Abstract
Although women appear to be more vulnerable to alcohol-induced pathophysiology than men, the neurobiological basis for sex differences is largely unknown, partially because most studies on alcohol drinking are conducted in male subjects only. The present study examined sex differences in alcohol consumption in two rat strains, Long Evans and Wistar, using multiple behavioral paradigms. The effects of the estrous cycle on alcohol consumption were monitored throughout the study. The results indicated that females drank more alcohol than males when given either continuous or intermittent access to alcohol (vs. water) in their home cages (voluntary drinking). Under operant conditions, no sex or strain differences were found in drinking prior to development of alcohol dependence. However, upon dependence induction by chronic, intermittent alcohol vapor exposure, Wistar rats of both sexes substantially escalated their alcohol intake compared with their nondependent drinking levels, whereas Long Evans rats only exhibited a moderate escalation of drinking. Under these conditions, the estrous cycle had no effect on alcohol drinking in any strain and drinking model. Thus, strain, sex, and drinking conditions interact to modulate nondependent and dependent alcohol drinking. The present results emphasize the importance of including sex and strain as biological variables in exploring individual differences in alcohol drinking and dependence.
Keywords: Alcohol use disorders, Alcoholism, Gender differences, Genetic differences, Ethanol, Drug addiction
1. Introduction
Alcoholism is a chronic, relapsing disorder that is marked by compulsive alcohol intake, an inability to control consumption, and the presence of a negative emotional state during abstinence. The National Institute on Alcohol Abuse and Alcoholism (2015) reported that 88,000 alcohol-related fatalities occur annually (26,000 women and 62,000 men) in the United States. An estimated 16.6 million adults suffer from alcohol use disorders (5.8 million women and 10.8 million men), but only 1.3 million receive treatment (444,000 women and 904,000 men). Women who engage in excessive drinking have higher rates of alcoholic hepatitis (liver inflammation), cardiomyopathy, and cancer (breast, throat, mouth, liver, colon, and esophagus) compared with men (National Institute on Alcohol Abuse and Alcoholism, 2015; Centers for Disease Control and Prevention, 2015).
Despite strong evidence of sex differences in alcohol consumption, there is a notable lack of preclinical studies that include female subjects. A search of the Web of Science Core Collection database using the terms “alcohol dependence” or “ethanol dependence” and “rat” revealed a total of 2094 research articles that were published between 1995 and 2014, but the number of retrieved articles decreased to only 154 when including the search term “female.” Although the number of research articles that included female subjects has been increasing over the past two decades, those that include females are still in the minority (7% of studies in our Web of Science sample).
Female rats generally drink more alcohol than male rats, but the results may vary depending on the rats’ genetic background and drinking conditions (Table 1). The decision to not use female subjects in preclinical studies has also been influenced by the argument that hormonal fluctuations that occur during the estrous cycle can affect the results (ter Horst et al., 2011). On June 9, 2015, the National Institutes of Health (NIH) issued notice NOT-OD-15-102 that acknowledged the role that sex plays in the way in which individuals respond to disease and preventative and therapeutic interventions. Rather than simply excluding females from experimental designs, all NIH-sponsored grants must now include female subjects unless there is “strong justification from the scientific literature, preliminary data, or other relevant considerations.”
Table 1.
Alcohol intake in adult male and female rats across various strains and drinking paradigms.
| Author | Strain | Housing | Paradigm | Results | Comments |
|---|---|---|---|---|---|
| Li and Lumeng, 1984 | NIH heterogeneous stock rats and eight inbred strains from which they were derived | Single | Two-bottle choice | Female > Male | Intake varied depending on the rat strain. The rats were given 24-h/day access to 10% (v/v) alcohol. |
| Lancaster and Spiegel, 1992 | Long-Evans | Single | Two-bottle choice | Female > Male | The rats were given 24-h/day access to 5% and 10% (unclear - likely v/v) beer. |
| Lancaster et al., 1996 | Sprague-Dawley | Single | Two-bottle choice | Female > Male | The rats were given 24-h/day access to 5% (v/v) beer. Neonatal estrogenization of females to produce a male-like phenotype abolished sex differences in drinking. Neither castration nor ovariectomy affected alcohol drinking. The rats were given 23-h/day access to 2–12% (unclear whether v/v) alcohol. |
| Almeida et al., 1998 | Wistar | Single | Two-bottle choice | Female > Male | |
| Juárez and Barrios de Tomasi, 1999 | Wistar | Single | Two-bottle choice | Female > Male | The rats were given 24-h/day access to 6% (v/v) alcohol + 2% sucrose. |
| Cailhol and Mormede, 2001 | Wistar Kyoto, Wistar Kyoto Hyperactive, and Spontaneously Hypertensive | Single | Two-bottle choice | Female > Male | Gonadectomy did not change alcohol intake. The rats were given 24-h/day access to 2–10% (v/v) alcohol. |
| Cailhol and Mormede, 2002 | Wistar Kyoto, Wistar Kyoto Hyperactive, and Spontaneously Hypertensive Rat (SHR) | Single | Two-bottle choice | Female > Male | The rats were given 24-h/day access to 15% (v/v) alcohol. |
| Vendruscolo et al., 2006 | Lewis, SHR, and four strains derived from a Lewis/SHR intercross | Single | Two-bottle choice | Female > Male | The rats were given 24-h/day access to 2.5–20% (v/v) alcohol. |
| Vendruscolo et al., 2008 | SHR | Single | Two-bottle choice | Female > Male | The rats were given 24-h/day access to 10% (v/v) alcohol. |
| De la Torre et al., 2015 | Wistar | Single | Two-bottle choice | Female > Male | The rats were given 23-h/day access to 2–10% (unclear – likely v/v) alcohol. Alcohol intake was higher in naïve females than in naïve males. Alcohol intake was greater in alcohol pre-exposed males than in alcohol pre-exposed females. |
| Dhaher et al., 2012 | High alcohol-drinking (HAD) replicate lines | Single | Two-bottle choice | Female ≥ Male | The rats were given 22-h/day access to 15% (v/v) alcohol in a lickometer setup. HAD-1 male and female rats drank similar amounts of alcohol, whereas HAD-2 female rats exhibited increased alcohol intake than HAD-2 male rats. |
| Bell et al., 2006 | Alcohol-preferring P rats | Single | Two-bottle choice | Female = Male | The rats were given 22-h/day access to 15% (v/v) alcohol in a lickometer setup. |
| Schramm-Sapyta et al., 2014 | CD (derived from Sprague-Dawley) | Single | Two-bottle choice | Female = Male | The rats were given 24-h/day access to 20% (v/v) alcohol on Mondays, Wednesdays, and Fridays. |
| Walker et al., 2008 | Wistar | Group (likely) | Two-bottle choice | Female > Male | The rats were given limited access (1-h/day) to 5% (unclear whether v/v) alcohol + 0.125% saccharin + 3% glucose. Testing occurred in novel cages. |
| Vetter-O’Hagen et al., 2009 | Sprague-Dawley | Group | Two-bottle choice | Female > Male | The rats were given limited 2 h/day access to 6–10% (v/v) alcohol with 0.1% saccharin. The rats were housed two per cage and a mesh divider separated them while tested for alcohol drinking. |
| Maldonado-Devincci et al., 2010 | Sprague-Dawley | Group | Two-bottle choice | Female > Male | The rats were given limited access (30-min/day sessions in different cages; i.e., not in their home cages) to 10% (v/v) alcohol + 0.5% saccharin. Phases of estrous cycle did not affect alcohol drinking. |
| Sluyter et al., 2000 | Wistar | Group | Two-bottle choice | Female ≥ Male | The rats were given intermittent (every other day) 24-h/day access to 2–10% (v/v) alcohol concentrations. |
| Varlinskaya et al., 2015 | Sprague-Dawley | Group | Two-bottle choice | Female > Male | The rats were given intermittent (every other day) limited access (30-min/day) sessions in novel cages to 10% (unclear whether v/v) alcohol + 0.125% saccharin + 3% sucrose. The same rats were given three drinking sessions alone counterbalanced with three social (grouped) drinking sessions. Adult rats of both sexes drank more when given access to alcohol in a social environment. |
| Morales et al., 2015 | Long Evans | Single | Two-bottle choice and alcohol vapor exposure | Female > Male | The rats were given 24-h/day access to 20% (v/v) alcohol on Mondays, Wednesdays, and Fridays for 5 weeks. The rats were then exposed to alcohol vapor (12 h on/12 h off; blood alcohol levels ~190–260 mg/dl) or air for 10 days. The rats were given access to 20% (v/v) alcohol again in free choice with water starting 96 h after removal from alcohol vapor. Alcohol vapor exposure increased drinking in male but not female rats. |
| Moore and Lynch, 2015 | Alcohol-preferring P rats | Single | Three-bottle choice. Operant oral self-administration | Female = Male | The rats were first given 24-h/day access three-bottle choice (water, 8% [v/v] and 16% [v/v] alcohol). The rats were then tested in a 1-h/day operant sessions for alcohol (10%, v/v) self-administration on fixed-ratio 1 and progressive ratio schedules of reinforcement. |
| Blanchard et al., 1993 | Long-Evans | Unclear | Operant oral self-administration | Female > Male | The rats were tested in 30-min/day sessions on a fixed-ratio 3 schedule of reinforcement. |
| van Haaren and Anderson, 1994 | Wistar | Group | Operant oral self-administration | Female = Male | Schedule-induced polydipsia in 45-min/day sessions. The rats were food-deprived. |
The present study investigated sex differences in alcohol drinking in Long Evans and Wistar rats using multiple behavioral paradigms and evaluated the influence of the estrous cycle on such behavior in free-cycling female rats. We compared Long Evans and Wistar rats because they are frequently used in behavioral studies, and these strains have been recently used for genetic manipulations (e.g., Cre lines) outside and inside the NIH. We employed three different paradigms of alcohol drinking: continuous voluntary two-bottle choice (10% alcohol vs. water), intermittent voluntary two-bottle choice (16% alcohol vs. water), and chronic intermittent alcohol vapor exposure combined with operant alcohol self-administration. These three paradigms are widely used in the alcohol field and frequently yield varying patterns of alcohol drinking and levels of intoxication and withdrawal. The impact of the estrous cycle on alcohol intake was monitored throughout the study in single- and group-housed females.
2. Materials and methods
2.1. Subjects
Twenty-four Long Evans rats (12 males and 12 females) and 24 Wistar rats (12 males and 12 females) were obtained from Charles River (Kingston, New York, USA). The females weighed 185–205 g at the beginning of the study and weighed 315–490 g at the end of the study. The males weighed 295–350 g at the beginning of the study and weighed 565 and 855 g at the end of the study. The rats were single-housed (Experiments 1 and 2) or group-housed by sex (Experiment 3; 4–6 per cage) in standard plastic cages that were lined with wood-chip bedding and maintained under a reverse 12 h/12 h light/dark cycle (lights on at 8:00 PM) at 21 ± 2 °C. The females were housed in the same room as the males. The animals had ad libitum access to food and water throughout the experiment, except during operant testing (Experiment 3). All of the procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Committee (protocol no. 14-INRB-6).
2.2. Experiment 1: continuous voluntary drinking (two-bottle choice)
For both Experiment 1 and 2 (below), the rats were single housed to allow us to accurately record fluid intake for each individual rat.
Over a 21-day period, single-housed rats (6 male and 6 female Long Evans rats and 6 male and 6 female Wistar rats) had continuous access to water and 10% (w/v) alcohol in their home cages. The bottles were routinely rotated to avoid side preferences. Beaded nozzles were installed on each bottle to reduce fluid loss from cage movement. Each bottle was weighed daily between 11:30 AM and 12:00 PM and immediately placed back in the home cage. The body weight of the rats was recorded at least once per week. The data are expressed in grams of alcohol/day/kg of body weight and percent preference for alcohol relative to water.
2.3. Experiment 2: intermittent voluntary drinking (two-bottle choice)
The same animals that were used in Experiment 1 were used in Experiment 2. Throughout a 10-week period, an adaptation of the Wise (1973) model was used. The animals had intermittent access to 16% (w/v) alcohol in their home cages for three weekly 24-h sessions. The rats were given a bottle of alcohol between 11:30 AM and 12:00 PM on Monday, Wednesday, and Friday every week. The bottles were removed the following day between 11:30 AM and 12:00 PM, and alcohol and water intake were recorded. The water and alcohol bottles were rotated to avoid side preferences. The rats were weighed weekly to calculate alcohol intake in grams per kilogram of body weight. The percent preference for alcohol relative to water was calculated.
2.4. Experiment 3: operant alcohol self-administration and alcohol vapor exposure
The rats were group-housed for this experiment for the following reasons: 1. Although housed in groups, rats were tested individually in the operant chambers; 2. We have limited space in the vapor chambers, thus individual housing would make this experiment difficult to be conducted in a timely manner; 3. Most studies using operant alcohol self-administration use group-housed subjects, thus allowing us direct comparison with previously published studies; and 4. NIDA-IRP animal care and use committee requires group housing unless otherwise strongly justified.
A separate group of group-housed rats that included 12 Long Evans rats (six males, six females) and 12 Wistar rats (six males, six females) were trained to lever press for access to alcohol or water in standard operant chambers (Med Associates, St. Albans, VT, USA). The rats were given free-choice access to alcohol (10% w/v) and water for 1 day in their home cages to habituate them to the taste of alcohol. The rats were then subjected to an overnight session in operant chambers with access to one lever (right lever) that delivered water. Food was freely available during this training. After 1 day off, the rats were subjected to a 2-h session and a 1-h session the next day, with one lever that delivered alcohol (right lever). All of the subsequent sessions lasted 30 min, and two levers were available (left lever: water; right lever: alcohol). The operant sessions were conducted on a fixed-ratio 1 schedule of reinforcement (i.e., each lever press resulted in fluid delivery). Upon stable levels of responding for alcohol, the rats were exposed to chronic, intermittent alcohol vapor to induce dependence as previously reported (Vendruscolo et al., 2012). Cycles of alcohol intoxication and withdrawal occurred daily for 10 weeks. Over a 24-h period, the alcohol vapor ran for 14 h consecutively, and operant alcohol self-administration (typically twice per week) occurred during the 10 h period without alcohol vapor between 6 and 8 h into withdrawal. In this model, the rats exhibit reliable signs of alcohol dependence, including a negative emotional-like state and somatic symptoms during withdrawal (for review, see Vendruscolo and Roberts, 2014). For the present experiments, the average blood alcohol levels during vapor exposure were as follows: 199.7 mg/dl for Long Evans males, 174.2 mg/dl for Wistar males, 115.4 mg/dl for Long Evans females and 209.2 mg/dl for Wistar females.
2.5. Estrous cycle phase determination
Vaginal smears were collected to determine whether hormonal fluctuations impacted alcohol drinking in Experiments 1–3. A cotton swab was moistened with sterile water and gently inserted approximately 4 mm into the vagina and slowly rotated clockwise to collect cell samples. The cells were transferred to slides and viewed under a light microscope to examine the stage of the estrous cycle. Three phases were used for categorization: 1. diestrus (predominance of leukocytes, small speckling), 2. proestrus (predominance of nucleated epithelial cells, large and round), and 3. estrus (predominance of cornified epithelial cells, jagged shape). The experimenters did not pharmacologically synchronize cycles; however, because of cage proximity, synchronization indeed occurred for some of the females. Estrous samples were obtained immediately after alcohol self-administration to avoid interference with behavior.
2.6. Statistical analysis
A power analysis for sample size calculations (estimated average for one group = 10, estimated average for the other group = 7; estimated standard deviation = 3; power goal = 0.80; α= 0.05) indicated that 10 animals per group was an adequate sample size to detect sex differences. The sample size was 12 per strain and sex to account for possible loss of animals because of a failure to self-administer alcohol or computer failure during testing.
The results are presented as mean ± SEM. The data were analyzed using two-way analysis of variance (ANOVA), with sex/strain and vapor exposure/strain as between-subjects factors. For estrous cycle phases, the data were analyzed using two-way ANOVA, with strain as the between-subjects factor and the estrous cycle phase as the within-subjects factor. Tukey’s post hoc test was used when appropriate. The level of significance was p ≤ 0.05. GraphPad Prism 6 and Statsoft Statistica 12 software were used for the statistical analyses.
3. Results
3.1. Experiment 1
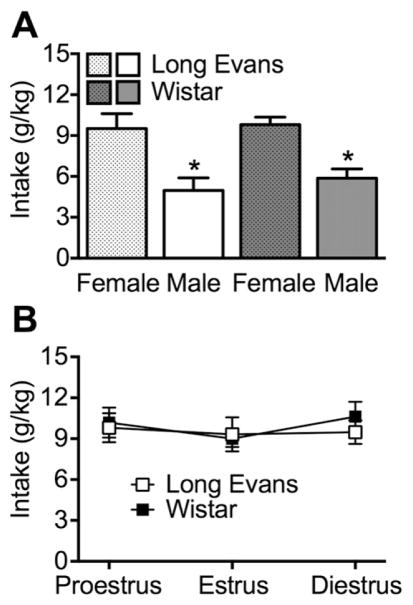
The rats were single-housed and males and females shared the same room. The average intake of continuous voluntary alcohol (10%, w/v) over 15 days is shown in Fig. 1. The two-way ANOVA revealed an overall effect of sex (F1,20 = 148.6, p < 0.0001) on alcohol intake (in g/kg), such that female rats, regardless of strain, drank more alcohol than male rats. For percent preference, the ANOVA revealed a sex × strain interaction (F1,20 = 9.2, p < 0.01). The post hoc analyses indicated that female Long Evans rats had a higher preference for alcohol compared with all of the other groups (p < 0.005; Table 2). Moreover, female Wistar rats exhibited a significantly greater total fluid intake (in ml/kg) compared with all the other groups (sex × strain interaction: F1,20 = 9.3, p < 0.01; post hoc test: p < 0.0001; Table 2).
Fig. 1.
Continuous, voluntary alcohol (10%, w/v) drinking. A, Regardless of strain, female rats exhibited an increase in alcohol intake (in g/kg) compared with male rats. B, The estrous cycle did not significantly impact alcohol intake in females of either strain (Long Evans and Wistar). *p < 0.05, different from female (overall effect of sex).
Table 2.
Percent preference for alcohol (continuous access to 10%, w/v, alcohol) and total fluid intake (ml/kg).
| Strain | Male | Female |
|---|---|---|
| Long Evans | 29.0% (±2.0) | 44.5%* (±2.1) |
| 176.5 ml/kg (±6.1) | 204.3 ml/kg (±2.1) | |
| Wistar | 30.7% (±2.2) | 34.0% (±1.8) |
| 186.7 ml/kg (±9.9) | 278.8* ml/kg (±17.4) |
p < 0.005, different from all other groups.
The two-way ANOVA did not reveal significant effects of estrous cycle stages on alcohol intake (in g/kg) in either female Long Evans or female Wistar rats (Fig. 1). However, a significant effect of strain on percent preference was observed (F1,10 = 11.2, p < 0.01), with Long Evans rats exhibiting higher alcohol preference compared with Wistar rats (Table 3).
Table 3.
Percent preference for alcohol (continuous access to 10%, w/v, alcohol) across phases of estrous cycle.
| Strain | Proestrus | Estrus | Diestrus |
|---|---|---|---|
| Long Evans | 44.8%* (±4.7) | 48.9%* (±4.3) | 43.7%* (±3.1) |
| Wistar | 37.8% (±2.9) | 34.0% (±1.8) | 34.7% (±3.5) |
p < 0.01, different from Wistar (overall effect of strain).
3.2. Experiment 2
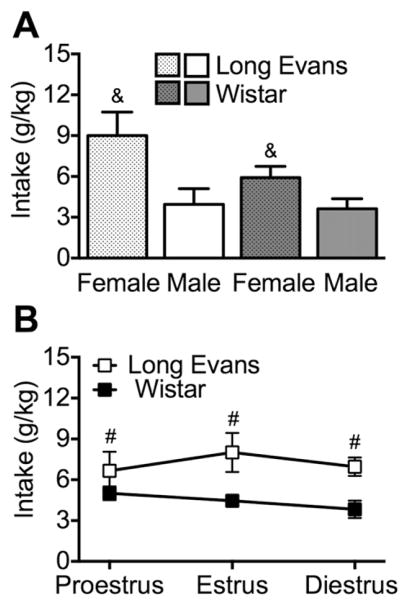
The same rats that were used in Experiment 1 were given intermittent access to alcohol (16%, w/v) in their home cages for 8 weeks (24 sessions). Males and females were single-housed and they shared the same holding room. The averages of all 24 sessions for each group are shown in Fig. 2. A significant sex × strain interaction was observed (F1,20 = 8.4, p < 0.01). The post hoc analyses indicated that female Long Evans rats drank more alcohol than all the other groups (p < 0.0005), and that Wistar female rats drank more alcohol than male Wistar and Long Evans rats (p < 0.05). For percent preference, an overall strain effect was observed (F1,20 = 9.3, p < 0.01), in which Long Evans rats exhibited higher preference than Wistar rats (Table 4). Moreover, female rats exhibited a significantly greater total fluid intake (in ml/kg) compared with male rats (sex effect: F1,20 = 64.2, p < 0.0001; Table 4).
Fig. 2.
Intermittent, voluntary alcohol (16%; w/v) drinking. A, Female rats drank significantly more alcohol (in g/kg) compared with male rats. Long Evans female rats drank significantly more alcohol compared with Wistar female rats. B, Long Evans rats drank more alcohol compared with Wistar rats throughout the estrous cycle. &p < 0.05, different from all other groups; #p < 0.05, different from Wistar (overall effect of strain).
Table 4.
Percent preference for alcohol (intermittent access to 16%, w/v, alcohol) and total fluid intake (ml/kg).
| Strain | Male | Female |
|---|---|---|
| Long Evans | 37.3% (±6.3) | 45.3%* (±2.1) |
| 65.5 ml/kg (±4.3) | 121.8 ml/kg** (±5.5) | |
| Wistar | 30.0% (±4.7) | 26.6% (±2.6) |
| 78.9 ml/kg (±8.0) | 126.5 ml/kg** (±7.5) |
p < 0.05, different from female Wistar.
p < 0.0001, different from males.
Across all stages of the estrous cycle, female Long Evans rats drank more alcohol (F1,10 = 12.4, p < 0.01; Fig. 2) and exhibited greater preference (F1,10 = 30.0, p < 0.0005; Table 5) compared with female Wistar rats.
Table 5.
Percent preference for alcohol (intermittent access to 16%, w/v, alcohol) across phases of estrous cycle.
| Strain | Proestrus | Estrus | Diestrus |
|---|---|---|---|
| Long Evans | 42.5%* (±4.3) | 54.3* (±5.7) | 46.3%* (±4.9) |
| Wistar | 28.0% (±3.3) | 30.6% (±1.7) | 27.1% (±3.8) |
p < 0.05, different from Wistar (overall effect of strain).
3.3. Experiment 3
A separate cohort of rats was trained to lever press for alcohol and then exposed to chronic, intermittent alcohol vapor to produce dependence. Prior to alcohol vapor exposure, the animals were group-housed by sex and males and females shared the same room. During alcohol vapor exposure, males and females shared the same room, but were housed in separate vapor chambers. The results are shown in Fig. 3. No sex differences were found for alcohol intake (g/kg) either before or during vapor exposure. The ANOVA revealed a vapor exposure × strain interaction (F1,20 = 9.6, p < 0.01). The post hoc tests indicated that vapor-exposed Wistar rats exhibited higher alcohol intake compared with Long Evans rats (p < 0.0001; Fig. 3). The ANOVA revealed that female Wistar rats ingested significantly more than Long Evans rats (F1,30 = 6.8, p < 0.05) across the estrous cycle, but the phases of the estrous cycle did not affect alcohol intake (Fig. 3).
Fig. 3.
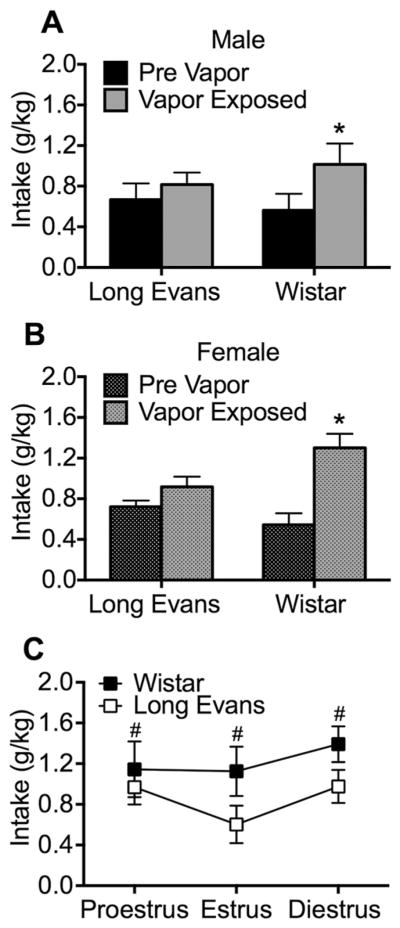
Operant alcohol (10%, w/v) self-administration. Exposure to alcohol vapor induces escalated levels of alcohol self-administration during withdrawal in male (A) and female (B) Wistar but not Long Evans rats. C, Across all stages of the estrous cycle, Wistar rats self-administered more alcohol compared with Long Evans rats. *p < 0.05, different from pre-vapor; #p < 0.05, different from Long Evans (overall effect of strain).
4. Discussion
In the present study, both female Wistar and Long Evans rats drank more alcohol than males when given continuous or intermittent access to alcohol (vs. water) in their home cages (voluntary drinking). During these experiments, the male and female rats were single-housed and shared the same holding room. Under operant conditions, the males and females were group-housed by sex and again shared the same holding room. Here, we found no sex or strain differences for drinking prior to the development of alcohol dependence. However, during dependence that was produced by chronic, intermittent alcohol vapor exposure, Wistar rats of both sexes substantially escalated their alcohol intake compared with their nondependent drinking levels. Long Evans rats of both sexes only exhibited a moderate escalation of drinking. The phases of the estrous cycle did not affect alcohol drinking in either strain or under any of the drinking conditions.
The majority of studies have reported that female rats voluntarily drink more alcohol than male rats, although a few studies have found no sex differences in drinking (Table 1). Our findings indicate that females exhibit higher intake of 10% alcohol in a continuous, voluntary two-bottle choice paradigm and in an intermittent, two-bottle choice (16% alcohol) paradigm after prolonged exposure to alcohol. This outcome is unlikely related to sex differences in the pharmacokinetics of alcohol. Although Robinson et al. (2002) reported that the levels of alcohol peaked slightly faster in the blood and brain in females (i.e., faster absorption and distribution) and that alcohol elimination was slightly faster in female rats than in male rats, the effects were small and not sufficient to account for the large difference in female versus male drinking that was observed in the present study and in the literature. Additionally, Morales et al. (2011) reported that male and female Sprague-Dawley rats exhibited similar levels of acute and chronic tolerance to the social suppressing effects of alcohol, thus supporting the hypothesis that sex differences in alcohol drinking observed in the present study were unrelated to tolerance to alcohol. For operant alcohol self-administration, we did not observe sex differences, consistent with some previous studies (van Haaren and Anderson, 1994; Moore and Lynch, 2015), although Blanchard et al. (1993) reported that females self-administered more alcohol (10%, w/v) in a fixed ratio 3 schedule of reinforcement compared with males. Importantly, the lack of sex differences in operant self-administration that was observed herein persisted after chronic intermittent alcohol vapor exposure, a model that produces reliable signs of alcohol dependence (Vendruscolo and Roberts, 2014). Indeed, both male and female rats equally escalated their intake when tested during acute alcohol withdrawal. Thus, despite some inconsistencies, the present study suggests that female rats drink more alcohol than males in situations of minimal workload (i.e., voluntary drinking in the home cage), whereas no sex differences were observed when some work was required (i.e., lever pressing) to obtain access to alcohol.
Housing conditions may differentially affect behavior in males and females (Becker and Koob, 2016). Females are more social and males are more territorial. Thus, single housing could be hypothesized to be more stressful for females than males, and this could affect drinking levels (e.g., increased drinking in females). However, housing conditions are unlikely responsible for sex differences in alcohol drinking. As shown in Table 1, Sluyter et al. (2000); Walker et al. (2008); Vetter-O’Hagen et al. (2009) and Maldonado-Devincci et al. (2010) reported that group-housed females voluntarily drank more alcohol than males, whereas Schramm-Sapyta et al. (2014) reported a lack of sex differences in voluntary drinking in single-housed rats. Moreover, Varlinskaya et al. (2015) reported that adult males and females drank more alcohol under social circumstances than alone. In the present study, single-housed females (Experiments 1 and 2) voluntarily drank more alcohol in their home cages compared with males, whereas no sex differences were observed in group-housed animals (Experiment 3) tested in operant conditions.
Sex differences in alcohol intake in rodent models appear to be somewhat different than that reported in the human condition. For example, in human populations, men consume alcohol at higher levels compared with women (National Institute on Alcohol Abuse and Alcoholism, 2015; Centers for Disease Control and Prevention, 2015), whereas many rodent studies, including the present study, found that females drink more alcohol and have a higher preference for alcohol compared with males (summary in Table 1). The translational differences between human and animal data may stem from multiple factors, including social and cultural pressures. Moreover, disparities in alcohol drinking by men and women have been decreasing over time (Keyes et al., 2008). Because of the paucity of studies that included both male and female subjects, it is currently difficult to pinpoint the contribution of environmental and biological factors that modulate sex differences in alcohol drinking in rodents and humans.
Some effects of alcohol are known to change throughout the estrous cycle. Decreases in alcohol consumption during estrus have been identified in female rats with synchronized cycles via gonadotropin-releasing hormone agonists (Roberts et al., 1998), and decreases in alcohol drinking have been reported during diestrus in adult female rats that were exposed to high alcohol levels during adolescence (Maldonado-Devincci et al., 2010). Progesterone and estrogen levels are known to fluctuate throughout the estrous cycle. Estrogen levels peak in the early phases of proestrus, and progesterone peaks in late proestrus (Smith et al., 1975; Simpson and Kelly, 2012). A previous microdialysis study (Dazzi et al., 2006) showed that alcohol causes the greatest increase in dopamine levels in the medial prefrontal cortex during estrus. The sedative effects of alcohol were less pronounced in proestrus and diestrus (Cha et al., 2006). However, previous studies reported that the estrous cycle does not substantially impact alcohol intake in naturally cycling rats (Roberts et al., 1998; Ford et al., 2002; Maldonado-Devincci et al., 2010; Moore and Lynch, 2015). Consistent with these findings, in the present study, we did not detect significant changes in alcohol intake or preference or lever pressing for access to alcohol across the stages of the estrous cycle in two rat strains and multiple paradigms of alcohol drinking and dependence. Thus, despite the fact that some behavioral and physiological measures (as described above and reviewed by Simpson and Kelly, 2012) appear to be affected by the estrous cycle, and differences can be observed when cycles are synchronized, the present results suggest that hormonal fluctuations have little impact on alcohol intake in models of nondependent drinking and escalated drinking under free-cycling conditions in which female rats were either group- (operant alcohol self-administration) or single-housed (voluntary two-bottle choice drinking in the home cages) and cohabitate in the same housing room as males.
A previous study reported that male Long Evans rats that were given intermittent (alternate-day) forced alcohol (5, 10, or 15%, v/v) or two-bottle choice (5, 10, or 15%, v/v, vs. water) generally consumed significantly more alcohol compared with Fischer 344, Sprague-Dawley, and Wistar rats (Khanna et al., 1990). In the present study, we found that Long Evans rats, particularly driven by females, consumed more alcohol (voluntary intermittent two-bottle choice) and had a higher preference for alcohol (both continuous and intermittent voluntary two-bottle choice) compared with Wistar rats. However, repeated cycles of alcohol vapor and withdrawal caused a substantial escalation of alcohol self-administration in Wistar rats, whereas only a marginal effect was observed in Long Evans rats. Morales et al. (2015) reported that 10 days of alcohol vapor exposure, followed by a 96-h abstinence period, caused a significant escalation of voluntary alcohol intake (intermittent access to two-bottle choice) in male Long Evans rats, whereas only a marginal effect was observed in females. These findings indicate that Long Evans rats may be more resistant than Wistar rats to neuroadaptations that are responsible for the escalation of alcohol self-administration, especially in this model (for review, see Vendruscolo and Roberts, 2014).
In conclusion, strain, sex and drinking conditions interact to modulate nondependent and dependent alcohol drinking, whereas hormonal fluctuations during different phases of the estrous cycle in females have little impact on drinking under free-cycling conditions. The present results highlight the need to include sex and strain as biological variables when exploring the importance of individual differences in alcohol drinking and dependence for the development of future personalized treatment for alcohol use disorders.
Acknowledgments
The National Institute on Drug Abuse, Intramural Research Program supported this work. The authors thank Michael Arends for editorial assistance.
References
- Almeida O, Shoaib M, Deicke J, Fischer D, Darwish M, Patchev V. Gender differences in ethanol preference and ingestion in rats: the role of the gonadal steroid environment. J Clin Investig. 1998;101(12):2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Koob G. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68(2):242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83(1):35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Blanchard B, Steindorf S, Wang S, LeFevre R, Mankes R, Glick S. Prenatal ethanol exposure alters ethanol-induced dopamine release in nucleus accumbens and striatum in male and female rats. Alcohol Clin Exp Res. 1993;17(5):974–981. doi: 10.1111/j.1530-0277.1993.tb05651.x. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25(4):594–599. [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63(1):91–99. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fact Sheets: Alcohol Use and Your Health. 2015 (Retrieved December 27, 2015, from) http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm.
- Cha Y, Li Q, Wilson W, Swartzwelder H. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res. 2006;30(1):113–118. doi: 10.1111/j.1530-0277.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Seu E, Cherchi G, Barbieri P, Matzeu A, Biggio G. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 2006;32:892–901. doi: 10.1038/sj.npp.1301150. [DOI] [PubMed] [Google Scholar]
- de la Torre M, Escarabajal M, Agüero Á. Sex differences in adult Wistar rats in the voluntary consumption of ethanol after pre-exposure to ethanol-induced flavor avoidance learning. Pharmacol Biochem Behav. 2015;137:7–15. doi: 10.1016/j.pbb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Dhaher R, McConnell K, Rodd Z, Mcbride W, Bell R. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav. 2012;102:540–548. doi: 10.1016/j.pbb.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M, Eldridge J, Samson H. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res. 2002;26(5):635–643. [PubMed] [Google Scholar]
- Juárez J, Barrios de Tomasi E. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19(1):15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Keyes K, Grant B, Hasin D. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93(1–2):21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna J, Kalant H, Shah G, Sharma H. Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol. 1990;7(5):429–434. doi: 10.1016/0741-8329(90)90027-a. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Lancaster F, Brown T, Coker K, Elliott J, Wren S. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period in Sprague-Dawley rats. Alcohol Clin Exp Res. 1996;20(6):1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Li T, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res. 1984;8(5):485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci A, Alipour K, Michael L, Kirstein C. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav. 2010;96(4):476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Lynch W. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav. 2015;132:1–9. doi: 10.1016/j.pbb.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–1624. doi: 10.1111/j.1530-0277.2011.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Mcginnis M, Mccool B. Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long–Evans rats. Pharmacol Biochem Behav. 2015;139:67–76. doi: 10.1016/j.pbb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse Alcoholism. Alcohol Facts and Statistics. 2015 Mar 1; (Retrieved September 20, 2015, from) http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics.
- Roberts A, Smith A, Weiss F, Rivier C, Koob G. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22(7):1564–1569. [PubMed] [Google Scholar]
- Robinson D, Brunner L, Gonzales R. Effect of gender and estrous cycle on the pharmacokinetics of ethanol in the rat brain. Alcohol Clin Exp Res. 2002;26(2):165–172. [PubMed] [Google Scholar]
- Schramm-Sapyta N, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn C. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology. 2014;231(8):1831–1839. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975 Jan;96(1):219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly J. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res. 2012;229:289–300. doi: 10.1016/j.bbr.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Hof M, Ellenbroek B, Degen S, Cools A. Genetic, sex, and early environmental effects on the voluntary alcohol intake in Wistar rats. Pharmacol Biochem Behav. 2000;67:801–808. doi: 10.1016/s0091-3057(00)00425-1. [DOI] [PubMed] [Google Scholar]
- ter Horst J, de Kloet E, Schächinger H, Oitzl M. Relevance of stress and female sex hormones for emotion and cognition. Cell Mol Neurobiol. 2011;32:725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaren F, Anderson K. Sex differences in schedule-induced alcohol consumption. Alcohol. 1994;11(1):35–40. doi: 10.1016/0741-8329(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E, Truxell E, Spear L. Ethanol intake under social circumstances or alone in Sprague-Dawley rats: impact of age, sex, social activity, and social anxiety-like behavior. Alcohol Clin Exp Res. 2015;39:117–125. doi: 10.1111/acer.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo L, Roberts A. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48(3):277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo L, Terenina-Rigaldie E, Raba F, Ramos A, Takahashi R, Mormede P. Evidence for a female-specific effect of a chromosome 4 locus on anxiety-related behaviors and ethanol drinking in rats. Genes Brain Behav. 2006;5:441–450. doi: 10.1111/j.1601-183X.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo L, Izídio G, Takahashi R, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behav Pharmacol. 2008;19:21–27. doi: 10.1097/FBP.0b013e3282f3cfbe. [DOI] [PubMed] [Google Scholar]
- Vendruscolo L, Barbier E, Schlosburg J, Misra K, Whitfield T, Jr, Logrip M, Rivier C, Repunte-Canonigo V, Zorrilla E, Sanna P, Heilig M, Koob G. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32(22):7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B, Walker J, Ehlers C. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]