 110% triglyceride accumulation and BBP exhibited minimal. Chemicals with the highest adipogenic activity in this study, TBPDP, DBP, and pyraclostrobin, exhibited high, minimal, and no PPARγ activities in previous studies, respectively 28, 62. Particularly notable, pyraclostrobin, a high-production pesticide 60, 61, was one of the most efficacious and potent adipogenic chemicals tested, though does not activate PPARγ 62. In an attempt to better inform potential causative receptor pathways, ToxCast assays (https://actor.epa.gov/dashboard/) were reviewed for a list of receptors that could regulate adipogenesis and/or lipogenesis (Table S2). Thirty-eight chemicals we have assessed for adipogenicity were included in the ToxCast database and exhibited a wide range of receptor activities, including modulation of the farnesoid X receptor, TR, AR, GR, RXR, liver X receptor, retinoic acid receptor, pregnane X receptor, low-density lipoprotein receptor, and PPARγ. Each of these receptors, or a combination of them, may be contributing to the observed triglyceride accumulation and cell proliferation effects and highlights the importance of assessing a more complex suite of pathways. Further work should attempt to more rigorously determine the specific receptor mechanisms driving the adipogenic activity of house dust, using gene expression and receptor agonist or antagonist co-treatment experiments.
In conclusion, the results described herein delineate significant concerns for human metabolic health, particularly in children. The observed adipogenic activity of house dust extracts, which are occurring at concentrations below exposure levels estimated by EPA, is concerning and should be interrogated further. While bioavailability following oral exposure to each of these chemicals is not well characterized, effects at a dust mass equivalence >10,000-fold below estimated child oral exposure levels (to total dust mass) suggests a need for further investigation. Notably, previous work demonstrated high bioaccessibility for PFRs in dust (~80%), decreasing with Log Kow values63. Notably, a wide range of chemicals that are commonly reported in house dust samples exhibited triglyceride accumulation and/or pre-adipocyte proliferation, highlighting that these chemicals (and likely others) are likely promoting the adipogenic activity described. Further work should carefully assess the relative contribution of these chemicals, at the concentrations they are found in house dust, to the total adipogenicity exhibited by these samples; there may be other chemicals present that contribute to the total bioactivity as well, and determining the magnitude of activity that these contaminants promote will help elucidate other potential contaminants of interest. This activity is likely due to disruption of several receptor pathways that regulate adipogenesis, many of which are activated by house dust samples 28, 30–32, and future studies should assess receptor activation and antagonist testing with house dust samples to better characterize these pathways.. While studies often highlight individual contaminants and classes of contaminants of concern for potential metabolic disruption, there is a critical need to more thoroughly assess realistic environmental mixtures that may be contributing to this and other adverse human health trends.
Supplementary Material
Acknowledgments
Funding: Project supported by a grant from the National Institute of Environmental Health Sciences (R01 ES016099-06). Additional support received from Fred and Alice Stanback, the Nicholas School of the Environment, and the Duke Cancer Institute.
We would like to thank all the participants that provided dust samples for this study. In addition, we would like to thank Anthony Luz (Duke University) for thoughtful discussions regarding the potential mechanism of action for the strobin family of fungicides.
Footnotes
Disclosure Statement: The authors declare they have no actual or potential competing financial interests.
Supporting Information. Supporting information is provided for positive control and paraben responses, strobin family cell viability response, correlations between PPARγ activities and adipogenic outcomes, chemical purity and ordering information, and a list of putative adipogenic pathways for tested chemicals.
References
- 1.The Endocrine Disruption Exchange (TEDX) [accessed: 1 June 2017];TEDX List of Potential Endocrine Disruptors. 2017 Available: http://endocrinedisruption.org/endocrine-disruption/tedx-list-of-potential-endocrine-disruptors/overview.
- 2.Heindel JJ, vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, Cohn BA, Fabbri E, Gioiosa L, Kassotis C, Legler J, La Merrill M, Rizzir L, Machtinger R, Mantovani A, Mendez MA, Montanini L, Molteni L, Nagel SC, Parmigiani S, Panzica G, Paterlini S, Pomatto V, Ruzzin J, Sartor G, Schug TT, Street ME, Suvorov A, Volpi R, Zoeller RT, Palanza P. Parma consensus statement on metabolic disruptors. Environmental health: a global access science source. 2015;14:54. doi: 10.1186/s12940-015-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janesick AS, Blumberg B. Obesogens: an emerging threat to public health. American journal of obstetrics and gynecology. 2016;214(5):559–65. doi: 10.1016/j.ajog.2016.01.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. 2010;3:285–95. doi: 10.2147/DMSOTT.S7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legler J, Fletcher T, Govarts E, Porta M, Blumberg B, Heindel JJ, Trasande L. Obesity, Diabetes, and Associated Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union. The Journal of clinical endocrinology and metabolism. 2015;100(4):1278–88. doi: 10.1210/jc.2014-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Services, U. D. o. H. a. H, editor. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. National Center for Health Statistics; 2015. [Google Scholar]
- 7.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 8.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3(2):127–33. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 9.Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, Vom Saal FS, Taylor JA. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:13. doi: 10.1016/j.reprotox.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environmental health perspectives. 2013;121(3):359–66. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. The Journal of steroid biochemistry and molecular biology. 2011;127(1–2):9–15. doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicological sciences: an official journal of the Society of Toxicology. 2005;84(2):319–27. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 13.Niemelä S, Miettinen S, Sarkanen JR, Ashammakhi N. Adipose Tissue and Adipocyte Differentiation: Molecular and Cellular Aspects and Tissue Engineering Applications. Topics in Tissue Engineering. 2008;4:1–26. [Google Scholar]
- 14.Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, Kato K, Shoeib M, Vieira VM, McClean MD. Polyfluorinated compounds in serum linked to indoor air in office environments. Environmental science & technology. 2012;46(2):1209–15. doi: 10.1021/es2038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environmental health perspectives. 2015;123(2):160–5. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudel RA, Dodson RE, Perovich LJ, Morello-Frosch R, Camann DE, Zuniga MM, Yau AY, Just AC, Brody JG. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environmental science & technology. 2010;44(17):6583–90. doi: 10.1021/es100159c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, Webster TF. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environmental health perspectives. 2011;119(9):1247–52. doi: 10.1289/ehp.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallaire F, Dewailly E, Muckle G, Ayotte P. Time trends of persistent organic pollutants and heavy metals in umbilical cord blood of Inuit infants born in Nunavik (Quebec, Canada) between 1994 and 2001. Environmental health perspectives. 2003;111(13):1660–4. doi: 10.1289/ehp.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houlihan J, Kropp T, Wiles R, Gray S, Campbell C. BodyBurden: The Pollution in Newborns. Environmental Working Group; 2005. [accessed 5 June 2017]. Available: http://www.ewg.org/research/body-burden-pollution-newborns. [Google Scholar]
- 20.Landrigan PJ, Sonawane B, Mattison D, McCally M, Garg A. Chemical contaminants in breast milk and their impacts on children’s health: an overview. Environmental health perspectives. 2002;110(6):A313–5. doi: 10.1289/ehp.021100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environmental science & technology. 2015;49(17):10466–73. doi: 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Environmental Protection Agency, Exposure Factors Handbook; US Environmental Protection Agency, editor. Washington, DC: 2011. [accessed 5 June 2017]. p. 2011. Edition (Final). Available: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252. [Google Scholar]
- 23.Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environmental science & technology. 2003;37(20):4543–53. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- 24.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Webster TF. Associations between PBDEs in office air, dust, and surface wipes. Environment international. 2013;59:124–32. doi: 10.1016/j.envint.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stapleton HM, Harner T, Shoeib M, Keller JM, Schantz MM, Leigh SD, Wise SA. Determination of polybrominated diphenyl ethers in indoor dust standard reference materials. Analytical and bioanalytical chemistry. 2006;384(3):791–800. doi: 10.1007/s00216-005-0227-y. [DOI] [PubMed] [Google Scholar]
- 26.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environmental science & technology. 2009;43(19):7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang M, Webster TF, Ferguson PL, Stapleton HM. Characterizing the peroxisome proliferator-activated receptor (PPARgamma) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environmental health perspectives. 2015;123(2):166–72. doi: 10.1289/ehp.1408522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang M, Webster TF, Stapleton HM. Activation of Human Peroxisome Proliferator-Activated Nuclear Receptors (PPARgamma1) by Semi-Volatile Compounds (SVOCs) and Chemical Mixtures in Indoor Dust. Environmental science & technology. 2015;49(16):10057–64. doi: 10.1021/acs.est.5b01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang M, Webster TF, Stapleton HM. Effect-Directed Analysis of Human Peroxisome Proliferator-Activated Nuclear Receptors (PPARgamma1) Ligands in Indoor Dust. Environmental science & technology. 2015;49(16):10065–73. doi: 10.1021/acs.est.5b01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou PH, Lee CH, Ko FC, Lin YJ, Kawanishi M, Yagi T, Li IC. Detection of Hormone-Like and Genotoxic Activities in Indoor Dust from Taiwan Using a Battery of in Vitro Bioassays. Aerosol and Air Quality Research. 2015;15:1412–1421. [Google Scholar]
- 31.Suzuki G, Takigami H, Nose K, Takahashi S, Asari M, Sakai S. Dioxin-like and transthyretin-binding compounds in indoor dusts collected from Japan: average daily dose and possible implications for children. Environmental science & technology. 2007;41(4):1487–93. doi: 10.1021/es061907l. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki G, Tue NM, Malarvannan G, Sudaryanto A, Takahashi S, Tanabe S, Sakai S, Brouwer A, Uramaru N, Kitamura S, Takigami H. Similarities in the endocrine-disrupting potencies of indoor dust and flame retardants by using human osteosarcoma (U2OS) cell-based reporter gene assays. Environmental science & technology. 2013;47(6):2898–908. doi: 10.1021/es304691a. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman K, Sosa JA, Stapleton HM. Exposure to flame retardant chemicals and the occurrence and severity of papillary thyroid cancer. Under Review. 2017 doi: 10.1016/j.envint.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environmental health perspectives. 2012;120(7):1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassotis CD, Masse L, Kim S, Schlezinger JJ, Webster TF, Stapleton HM. Characterization of adipogenic chemicals in three different cell culture systems: implications for reproducibility based on cell source and handling. Scientific reports. 2017;7:42104. doi: 10.1038/srep42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, Zota AR. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environmental science & technology. 2016;50(19):10661–10672. doi: 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue J, Wan Y, Kannan K. Occurrence of bisphenols, bisphenol A diglycidyl ethers (BADGEs), and novolac glycidyl ethers (NOGEs) in indoor air from Albany, New York, USA, and its implications for inhalation exposure. Chemosphere. 2016;151:1–8. doi: 10.1016/j.chemosphere.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 39.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol. 2008;18(1):2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 40.Weschler CJ, Beko G, Koch HM, Salthammer T, Schripp T, Toftum J, Clausen G. Transdermal Uptake of Diethyl Phthalate and Di(n-butyl) Phthalate Directly from Air: Experimental Verification. Environmental health perspectives. 2015;123(10):928–34. doi: 10.1289/ehp.1409151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pawar G, Abdallah MA, de Saa EV, Harrad S. Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics. J Expo Sci Environ Epidemiol. 2016;27:100–105. doi: 10.1038/jes.2015.84. [DOI] [PubMed] [Google Scholar]
- 42.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27(2):124–36. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao X, Kim Y, Kim D, Yoon KS, Clark JM, Park Y. A Pyrethroid Pesticide, Permethrin, Alters Lipid Metabolism and Voluntary Activities in Mice. The FASEB Journal. 2015;29(1 Supplement) [Google Scholar]
- 44.Jin Y, Lin X, Miao W, Wu T, Shen H, Chen S, Li Y, Pan Q, Fu Z. Chronic exposure of mice to environmental endocrine-disrupting chemicals disturbs their energy metabolism. Toxicology letters. 2014;225(3):392–400. doi: 10.1016/j.toxlet.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environmental health perspectives. 2012;120(8):1123–9. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoppe AA, Carey GB. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. Obesity (Silver Spring) 2007;15(12):2942–50. doi: 10.1038/oby.2007.351. [DOI] [PubMed] [Google Scholar]
- 47.Kamstra JH, Hruba E, Blumberg B, Janesick A, Mandrup S, Hamers T, Legler J. Transcriptional and epigenetic mechanisms underlying enhanced in vitro adipocyte differentiation by the brominated flame retardant BDE-47. Environmental science & technology. 2014;48(7):4110–9. doi: 10.1021/es405524b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tung EW, Boudreau A, Wade MG, Atlas E. Induction of adipocyte differentiation by polybrominated diphenyl ethers (PBDEs) in 3T3-L1 cells. PloS one. 2014;9(4):e94583. doi: 10.1371/journal.pone.0094583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J Med Toxicol. 2007;3(3):89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, Boergesen M, Mandrup S, Vinggaard AM. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Molecular and cellular endocrinology. 2012;361(1–2):106–15. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Guo LW, Wu Q, Green B, Nolen G, Shi L, Losurdo J, Deng H, Bauer S, Fang JL, Ning B. Cytotoxicity and inhibitory effects of low-concentration triclosan on adipogenic differentiation of human mesenchymal stem cells. Toxicology and applied pharmacology. 2012;262(2):117–23. doi: 10.1016/j.taap.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Sun Q, Qi W, Yang JJ, Yoon KS, Clark JM, Park Y. Fipronil promotes adipogenesis via AMPKalpha-mediated pathway in 3T3-L1 adipocytes. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2016;92:217–23. doi: 10.1016/j.fct.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. Effects of parabens on adipocyte differentiation. Toxicological sciences: an official journal of the Society of Toxicology. 2013;131(1):56–70. doi: 10.1093/toxsci/kfs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand binding and activation of PPARgamma by Firemaster(R) 550: effects on adipogenesis and osteogenesis in vitro. Environmental health perspectives. 2014;122(11):1225–32. doi: 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002;128(4):1271–81. doi: 10.1104/pp.010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 57.Vankoningsloo S, Piens M, Lecocq C, Gilson A, De Pauw A, Renard P, Demazy C, Houbion A, Raes M, Arnould T. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J Lipid Res. 2005;46(6):1133–49. doi: 10.1194/jlr.M400464-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B. The strobilurin fungicides. Pest Management Science. 2002;58(7):649–662. doi: 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- 59.Foley B, Doheny DL, Black MB, Pendse SN, Wetmore BA, Clewell RA, Andersen ME, Deisenroth C. Screening ToxCast Prioritized Chemicals for PPARG Function in a Human Adipose-Derived Stem Cell Model of Adipogenesis. Toxicological sciences: an official journal of the Society of Toxicology. 2017;155(1):85–100. doi: 10.1093/toxsci/kfw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.United States Geological Survey (USGS) [accessed 21 November 2016];Estimated Annual Agricultural Pesticide Use - Pyraclostrobin. 2016 https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2014&map=PYRACLOSTROBIN&hilo=L.
- 61.Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nature communications. 2016;7:11173. doi: 10.1038/ncomms11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janesick AS, Dimastrogiovanni G, Vanek L, Boulos C, Chamorro-García R, Tang W, Blumberg B. On the Utility of ToxCast and ToxPi as Methods for Identifying New Obesogens. Environmental health perspectives. 2016;124(8):1214–1226. doi: 10.1289/ehp.1510352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang M, Stapleton HM. Evaluating the bioaccessibility of flame retardants in house dust using an in vitro Tenax bead-assisted sorptive physiologically based method. Environmental science & technology. 2014;48(22):13323–30. doi: 10.1021/es503918m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.