Abstract
Increased left ventricular mass (LVM) is an early precursor of target organ damage attributable to hypertension. Diminished parasympathetic cardiac control has been linked to both hypertension onset and left ventricular impairment; however, emerging evidence suggests that this pattern might be different in African Americans. The present study sought to determine whether race impacts the relationship between parasympathetic cardiac control and LVM. The LVM was assessed via echocardiography in a sample (n = 148) of African American and White adults (mean age 33.20 ± 5.71 years) with normal or mildly elevated blood pressure. Parasympathetic cardiac control was assessed by a measure of high-frequency heart rate variability (HF-HRV) determined from ECG recordings during 5 min of rest. In regression analysis, greater HF-HRV was associated with greater LVM among African Americans (P = 0.002) but was not related to LVM in Whites (P = 0.919). These are the first data to demonstrate that race moderates the relationship between HRV and LVM and further suggest that race might be an important factor in the association between parasympathetic cardiac control and other cardiovascular disease risk factors.
Introduction
Left ventricular hypertrophy (LVH), defined as increased left ventricular mass (LVM), is an important manifestation of hypertension (Burchfiel et al. 2005). Left ventricular mass is associated with an increased risk of cardiovascular disease (CVD) morbidity and mortality in a graded and continuous fashion (Burchfiel et al. 2005; Drazner et al. 2005), and both cross-sectional and longitudinal data have shown that African Americans tend to exhibit greater LVM and develop LVH at an earlier age compared with Whites (Hanevold et al. 2004; Lai et al. 2014). In the general population, the prevalence of LVH is between two and five times greater among African Americans compared with Whites, even after accounting for age, blood pressure, body composition and socioeconomic factors (Drazner et al. 2005). It is well established that African Americans experience a higher prevalence of hypertension than Whites and other ethnic groups, and hypertension is one of the strongest predictors of LVH (Burchfiel et al. 2005).
Models of hypertension pathogenesis have implicated autonomic imbalance, with sympathetic nervous system (SNS) hyperactivity as the primary mechanism underlying increased blood pressure (BP; Brook & Julius, 2000; Grassi, 2010). However, impaired parasympathetic nervous system (PNS) function also is implicated in elevated CVD risk and end-organ damage (Thayer et al. 2010). Heart rate variability (HRV) is an important index of parasympathetic (i.e. vagal) cardiac influence, determined from the intervals in time from one heart beat to the next (i.e. R–R interval) using continuous recordings of the ECG. Lower HRV is an independent risk factor for all-cause and cardiovascular mortality and is further associated with established CVD risk factors including age, obesity, smoking, total and low-density cholesterol, positive family history of CVD, and diabetes (Thayer et al. 2010). Low HRV is predictive of hypertension onset (Schroeder et al. 2003) and is diminished in both early and later stages of hypertension (Konrady et al. 2001; Kowalewski et al. 2005; Shehab & Abdulle, 2011; Kilit et al. 2015; Ozel et al. 2015). Importantly, previous research has also shown a consistent inverse relationship between HRV and LVH (Kohara et al. 1995; Kowalewski et al. 2005; Melillo et al. 2012) and this association has been demonstrated to be independent of other typically robust risk factors, including age and disease aetiology (Alter et al. 2006).
It has been suggested that lower HRV may be one factor accounting for the excess CVD burden among African Americans (Lampert et al. 2005). Although several studies reported that HRV was reduced in African Americans (Lampert et al. 2005; Choi et al. 2006), more recent findings have suggested that African Americans more typically exhibit higher HRV in comparison to Whites. Notably, in a recent meta-analysis of 17 studies spanning both clinical and non-clinical populations (composite n > 11,000), HRV was found to be higher among African Americans compared with Whites (Hill et al. 2015). This pattern remained robust even after accounting for significant covariates, including age and health status. Interestingly, higher HRV among African Americans compared with Whites has been observed as early as the first 6 months of life (Propper et al. 2008). Others have reported higher HRV in African American adolescent twins compared with Whites and shown that this difference persists over time (Li et al. 2009). Despite these findings, the literature regarding the implications of higher HRV among African Americans is scant; thus, it is not yet clear whether this counterintuitive pattern is clinically meaningful. Based upon previous research, we hypothesized that African Americans would exhibit both greater HRV and greater LVM compared with Whites. We also sought to determine whether the association between HRV and LVM differed as a function of race.
Methods
Ethical approval
The study protocol and all procedures were approved by Duke University Medical Center Institutional Review Board (protocol no. 00012323) and in accord with the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects before participation.
Participants and procedures
Participants were 148 employed men and women between the ages of 25 and 45 years who participated in the Duke Biobehavioral Investigation of Hypertension (BIOH). Details of the study and primary results were published previously (Sherwood et al. 2002). No subjects had a screening blood pressure > 160/95 mmHg or weighed >125% of ideal body weight. Participant ethnicity was based on self-report. Use of cardiovascular medications or tobacco products were exclusion criteria, and all participants were instructed to avoid strenuous exercise or use of caffeine before the study session. The present study included individuals with either normal BP or untreated clinic BP in the range of 130–160/85–99 mmHg.
Measures
Echocardiogram
A two-dimensional echocardiogram was performed on all patients by an experienced sonographer using a Hewlett-Packard imaging system, equipped with a 2.5 MHz phased array transducer. Images were obtained using harmonic imaging with the patient in the partial left lateral decubitus position, and were recorded on S-VHS videotape. These studies were subsequently quantified by a single experienced, blinded observer. Left ventricular end-diastolic diameter, posterior wall thickness and interventricular septal thickness were measured at end diastole using a leading edge to leading edge convention. Left ventricular mass was estimated using a cube function model. To adjust for variations in heart size attributable to differences in body size, the left ventricular mass index (LVMI) was calculated as ventricular mass/height2.7, as previously described (de Simone et al. 1995).
Heart rate variability
Methods for the derivation of HRV in the present sample have been described previously (Watkins et al. 1996). Briefly, after 20 min resting in the supine posture, 5 min of continuous R–R interval measurements were recorded via ECG at a sampling rate of 1000 Hz for assessment of HRV. The R–R intervals were edited for artifacts, linearly interpolated, and resampled at a frequency of 4 Hz. A fast Fourier transform was then applied to the interpolated data after detrending and application of a Hanning filtering window. Power spectra were derived using the Welch algorithm, and HRV was estimated from the R–R interval power summed across the high-frequency (HF) power band (i.e. 0.15–0.45 Hz). The HF-HRV power was logarithmically transformed (i.e. lnHF-HRV) before analysis in order to normalize skewness.
Covariates
Previous research suggests that ambulatory blood pressure (ABP) more strongly reflects daily pressure-related afterload on the left ventricle compared with clinic BP (Sherwood et al. 2002); therefore, ABP was obtained from all participants during a typical workday. The AccuTracker II ABP Monitor (Suntech AccuTracker II Raleigh, NC, USA), an ausculatory, non-invasive device, was worn for 24 h, from between 08.00 and 10.00 h until the same time the following morning. The device was programmed to take four BP measurements per hour at random intervals during the day and to take two BP readings per hour during sleeping hours. All BP readings were reviewed and artifactual readings deleted following criteria previously described (Sherwood et al. 2002). Mean 24 h systolic blood pressure (SBP24) and diastolic blood pressure (DBP24) values were computed based on all valid readings obtained during waking hours and during nighttime sleep. In addition to ABP, we also controlled for age, sex and body mass index (BMI), as men tend to have greater BMI and larger LVM compared with women. The BMI was determined from height and weight measurements taken for each participant at the time of recruitment using a clinical scale and stadiometer.
Data analysis
Analysis of variance and χ2 tests were used to assess differences in demographic, haemodynamic and echocardiographic characteristics between African American and White subjects. Differences in HF-HRV and LVMI between African Americans and Whites also were compared; age, sex, BMI and ABP served as covariates. Hierarchical regression models were used to examine whether HF-HRV, race and/or their interaction were related to LVMI, while accounting for the influence of demographic factors and BP. All analyses were performed using the SAS (Cary, NC, USA) software system; significance was set at P < 0.05.
Results
Comparison of sample characteristics by race
Sample characteristics (mean + SD, or percentage) are presented separately by race in Table 1. The White subsample was composed of a larger proportion of men (65%) compared with the African Americans (47%; P = 0.031). Body mass index was higher among African Americans compared with Whites (P = 0.007). There were no differences in age, ambulatory BP, HF-HRV or LVMI between African Americans and Whites in unadjusted models.
Table 1.
Comparison of demographic, haemodynamic and echocardiographic characteristics between African American and White subjects
| Variable | African American (n = 74)
|
White (n = 74)
|
Range | P-value | ||
|---|---|---|---|---|---|---|
| Mean or percentage | SD | Mean or percentage | SD | |||
| Age (years) | 33.78 | 5.88 | 32.64 | 5.51 | 25–44 | 0.222 |
| Sex (% male) | 47% | – | 65% | – | – | 0.031 |
| BMI (kg m−2) | 26.76 | 3.78 | 25.29 | 2.62 | 18–38 | 0.007 |
| SBP24h (mmHg) | 123.32 | 12.38 | 120.39 | 12.34 | 70–175 | 0.151 |
| DBP24h (mmHg) | 75.37 | 9.20 | 73.82 | 8.31 | 43–119 | 0.281 |
| lnHF-HRV (ms2) | 6.52 | 1.16 | 6.34 | 1.12 | 3.2–9.8 | 0.337 |
| LVMI (g m−2.7) | 40.92 | 8.06 | 39.09 | 8.93 | 25–71 | 0.194 |
Mean 24 hour systolic blood pressure (SBP24), Mean 24 hour diastolic blood pressure (DBP24). Natural logarithmic transformation of high-frequency heart rate variability (lnHF-HRV), left ventricular mass indexed (LVMI) to weight (in grams) and height (in metres).
Abbreviations: BMI, body mass index.
Left ventricular mass
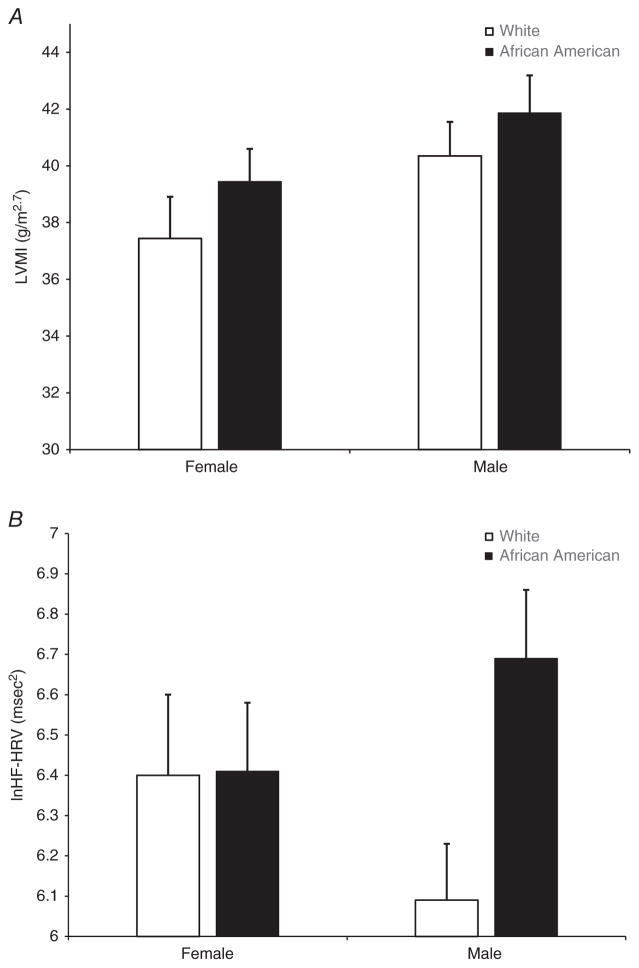
For LVMI, there were no significant main effects for age [F(1,147) = 0.00, P = 0.95], BMI [F(1,147) = 2.03, P = 0.16], 24 h SBP [F(1,147) = 2.38, P = 0.12], 24 h DBP [F(1,147) = 0.03, P = 0.85] or race [F(1,147) = 1.06, P = 0.31]. There was a significant main effect for sex [F(1,147) = 4.24, P = 0.041]; however, the race × sex interaction was not significant [F(1,147) = 0.00, P = 0.95]. As depicted in Fig. 1A, irrespective of race, men had greater LVMI compared with women.
Figure 1. Bar graphs of mean left ventricular mass index (LVMI) and mean resting high-frequency heart rate variability (HF-HRV) by race and sex.
A, there was a significant main effect for sex, with men exhibiting greater LVMI compared with women (P = 0.041). B, there were no sex differences in HF-HRV. There was a non-significant trend for the race × sex interaction (P = 0.109), suggesting that African American men exhibited higher HF-HRV compared with White men. Error bars reflect SEM.
Heart rate variability
For HF-HRV, there were no significant main effects for sex [F(1,147) = 0.01, P = 0.92], BMI [F(1,147) = 0.81, P = 0.37] or race [F(1,147) = 2.00, P = 0.16]. There were significant main effects for age [F(1,147) = 14.92, P = 0.0002], 24 h SBP [F(1,147) = 5.36, P = 0.02] and 24 h DBP [F(1,147) = 8.02, P = 0.005]. There was also a non-significant trend for the race × sex interaction [F(1,147) = 2.60, P = 0.109]. As shown in Fig. 1B, there was virtually no difference in HF-HRV between African American and White women; however, African American men exhibited higher resting HF-HRV compared with White men.
Multivariate associations with left ventricular mass
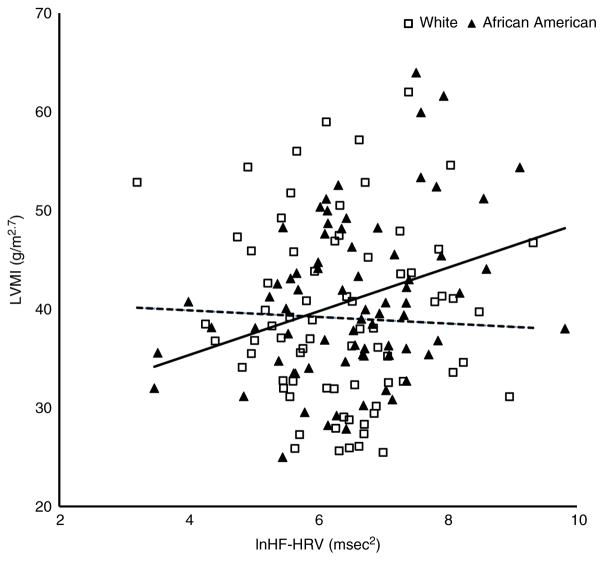
As shown in the hierarchical regression results in Table 2, sex accounted for 5% of the variance in LVMI (β = 0.24, standard error (SE) = 1.41, P < 0.01) in model 1. Body mass index, ABP and HF-HRV were entered in model 2, with HF-HRV emerging as the only additional significant predictor (β = 0.19, SE = 0.65, P < 0.05) of LVMI. This model explained an additional 9% of the variance in LVMI. The HF-HRV × race interaction term was entered alone in model 3 and significantly (β = 1.06, SE = 1.16, P < 0.05) predicted an additional 2% of the variance in LVMI. As depicted in Fig. 2, the relationship between HF-HRV and LVMI differed by race. An examination of the simple slopes revealed that HF-HRV was positively and significantly associated with LVMI among African Americans (b = 2.76, SE = 0.86, 95% confidence interval [1.06, 4.47]) but this effect was not significant in Whites (b = 0.09, SE = 0.87, 95% confidence interval [−1.63, 1.81]).
Table 2.
Hierarchal regression analysis of age, sex, race, body mass index, ambulatory blood pressure and the heart rate variablility × race interaction as predictors of left ventricular mass index
| Predictors | Model 1 b (SE) [β] | Model 2 b (SE) [β] | Model 3b (SE) [β] |
|---|---|---|---|
| Age | 0.07 (0.12) [0.05] | 0.08 (0.13) [0.05] | 0.06 (0.13) [0.04] |
| Sex | 4.21 (1.41) [0.24]** | 3.07 (1.48) [0.18]** | 2.45 (1.49) [0.14]* |
| Race | 2.48 (1.39) [0.15] | 1.00 (1.37) [0.06] | −16.34 (7.68) [−0.96] |
| BMI | 0.38 (0.22) [0.15] | 0.41 (0.22) [0.16] | |
| SBP24h | 0.12 (0.11) [0.18] | 0.13 (0.11) [0.18] | |
| DBP24h | 0.06 (0.16) [0.06] | 0.08 (0.16) [0.08] | |
| lnHF-HRV | 1.44 (0.65) [0.19]* | 0.09 (0.87) [0.01] | |
| lnHF-HRV × race | 2.67 (1.16) [1.06]* | ||
| R2 | 0.05** | 0.14** | 0.16** |
| 3R2 | 0.05** | 0.09** | 0.02** |
Abbreviations: BMI, body mass index; SBP24, mean 24 hour systolic blood pressure; DBP24, mean 24 hour diastolic blood pressure; HF-HRV, high-frequency heart rate variability.
P < 0.05,
P < 0.01.
Figure 2. Scatterplot of left ventricular mass index (LVMI) and resting high-frequency heart rate variability (HF-HRV) in African Americans (filled triangles, continous line) compared with Whites (open squares, dashed line).
The simple slope for HF-HRV predicting LVMI was significant for African Americans (P = 0.002) but not for Whites (P = 0.919).
We considered the possibility that differential rates of high blood pressure among Whites, relative to African Americans in our sample, might have accounted for the observed association between HF-HRV and LVMI. Separate regression models were computed for individuals with normal pressure and those with hypertension defined using JNC-7 criteria of daytime ambulatory SBP ≥ 135 mmHg or DBP ≥ 85 mmHg. Notably, only 33 (19 African Americans and 14 Whites) of the 148 participants met these more stringent criteria for hypertension that excludes those with high clinic BP attributable to the ‘white coat’ effect. The HF-HRV × race interaction was not statistically significant for those with either normal (β = 0.89, SE = 1.23, P = 0.089) or high blood pressure (β = 2.05, SE = 3.13, P = 0.087), but in both instances, trended in the expected direction and was consistent with the pattern observed in the whole sample.
Discussion
The purpose of the present study was to examine racial differences in HRV and LVM and to determine whether the association between HRV and LVM differed as a function of race. Although there were no differences in either HF-HRV or LVMI as a function of race, regression analysis revealed a significant interaction between race and HF-HRV such that HF-HRV was positively associated with LVMI among African Americans but was unrelated to LVMI in Whites. This pattern was observed while accounting for the potential influence of several factors (i.e. age, sex, BMI and ABP), which have previously been shown to influence both HRV and LVM.
Although research has typically conceptualized autonomic imbalance primarily from the perspective of heightened SNS activity, there has been resurgent interest in further explicating the role of parasympathetic functioning, and particularly HRV, in disease onset and trajectory (Thayer et al. 2010). Although regulation of the heart involves dynamic interactions of both SNS and PNS activity, previous research has shown that in resting conditions, parasympathetic activity is predominant (Berntson et al. 1997). There is consistent evidence that lower PNS activity as indexed by HRV is associated with increased CVD risk, including hypertension onset, CVD and all-cause mortality (Thayer et al. 2010). In contrast, higher HRV is generally considered to be cardioprotective and is regarded as an indicator of better health and well-being (Kemp & Quintana, 2013). Based on this conceptualization, it would be expected that higher HRV among African Americans should convey some cardioprotective benefit. However, weighed against the well-documented evidence of a greater CVD risk profile, including greater rates of hypertension and the strong association between hypertension and greater LVM, our findings of a positive association between HF-HRV and LVM among African Americans, seemingly, contradicts this notion. Despite this, there is modest evidence supporting a differential relationship between HRV and other CVD risk factors among African Americans. For example, one study reported a pattern of both higher systemic vascular resistance, an index of microvascular function, and higher HRV at baseline in a sample of middle-aged African American men compared with White men of a similar age (Dorr et al. 2007). Other research has shown that the link between HRV and psychosocial CVD risk factors may also be divergent among African Americans. Notably, in studies of predominately White or European samples, depression is associated with lower HRV, increased risk of CVD onset and worse prognosis among those with CVD (Thayer et al. 2010; Kemp & Quintana, 2013; Keen et al. 2015). However, a recent study in middle-aged African American adults observed a positive association between HRV and self-reported depressive symptoms (Keen et al. 2015). Our findings add to this small body of research documenting that greater HRV is positively associated with markers of increased CVD risk among African Americans.
As others have indicated, a number of factors, including age, sex and relative health status, may moderate the relationship between race and HRV. Indeed, sex differences in HRV have been shown, with women typically exhibiting higher HRV relative to men, irrespective of race (Hill et al. 2015). In the present study, although there was not a statistically significant race × sex interaction, there was some suggestion that HF-HRV was higher among African American compared with White men, but similar amongst women of both races (Fig. 1B). As these results suggest, racial differences in HRV might be more pronounced among men relative to women, although further research is needed to explore this possibility. In addition, other factors, such as genetics, might also play an important role. For instance, a recent study in a Brazilian cohort (n >11,000) found that individuals who identified as ‘Black’ exhibited higher resting state HRV compared with individuals who identified as ‘Brown’, and both groups displayed higher HRV relative to Brazilians who identified as ‘White’ (Kemp et al. 2016). Importantly, it has been established that Brazilians and African Americans share common genetic ancestry, and similar disparities in epidemiological data have been noted in rates of hypertension and CVD among Brazilians as observed for African Americans in the USA (Kemp et al. 2016). Although previous research has found no racial differences in the relative contribution of genetic influences on HRV (Li et al. 2009), other work has suggested that genetics may more strongly contribute to vascular functioning among African Americans compared with Whites (Hill et al. 2014). This raises the possibility that genetic influences may account, at least in part, for the racial difference in HRV.
Our findings for the effect of sex on LVMI are consistent with previous studies, showing relatively greater LVM to be evident among men compared with women (Burchfiel et al. 2005; Drazner et al. 2005; Lai et al. 2014). Although previous research has found greater LVM among African Americans relative to Whites (e.g. Hanevold et al. 2004; Burchfiel et al. 2005; Drazner et al. 2005; Lai et al. 2014), similar differences in our sample were not robust. In addition, prior studies conducted with older and/or more clinically impaired samples also have shown a significant inverse relationship between HRV and LVM in African Americans and Whites (Narayan et al. 2002). In contrast, our study was composed of relatively young and healthy participants. Only about one-third of our sample exhibited BP in the hypertensive range, and accordingly, the extent of left ventricular remodelling typically did not meet criteria for LVH. We also examined whether differences in relative rates of high blood pressure might have been driving our observed association between HF-HRV and LVMI. In exploratory analyses, the HF-HRV × race interaction was not statistically significant among individuals with either normal or high blood pressure; however, in both groups, results were similar to those observed across the entire sample. These additional observations indicate that the differing relationship between HF-HRV and LVMI observed among African Americans and Whites in our study was independent of the presence of hypertension.
Despite previous evidence indicating greater HRV among African Americans compared with Whites (Hill et al. 2015), the aetiology of this pattern is not well understood. According to classical perspectives on the early pathophysiology of hypertension, the resulting state of autonomic imbalance is characterized by SNS hyperarousal and attenuated parasympathetic cardiac activity (i.e. lower HRV; Brook & Julius, 2000). However, the studies that guided the development of this model were largely and/or exclusively based on White populations; subsequent evidence has shown a different developmental pattern amongst African Americans. Indeed, for African Americans vascular dysfunction, including elevated systemic vascular resistance, appears to precede the development of hypertension (Taherzadeh et al. 2010). In the context of a more vascular origin for hypertension, it is feasible that there exists an alternative mode of autonomic imbalance, in which central and peripheral SNS activity and cardiac PNS activity are both elevated. Overall, an interpretation of the aetiology of racial differences in HRV and the significance of this pattern in relationship to disease risk remains speculative. One clear indication from the emerging evidence is that current interpretative assumptions regarding the meaning of higher HRV may not be universal for all groups. This may have significant implications for the utility and information value of HRV in risk stratification, particularly among African Americans, and underscores the need for additional research on the relationships among race, HRV and other CVD risk factors in both healthy and clinical populations.
Given that this was a cross-sectional study, the data do not permit inference regarding the cause–effect relationship between HRV and LVM. Although our relatively large sample size and inclusion of stringent covariates (i.e. ABP) do provide support for the robustness of our observations, our sample was composed of a larger proportion of men than women. This sex difference might have contributed to a lack of statistical power in tests of the sex × race interaction for LVMI and HRV. In addition, we only assessed HF-HRV, a single frequency domain measure of HRV. Nonetheless, HF-HRV derived from short-term ECG recordings is a widely used index of parasympathetic cardiac modulation, and previous research has shown a strong correlation between HF-HRV and time domain HRV metrics (Berntson et al. 1997). Replication of our results using a wider range of HRV parameters is clearly warranted.
To our knowledge, these are the first data to demonstrate that race moderates the relationship between HRV and LVM. These results extend previous research by demonstrating that race may be an important factor in research examining the association between parasympathetic cardiac control and other CVD risk factors. Further research examining the relationship between race and cardiac autonomic function is needed to determine whether the apparent paradox of greater HRV among African Americans is cardioprotective or a physiological epiphenomenon.
New Findings.
What is the central question of this study?
Decreased heart rate variability (HRV) is associated with increased cardiovascular disease (CVD) risk, including greater left ventricular mass (LVM). Despite their enhanced CVD risk profile, African Americans have been shown to exhibit higher HRV, relative to Whites; however, it is unclear whether this pattern extends to the association between HRV and LVM.
What is the main finding and its importance?
Using ECG and echocardiographic data, HRV was positively associated with LVM in a non-clinical sample of African Americans. These findings suggest that current assumptions regarding the meaning of higher HRV might not be universal, which might have implications for HRV as a risk marker among African Americans.
Acknowledgments
Funding
This work was supported by funding from the National Heart, Lung and Blood Institute (HL49427, HL50774 and HL121708).
Footnotes
Competing interests
None declared.
Author contributions
A.S. and A.L.H. conceptualized the research design and methods. A.S., A.L.H. and L.L.W. collected and analysed the data. L.K.H., L.L.W., A.J.H., J.A.B. and A.S. prepared, edited and revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- Alter P, Grimm W, Vollrath A, Czerny F, Maisch B. Heart rate variability in patients with cardiac hypertrophy—relation to left ventricular mass and etiology. Am Heart J. 2006;151:829–836. doi: 10.1016/j.ahj.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens. 2000;13:112S–122S. doi: 10.1016/s0895-7061(00)00228-4. [DOI] [PubMed] [Google Scholar]
- Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, Taylor HA., Jr Metabolic syndrome and echocardiographic left ventricular mass in blacks: the atherosclerosis risk in communities (ARIC) study. Circulation. 2005;112:819–827. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- Choi JB, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, Dimsdale JE. Age and ethnicity differences in short-term heart-rate variability. Psychosom Med. 2006;68:421–426. doi: 10.1097/01.psy.0000221378.09239.6a. [DOI] [PubMed] [Google Scholar]
- de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- Dorr N, Brosschot JF, Sollers JJ, 3rd, Thayer JF. Damned if you do, damned if you don’t: the differential effect of expression and inhibition of anger on cardiovascular recovery in black and white males. Int J Psychophysiol. 2007;66:125–134. doi: 10.1016/j.ijpsycho.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens. 2010;23:1052–1060. doi: 10.1038/ajh.2010.154. [DOI] [PubMed] [Google Scholar]
- Hanevold C, Waller J, Daniels S, Portman R, Sorof J International Pediatric Hypertension Association. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–333. doi: 10.1542/peds.113.2.328. [DOI] [PubMed] [Google Scholar]
- Hill LK, Hu DD, Koenig J, Sollers JJ, 3rd, Kapuku G, Wang X, Snieder H, Thayer JF. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosom Med. 2015;77:16–25. doi: 10.1097/PSY.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LK, Sollers JJ, 3rd, Edwards CL, Thayer JF, Whitfield KE. A validation of estimated total peripheral resistance using twin data. Biomed Sci Instrum. 2014;50:210–218. [PMC free article] [PubMed] [Google Scholar]
- Keen L, 2nd, Turner AD, Mwendwa D, Callender C, Campbell A., Jr Depressive symptomatology and respiratory sinus arrhythmia in a non-clinical sample of middle-aged African Americans. Biol Psychol. 2015;108:56–61. doi: 10.1016/j.biopsycho.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Koenig J, Thayer JF, Bittencourt MS, Pereira AC, Santos IS, Dantas EM, Mill JG, Chor D, Ribeiro AL, Benseñor IM, Lotufo PA. Race and resting-state heart rate variability in Brazilian civil servants and the mediating effects of discrimination: an ELSA-Brasil cohort study. Psychosom Med. 2016;78:950–958. doi: 10.1097/PSY.0000000000000359. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol. 2013;89:288–296. doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Kilit C, Pasali Kilit T, Onrat E. Autonomic modulation in hypertension without hypertrophy. Acta Cardiol. 2015;70:721–727. doi: 10.2143/AC.70.6.3120186. [DOI] [PubMed] [Google Scholar]
- Kohara K, Hara-Nakamura N, Hiwada K. Left ventricular mass index negatively correlates with heart rate variability in essential hypertension. Am J Hypertens. 1995;8:183–188. doi: 10.1016/0895-7061(94)00190-M. [DOI] [PubMed] [Google Scholar]
- Konrady AO, Rudomanov OG, Yacovleva OI, Shlyakhto EV. Power spectral components of heart rate variability in different types of cardiac remodelling in hypertensive patients. Med Sci Monit. 2001;7:58–63. [PubMed] [Google Scholar]
- Kowalewski M, Baszuk-Stefaniuk E, Urban M, Peczynska J. Heart rate variability and left ventricular mass in slim children and young adults with hypertension. Kardiol Pol. 2005;63:605–610. discussion 611–602. [PubMed] [Google Scholar]
- Lai CC, Sun D, Cen R, Wang J, Li S, Fernandez-Alonso C, Chen W, Srinivasan SR, Berenson GS. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64:1580–1587. doi: 10.1016/j.jacc.2014.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150:153–160. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Li Z, Snieder H, Su S, Ding X, Thayer JF, Treiber FA, Wang X. A longitudinal study in youth of heart rate variability at rest and in response to stress. Int J Psychophysiol. 2009;73:212–217. doi: 10.1016/j.ijpsycho.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo P, Izzo R, De Luca N, Pecchia L. Heart rate variability and target organ damage in hypertensive patients. BMC Cardiovasc Disord. 2012;12:105. doi: 10.1186/1471-2261-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Papademetriou V, Devereux RB, Wachtell K, Dahlof B. P-374: Differences in heart rate variabilty in African-American vs Caucasian hypertensives with left ventricular hypertrophy: the LIFE study. Am J Hypertensi. 2002;15:166A. [Google Scholar]
- Ozel E, Tastan A, Ozturk A, Ozcan EE. Relationship between sympathetic overactivity and left ventricular hypertrophy in resistant hypertension. Hellenic J Cardiol. 2015;56:501–506. [PubMed] [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, Carbone MA, Cox M. Gene–environment contributions to the development of infant vagal reactivity: the interaction of dopamine and maternal sensitivity. Child Dev. 2008;79:1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- Shehab A, Abdulle A. Cognitive and autonomic dysfunction measures in normal controls, white coat and borderline hypertension. BMC Cardiovasc Disord. 2011;11:3. doi: 10.1186/1471-2261-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Gullette EC, Hinderliter AL, Georgiades A, Babyak M, Waugh RA, Blumenthal JA. Relationship of clinic, ambulatory, and laboratory stress blood pressure to left ventricular mass in overweight men and women with high blood pressure. Psychosom Med. 2002;64:247–257. doi: 10.1097/00006842-200203000-00007. [DOI] [PubMed] [Google Scholar]
- Taherzadeh Z, Brewster LM, van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J Clin Hypertens (Greenwich) 2010;12:431–438. doi: 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P, Sherwood A. Noninvasive assessment of baroreflex control in borderline hypertension. Comparison with the phenylephrine method. Hypertension. 1996;28:238–243. doi: 10.1161/01.hyp.28.2.238. [DOI] [PubMed] [Google Scholar]