Abstract
Objective
In Stiff-Person Syndrome (SPS), an antibody-mediated impaired GABAergic neurotransmission is believed to cause muscle stiffness and spasms. Patients improve with GABA-enhancing drugs and IVIg, but several respond poorly and remain disabled. The need for more effective therapy prompted a trial with the anti-CD20 monoclonal antibody rituximab.
Methods
This was a placebo-controlled randomized trial of rituximab (two bi-weekly infusions of 1gr each). The primary outcome was a change in stiffness scores at 6 months. Secondary outcomes were changes in heightened-sensitivity and quality of life scores. Enrolling 24 patients was calculated to detect 50% change in stiffness scores.
Results
Randomization was balanced for age, sex, disease duration and GAD autoantibody titers. No significant changes were noted at 6 months after treatment in all outcomes. Specifically, no differences were noted in the stiffness index, the primary outcome, or sensitivity scores, the secondary outcome, at 3 or 6 months. Quality of life scores improved significantly (p<0.01) at 3 months in both groups, but not at 6 months, denoting an early placebo effect. Blinded self-assessment rating of the overall stiffness for individual patients revealed improvement in four patients in each group. At 6 months, improvement persisted in one patient in the placebo group vs. three out of 4 in the rituximab group, where these meaningful improvements were also captured by video recordings.
Interpretation
This is the largest controlled trial conducted in SPS patients demonstrating no statistically significant difference in the efficacy measures between rituximab and placebo. The lack of rituximab’s efficacy could be due to a considerable placebo effect; insensitivity of scales to quantify stiffness especially in the less severely affected patients; or drug effectiveness only in a small patient subset.
INTRODUCTION
Stiff Person Syndrome (SPS) is an immune-mediated disorder characterized by rigidity of the axial and proximal limb muscles, superimposed upon disabling spasms and heightened sensitivity to external stimuli1–3. Although thought to be rare, the disease is largely under-diagnosed. SPS responds to immunotherapies3–5 and is frequently associated with other autoimmune diseases and autoantibodies1–5. More than 80 % of SPS patients have high titer antibodies against glutamic acid decarboxylase (GAD65), the rate-limiting enzyme for the synthesis of gamma-aminobutyric acid (GABA)1–8 and up to 15% have antibodies to glycine receptor α-subunit9,10.
Although the causative link between SPS and these antibodies is still uncertain, several studies suggest that: a) GAD activity and GABA synthesis are directly inhibited in vitro by anti-GAD specific IgG from SPS patients11,12 b) anti-GAD antibodies are produced intrathecally and they are associated with low GABA level in the brain and CSF2,13,14 c) GAD immunization of animals under specific conditions induces GABAergic neuronal loss15; d) GABA-receptor associated protein (GABARAP) IgG antibodies form GAD-positive patients inhibit the expression of GABAA receptors in cerebellar neurons16; and e) disease-specific monoclonal antibodies are able to impair GABAergic neurotransmission and affect behavior in laboratory animals17. Even though the pathogenic role of anti-GAD antibodies needs further confirmatory evidence18, 19, their presence in more than 80% of the patients suggests that B cells are activated and may participate in the disease, possibly exerting multiple immunoregulatory effects in the periphery or within the CNS, as proposed for other CNS disorders20, 21.
Accordingly, a drug that affects B cells may be beneficial. SPS patients usually respond favorably to GABA-enhancing drugs and periodic infusions of IVIg4,22,23, but in many of them the improvement is modest or short-lived necessitating the need for exploring other more specific therapies. Rituximab is a genetically engineered monoclonal antibody directed against CD20, that causes a swift and sustained depletion of B cells; the drug is promising in the treatment of several autoimmune neurological diseases including multiple sclerosis20,21. The sustained effect of rituximab in other antibody-mediated disorders, prompted us to examine its efficacy in anti-GAD positive SPS patients.
METHODS
Patient recruitment, randomization and exclusion criteria
The study was conducted at the National Institutes of Health (NIH) under a CRADA between the National Institute of Neurological Diseases and Stroke (NINDS)-(MC Dalakas, Principal Investigator) and Genentech between 2003-2006. The trial was registered at ClinicalTrials.gov (ClinicalTrials.gov number, NCT00050245 and was conducted under a protocol approved by the NINDS Institutional Review Board and after granting an IND to the principal investigator from the Food and Drugs Administration (FDA). All patients were admitted to the NIH Clinical Center and signed an informed study consent.
A total of twenty-four patients with confirmed diagnosis of Stiff Person Syndrome fulfilling previously established clinical criteria, were enrolled. All patients were selected among more than 40 patients followed in the outpatient clinic at the NIH Clinical Center, as published in previous series1, 2, 13, 14,22, but also from newly recruited patients specifically for this study. All patients were symptomatic with a varying degree of stiffness and spasms, quantified according to the scales described previously22 and below. All patients had high serum anti-GAD antibody titers (measured commercially), consistent with SPS. GAD titers were also measured and quantified blindly at the end of the study in serum samples taken before enrollment and at 6 months after treatment in the Neuroimmunology Unit, Medical School, University of Athens, using an ELISA assay as reported24. Patients with previous history of B cell or other type of malignancies, and those who have received any kind of immunosuppressive therapy over the six-month period prior to enrollment were excluded.
Randomization and Treatment Plan
Patients were allowed to continue receiving one of the non-immunosuppressive drugs used to treat SPS such as Diazepam, Neurontin or Baclofen but at the lowest possible dose that provided tolerable daily function. The doses of these drugs, lowered to the minimum specifically for the study, remained stable throughout the study and unchanged for 6 weeks prior to enrollment. Randomization was performed by the NIH pharmacy. After randomization, two bi- weekly intravenous cycles of 1 gram of rituximab or placebo (consisting of a normal saline solution) were administered. All patients were under continuous monitoring following standard safety guidelines, as previously described for our other rituximab trial25. Patients were pre-medicated with acetaminophen 650 mg and diphenhydramine 25 mg before infusions. Both, drug and placebo were supplied by the NIH pharmacy and sent to the floor covered so that all investigators, assessors, evaluators and nurses remained blinded to the study code. A DSMB was established to monitor safety.
Efficacy and monitoring
Clinical efficacy was determined on the basis of changes in the Stiffness Index and Heightened Sensitivity scores, which measure clinical severity and have been validated and utilized in other trials22, at months 3 and 6 after the infusions. These scales measure the following:
-
The distribution of stiffness index. This validated index score was used as the study’s primary outcome measure. The scale determines and quantifies stiffness as follows:
0: Absent stiffness; 1: Stiffness in face; 2: Stiffness in arms; 3: Stiffness in upper trunk; 4: Stiffness in abdomen; 5: Stiffness in lower trunk; 6: Stiffness in legs
The presence of each item adds one point (maximum score 6). A 2.5 decrease in the distribution of stiffness at month 6 was considered as a sign of efficacy.
-
The heightened sensitivity scale. This validated scale-used as a secondary outcome measure- determines and quantifies the events that trigger stiffness and spasms as follows:
1: Noise -induced stiffness and cramps; 2: Visually induced stiffness and cramps; 3: Somato-sensory-induced stiffness and cramps; 4: Voluntary activity induced spasms; 5: Emotional upset and “stress”-induced spasms; 6: Awakening due to nocturnal spasms; 7: Untriggered cramps and spasms. The presence of each item adds one point (maximum score 7).
Ancillary outcome measures
To enhance the information obtained from the aforementioned scales, we used the following additional ancillary outcome measures:
Intensity of stiffness. We utilized an examination-based, but empirical, stiffness severity scale that we called Overall stiffness intensity, rated from 0 (absent), 1 (mild), 2 (moderate), 3 (severe), combined with self-reported changes of stiffness, to appreciate the overall degree of stiffness at the end of the study. We reasoned that this might provide, in addition to the number of stiff areas noted above, some quantitative information on the overall stiffness severity.
The quality of life questionnaire (Supplementary Table 1). These scores were collected at baseline and in three subsequent measurements (on days 30, 90, and 180) following therapy.
Telephone Survey questionnaire. Before breaking the study code, but several months after study completion, the patients were contacted on the phone by two of us (MCD and BM) simultaneously, and were asked about the overall benefit they had experienced from the study drug using a standardized questionnaire. Specific questions in the questionnaire included the following: how did you rate your overall stiffness, on a scale from 1 (normal) to 10 (worst), at baseline and during the 6-month study period; when did improvement or deterioration start; how long did it last; and what is your status “now”, referring to several months after study completion when the interview was conducted. This survey was felt useful given the subjective nature of some SPS symptoms, especially stiffness, and the inherent difficulty in quantifying them. The patients were also asked to guess whether they thought they had received the drug or placebo and justify their reasoning.
Outcome Measures and data analysis
As per protocol, patient outcomes were evaluated on a continuous basis. The protocol was designed to test the superiority of rituximab compared to placebo. The primary outcome measure was a change in the Stiffness Index and the secondary outcome a change in Heightened Sensitivity at month 6. Based on our published data of stiffness index scores for untreated patients22, we assumed a variance of 0.83 using a repeated measures (4 time-periods) analysis of variance with Greenhouse-Geisser correlation; a total sample size of 24 patients (12 in each group) was deemed sufficient to provide the basis for detecting a change of 50% or greater with power greater than 0.80 and probability of Type-I error no more than 0.05. Accordingly, 24 patients were enrolled and randomized.
Efficacy was based on the difference in scores from baseline to end of treatment employing an intention to treat analysis. Comparison of the differences in scores between the placebo-randomized patients and those randomized to rituximab was carried out employing the Wilcoxon signed-rank test for analysis.
Monitoring of Anti-GAD antibody titers
Anti-GAD antibody titers were monitored with an ELISA assay (Euroimmun) that was set up at the Neuroimmunology Unit, University of Athens after the trial, as described24. Anti-GAD titers were measured in samples taken before enrolment (baseline) and at month 6 following either rituximab or placebo infusion.
RESULTS
Randomization and baseline evaluation
Twenty-four patients were enrolled; 12 were randomized to rituximab and 12 to placebo. Baseline characteristics were balanced between treatment groups regarding age, disease duration, GAD antibody titers, stiffness index, sensitivity scales, concomitant autoimmune diseases and diabetes (Table 1).
Table 1.
BASELINE MEASURES AT RANDOMIZATION
| Placebo (n=12) | Rituximab (n=12) | |
|---|---|---|
| Age (Years/SD) | 45.9 (10.3) | 50.8 (8.4) |
| SPS Duration (Years/SD) | 6.1 (3.6) | 8.0 (4.3) |
| Mean Baseline GAD titers (IU/ml) | 1,235,000 | 1,003,000 |
| Female (%) | 9 (75.0) | 6 (50.0) |
| Hyperthyroid (%) | 5 (41.7) | 3 (25.0) |
| Diabetes (%) | 3 (25.0) | 4 (33.3) |
Clinical evaluations
At baseline, the stiffness index was the same in both groups. One month after treatment, there was a non-significant reduction in the stiffness index in both groups; at 3 months a reduction in stiffness was observed in the placebo group but the difference was not significant compared to rituximab; at 6 months the stiffness index (the primary outcome measure) was equally reduced in both groups (Figure 1A). The heightened sensitivity index also did not capture any differences between both groups; some early worsening was observed in the placebo group but the difference was not significant compared to rituximab (Figure 1B). The Quality of Life index improved significantly (p<0.01) in both groups at month 3 denoting an early strong placebo effect; at month 6 the scores were moving upward in the placebo group but continued to go down, in the rituximab group but the difference was not significant (Figure 1C). The F-statistics (with 1 and 18 degrees of freedom) p-values associated with the repeated measure ANOVA were: 0,559 for stiffness; 0.433 for hypersensitivity and 0,486 for quality of life. For each of these 3 responses there appears to be a placebo effect as apparent in the figures.
Figure 1.
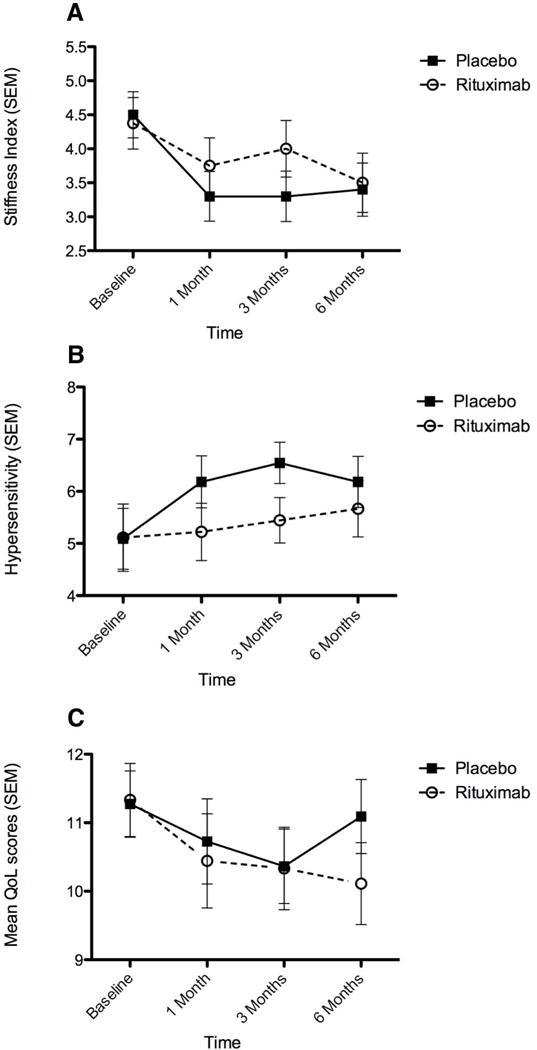
Graphic representation of stiffness (A), heightened sensitivity (B) and Quality of life (C) indexes. No statistical significance difference is noted not only for the stiffness index (the primary end-point) but also for the other measures. It is of interest however that the quality of life scales improved significantly at month 3 for both groups denoting a strong early placebo effect.
The following minor missing data that did not affect the results (even when the closest data were used) should be noted; in one placebo-randomized patient, the baseline data and in another the 3-month data were missing; in the rituximab group, in one patient the stiffness index was missing for the month 3 and for two others were missing for the month 6 (one had dropped off the study at month 4).
Overall stiffness intensity score
This scale, used as an ancillary measure to capture possible changes in the degree of stiffness, also failed to detect any difference between the two groups. Based on the examination-based scores [0 (absent), 1 (mild), 2 (moderate), 3 (severe)], combined with the question of whether the patients felt better, worse or no response, the following information was obtained: a) number of patients who felt better, experiencing mild-to moderate improvement in their stiffness: six of them had received placebo and four rituximab; b) number of patients who felt worse: two patients, both of whom had received placebo; and c) number of patients who felt “no response”: eight of them had received rituximab and four placebo.
Effect of Rituximab on anti-GAD antibody levels
A marked reduction in the anti-GAD titers was observed in the rituximab arm at month 6, but the effect was not statistically significant (p=0.0872, paired t-test). There was no difference in titers pre- and post-placebo (p=0.6321) (Figure 2). Further, 3 patients (2 in the placebo arm and 1 in the rituximab arm) were also positive for anti-glycine receptor antibodies, as reported previously9. No significant changes in the GAD antibody titers were observed in the 4 patients with a clinically observed response to rituximab, compared to the rest.
Figure 2.
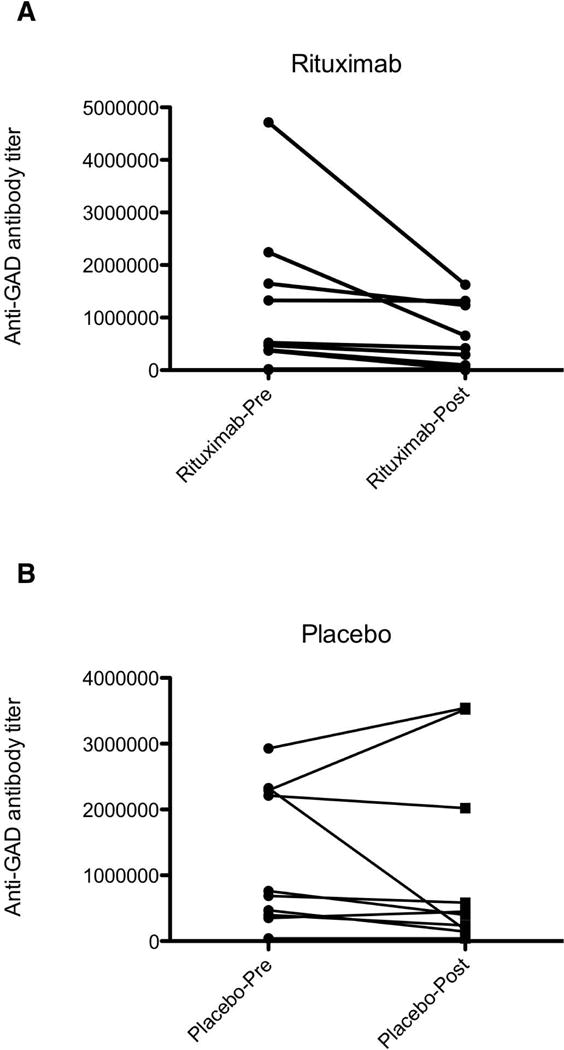
Antibody titers pre- and 6 months post- Rituximab (A) or placebo (B). Although the GAD titers are clearly reduced in the rituximab arm, the reduction was not significant compared to placebo.
Assessment of patients’ own response to therapy before breaking the study code
Telephone Survey questionnaire
Details of the patient’s own assessment before and after completion of the study, were clearly reflected on the telephone interview conducted several months after completion of the study but before breaking the study code using a standardized questionnaire. As shown in supplementary Table 2, no overall substantial differences were reported between the rituximab and the placebo group in any of the questions aimed to capture efficacy, quality of life, functional status or side effects.
Plots of self-assessed stiffness rates for individual patients during the 6-month study period are shown in Figure 3A. Four patients on rituximab (numbers 2,12,18,19, in supplementary table 2) reported clinical improvement in overall stiffness, starting 2-3 months after treatment initiation and peaking at 6 months; in some of these patients, video analysis also captured the improvements in gait and bodily functions (data not shown). Similarly, four patients on placebo (numbers 3, 5, 20,22 in supplementary Table 2), also reported clinical improvement in their overall stiffness but, in contrast to the rituximab group, these patients experienced the improvements somewhat earlier and in only one patient persisted at 6 months (Figure 3B).
Figure 3.
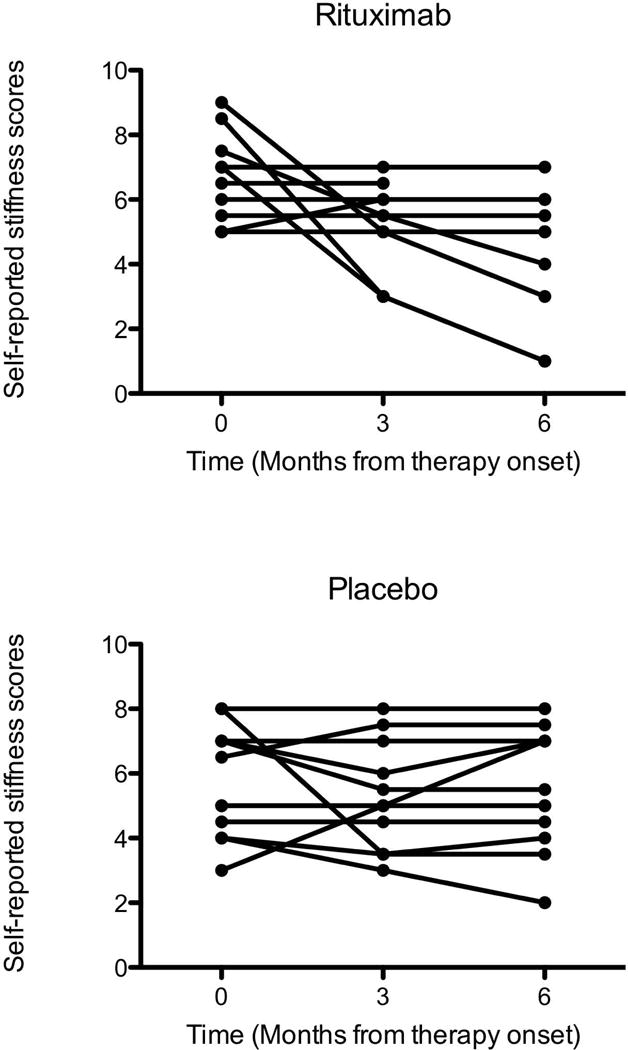
Scatter plots of self-reported clinical improvement or deterioration of the overall stiffness, rated 1 (best) to 10 (worst), in individual subjects at three time points. (A) In the rituximab arm, four patients experienced improvement prominent at 6 months. (B) In the placebo arm, some patients reported improvement, particularly within the first 3 months of the study, consistent with a placebo effect.
The patients were also asked to guess whether they received rituximab or placebo. Three of 12 patients randomized to placebo stated they received the drug and improved, one was uncertain and 8 guessed correctly that they received placebo. Similar results were reported in the rituximab group; 7 patients felt they received placebo, one that received the drug only because of a rash but without any change throughout the study while four guessed correctly that they received rituximab and improved providing clinical details (Figure 3A and supplementary Table 2).
DISCUSSION
This is the largest randomized controlled trial conducted in patients with SPS. The study demonstrated lack of efficacy of rituximab compared to placebo. In several other B-cell-mediated neurological disorders, such as multiple sclerosis, autoimmune encephalitis or myasthenia gravis, rituximab seems effective or promising20, 25–28. In contrast, in SPS, in spite of the presumed role of antibodies in disease pathogenesis, rituximab was not superior to placebo. The results are not necessarily in contrast to a few anecdotal reports29–31 where rituximab was reported efficacious in SPS, because four of 12 patients in our series who received rituximab reported meaningful clinical improvements, which in some cases were captured by video-recordings. There are several reasons for the noted lack of statistically significant efficacy in the present placebo-controlled study that may also shed light on the value of placebo in randomized trials.
First, the study showed a strong placebo effect, as evidenced by the improvement observed in both groups, and supported by the patients’ own assessment after completion of the study, in spite of being less prominent compared to rituximab (Figure 3 A,B). Although placebo effect is a factor in many placebo-controlled trials, it was very prominent in the present study probably because of the subjective nature of the patients’ symptomatology, the frequent fluctuations and the underlying anxiety, which for some patients is pronounced32 creating a strong anticipatory effect. Fear and anxiety in several SPS patients exhibit a day-to day variation that may aggravate stiffness and spasms and have an overall impact on assessing response to therapies.
Second, the scales used may be still insensitive and, to a certain degree subjective, even when recorded by the same neurologist, as we did throughout the study. Although we had used the same validated scales to demonstrate efficacy of IVIg in SPS patients22, in the rituximab study we included patients with overall less severe symptomatology where the scales used might not have been sensitive enough to capture minor changes.
Third, the degree of stiffness may fluctuate, even from day-to-day and is influenced, in addition to emotional stress, by various external factors difficult to control for. The advantage of the crossover trial design, that we used in the previous IVIG trial22, is that it mitigates the effect of such factors because these fluctuations in the very same patient are probably balanced out during both treatment periods.
Finally, rituximab may only help a subset of patients, as observed in the present study and documented by video analysis in some patients, implying that there is heterogeneity among SPS patients and larger patient numbers may be needed to reach statistical significance. A similar experience was noted in two prior trials with rituximab in patients with another rare disease, anti-MAG neuropathy.25, 33 In both of these studies, no statistically significant effect was noted, but individual patients experienced an apparent or even dramatic benefit, suggesting that a per-patient assessment by objective functional scales or video-recordings may have merit in small-size trials, necessitated by disease rarity.
Rituximab had no statistically significant effect on anti-GAD antibody titers, even though titers were markedly reduced. This was not unexpected but still does not provide an explanation for the lack of clinical efficacy because in SPS the serum antibody titers do not correlate with disease severity2, 14. Rituximab affects B cells but not plasma cells and the IgG titers may not substantially change. We did not observe any correlation between GAD titer reduction and clinical improvement in those 4 patients who clinically improved. Rituximab exerts its greater clinical effect in diseases where B-cells have a key pathogenic role, like multiple sclerosis and myasthenia gravis20,26–28, not necessarily by autoantibody reduction but rather by modulating the role of B cells in antigen presentation, T cell activation and production of pro-inflammatory cytokines21.
The drug was well tolerated. Some infusion-related reactions were equally balanced in both groups and none of the side effects were obvious or sufficiently distinct from the placebo to unblind the observers (supplementary Table 2). Similar observations were made in larger trials of rituximab in Multiple Sclerosis20 and in anti-MAG demyelinating neuropathies25,33.
An obvious question is whether the outcome of this negative trial, can be definitively interpreted that rituximab is ineffective in SPS patients, especially those with severe disease. Because in our series four patients with severe disease reported meaningful improvements, some of which captured in video recordings, it seems logical to entertain the impression that the drug might be useful for a subset of patients who have failed previous therapies with GABA-enhancing drugs and IVIg; anecdotal reports support this view29–31. We have also witnessed an effect of rituximab in the SPS variant, Progressive Encephalomyelitis with Rigidity and Myoclonus (PERM)34 where a patient positive for anti-glycine receptor antibodies, dramatically improved after a series of rituximab infusions. Whether a second set of infusions 6 months later could have made a difference, cannot be excluded but in our experience with other neuromuscular diseases, like myasthenia gravis and anti-MAG neuropathy, it seems unlikely that continuation of therapy will be of additional value if there is no convincing benefit 6-8 months after the first set of infusions. In one of the patients who dramatically improved (# 12 in supplementary Table 2), the improvement lasted 6-7 months (consistent with the mode of action of rituximab and the experience from other conditions20,21), prompting a request for a new infusion.
The study, although well conducted, has a few inherent limitations. Even if the series represents the largest ever-controlled study in SPS, the number of patients may still be small to establish efficacy using subjective and non-linear scales. Analysis of video recordings may be helpful but not solely sufficient or without a subjective bias. This is especially pertinent when including patents with less severe disease, as we did in this study, where appreciation of small changes may be objectively difficult. Perhaps, the newer anti-B cell agents such as occrelizumab, ofatumumab or obinutuzumab as well as agents affecting B regulatory functions, such as those directed against IL-6, may be more promising agents in SPS patients, as discussed21. Alternatively, the study results cannot exclude the possibility that SPS may be predominantly mediated by sensitized T cells rather than B cells and pathogenic autoantibodies, as has been already suggested3,6.
Supplementary Material
Acknowledgments
Financial support: NINDS (intramural program-Dr Dalakas) and Genentech through a CRADA with NINDS
We express our deepest appreciation to all the patients who participated in the study and those who came for screening but not enrolled. We also express our thanks to the research fellows and technicians of the Neuromuscular Diseases Section who helped with patient screening before enrollment, the nursing staff of the Clinical Center for the excellent care and Sofia Akrivou from the University of Athens for performing the GAD ELISA assay.
Footnotes
Author contributions
Study concept and design: MCD
Data acquisition and analysis: GR, BME, JMD, HA, MCD
Drafting the manuscript and figures: MCD, HA
Conflicts of Interest
The authors report no conflicts of interest pertinent to this study
References
- 1.Dalakas MC, Fujii M, Li M, McElroy B. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology. 2000;55:1531–1535. doi: 10.1212/wnl.55.10.1531. [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC, Li M, Fujii M, Jacobowitz DM. Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology. 2001;57:780–784. doi: 10.1212/wnl.57.5.780. [DOI] [PubMed] [Google Scholar]
- 3.McKeon A, Vincent A. Autoimmune movement disorders. Handb Clin Neurol. 2016;133:301–315. doi: 10.1016/B978-0-444-63432-0.00017-7. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol. 2009;11:102–110. doi: 10.1007/s11940-009-0013-9. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos H, Dalakas MC. A critical update on the immunopathogenesis of Stiff Person Syndrome. Eur J Clin Invest. 2010;40:1018–1025. doi: 10.1111/j.1365-2362.2010.02340.x. [DOI] [PubMed] [Google Scholar]
- 6.Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–772. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos H, Dalakas MC. Immunology of stiff person syndrome and other GAD-associated neurological disorders. Expert Rev Clin Immunol. 2013;9:1043–1053. doi: 10.1586/1744666X.2013.845527. [DOI] [PubMed] [Google Scholar]
- 8.McKeon A, Tracy JA. GAD65 neurological autoimmunity. Muscle Nerve. 2017 doi: 10.1002/mus.25565. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos H, Akrivou S, Dalakas MC. Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders. Neurology. 2013;81:1962–1964. doi: 10.1212/01.wnl.0000436617.40779.65. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Hernandez E, Arino H, McKeon A, et al. Clinical and Immunologic Investigations in Patients With Stiff-Person Spectrum Disorder. JAMA Neurol. 2016;73:714–720. doi: 10.1001/jamaneurol.2016.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinkel K, Meinck HM, Jury KM, et al. Inhibition of gamma-aminobutyric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. Ann Neurol. 1998;44:194–201. doi: 10.1002/ana.410440209. [DOI] [PubMed] [Google Scholar]
- 12.Raju R, Foote J, Banga JP, et al. Analysis of GAD65 autoantibodies in Stiff-Person syndrome patients. J Immunol. 2005;175:7755–7762. doi: 10.4049/jimmunol.175.11.7755. [DOI] [PubMed] [Google Scholar]
- 13.Levy LM, Dalakas MC, Floeter MK. The stiff-person syndrome: an autoimmune disorder affecting neurotransmission of gamma-aminobutyric acid. Ann Intern Med. 1999;131:522–530. doi: 10.7326/0003-4819-131-7-199910050-00008. [DOI] [PubMed] [Google Scholar]
- 14.Rakocevic G, Raju R, Dalakas MC. Anti-glutamic acid decarboxylase antibodies in the serum and cerebrospinal fluid of patients with stiff-person syndrome: correlation with clinical severity. Arch Neurol. 2004;61:902–904. doi: 10.1001/archneur.61.6.902. [DOI] [PubMed] [Google Scholar]
- 15.Chang T, Alexopoulos H, Pettingill P, et al. Immunization against GAD induces antibody binding to GAD-independent antigens and brainstem GABAergic neuronal loss. PLoS One. 2013;8:e72921. doi: 10.1371/journal.pone.0072921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raju R, Rakocevic G, Chen Z, et al. Autoimmunity to GABAA-receptor-associated protein in stiff-person syndrome. Brain. 2006;129:3270–3276. doi: 10.1093/brain/awl245. [DOI] [PubMed] [Google Scholar]
- 17.Manto M, Honnorat J, Hampe CS, et al. Disease-specific monoclonal antibodies targeting glutamate decarboxylase impair GABAergic neurotransmission and affect motor learning and behavioral functions. Front Behav Neurosci. 2015;9:78. doi: 10.3389/fnbeh.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arino H, Hoftberger R, Gresa-Arribas N, et al. Paraneoplastic Neurological Syndromes and Glutamic Acid Decarboxylase Antibodies. JAMA Neurol. 2015;72:874–81. doi: 10.1001/jamaneurol.2015.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalakas MC. Progress and stiff challenges in understanding the role of GAD-antibodies in stiff-person syndrome. Exp Neurol. 2013;247:303–307. doi: 10.1016/j.expneurol.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Hauser SL, Waubart E, Arnold DL, et al. B cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 21.Dalakas MC. Inhibition of B cell functions: implications for neurology. Neurology. 2008;70:2252–2260. doi: 10.1212/01.wnl.0000313840.27060.bf. [DOI] [PubMed] [Google Scholar]
- 22.Dalakas MC, Fujii M, Li M, et al. High-dose intravenous immune globulin for stiff-person syndrome. N Engl J Med. 2001;345:1870–1876. doi: 10.1056/NEJMoa01167. [DOI] [PubMed] [Google Scholar]
- 23.Prud’homme GJ, Glinka Y, Wang Q. Immunological GABAergic interactions and therapeutic applications in autoimmune diseases. Autoimmun Rev. 2015;14:1048–1056. doi: 10.1016/j.autrev.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Fouka P, Alexopoulos H, Akrivou S, et al. GAD65 epitope mapping and search for novel autoantibodies in GAD-associated neurological disorders. J Neuroimmunol. 2014;281:73–77. doi: 10.1016/j.jneuroim.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Dalakas MC, Rakocevic G, Salajegheh M, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009;65:286–293. doi: 10.1002/ana.21577. [DOI] [PubMed] [Google Scholar]
- 26.Lee WJ, Lee ST, Byun JI, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology. 2016;86:1683–1691. doi: 10.1212/WNL.0000000000002635. [DOI] [PubMed] [Google Scholar]
- 27.Anderson D, Phan C, Johnston WS, Siddiqi ZA. Rituximab in refractory myasthenia gravis: a prospective, open-label study with long-term follow-up. Ann Clin Transl Neurol. 3:552–555. doi: 10.1002/acn3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robeson KR, Kumar A, Keung B, et al. Durability of the Rituximab in Acetylcholine Receptor Autoantibody-positive Myasthenia Gravis. JAMA Neurol. 2017;74:60–66. doi: 10.1001/jamaneurol.2016.4190. [DOI] [PubMed] [Google Scholar]
- 29.Fekete R, Jankovic J. Childhood stiff-person syndrome improved with rituximab. Case Rep Neurol. 2012;4:92–96. doi: 10.1159/000339446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qureshi A, Hennessy M. Stiff person syndrome (SPS) complicated by respiratory failure: successful treatment with rituximab. J Neurol. 2012;259:180–181. doi: 10.1007/s00415-011-6123-9. [DOI] [PubMed] [Google Scholar]
- 31.Rizzi M, Knoth R, Hampe CS, et al. Long-lived plasma cells and memory B cells produce pathogenic anti-GAD65 autoantibodies in Stiff Person Syndrome. PLoS One. 2010;5:e10838. doi: 10.1371/journal.pone.0010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ameli R, Snow J, Rakocevic G, Dalakas MC. A neuropsychological assessment of phobias in patients with stiff person syndrome. Neurology. 2005;64:1961–1963. doi: 10.1212/01.WNL.0000163984.71993.FE. [DOI] [PubMed] [Google Scholar]
- 33.Leger JM, Viala K, Nicolas G, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein neuropathy. Neurology. 2013;80:2217–2225. doi: 10.1212/WNL.0b013e318296e92b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magira EE, Alexopoulos H, Charitatos E, et al. Progressive encephalomyelitis with rigidity and myoclonus (PERM): brucellosis as a possible triggering factor and long-term follow-up therapy with rituximab. Ther Adv Neurol Disord. 2016;9:69–73. doi: 10.1177/1756285615614812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


