Abstract
Importance
Whether sustained physical activity prevents cardiovascular disease (CVD) events in older adults is uncertain.
Objective
To test the hypothesis that cardiovascular morbidity and mortality was reduced in participants in a long-term physical activity program.
Design, Setting, Participants
The Lifestyle Interventions and Independence for Elders (LIFE) study was a multicenter, randomized trial. Participants were recruited at 8 centers in the United States. We randomized 1635 sedentary men and women aged 70 to 89 years with Short Physical Performance Battery (SPPB) score of 9 or below, but able to walk 400 m.
Interventions
The PA intervention was a structured moderate intensity program, predominantly walking two times per week on site for 2.6 years on average. The SA intervention consisted of weekly health education sessions for six months, then monthly.
Main Outcome
Total CVD events, including fatal and non-fatal myocardial infarction (MI), angina, stroke, transient ischemic attack, and peripheral artery disease, were adjudicated by committee and silent MI was assessed by serial electrocardiograms. A limited outcome of MI, stroke, and CVD death was also studied. Outcome assessors and adjudicators were blinded to intervention assignment.
Results
New CVD events occurred in 14.8% (n=121/818) of PA and 13.8% (113/817) of SA participants (HR=1.10, 95%CI: 0.85 to 1.42). For the more focused combined outcome of MI, stroke or cardiovascular death, rates were 4.6% in PA and 4.5% in the SA group (HR = 1.05 (95%CI: 0.67 to 1.66). Among frailer participants with a SPPB <8, total CVD rates were 14.2% in PA vs 17.7% in SA with HR = 0.76 (95%CI: 0.52 to 1.10), compared with 15.3% vs. 10.5% with HR = 1.59 (95%CI: 1.09 to 2.30) among those with SPPB of 8 or 9 (p for interaction =0.006). With the limited endpoint, the interaction was not significant (p=0.59), with HR = 0.94 (95% CI: 0.50–1.75) for SPPB < 8 and HR = 1.20 (95% CI, 0.62–2.34) for SBBP of 8 or 9).
Conclusions and Relevance
Among participants in the LIFE Study, an aerobically-based, moderately intensive PA program did not reduce cardiovascular events in spite of the intervention’s previously documented ability to prevent mobility disability.
Keywords: Exercise, cardiovascular disease, clinical trials, myocardial infarction, stroke, elderly, aged
Introduction
There are few randomized, clinical trials testing the ability of physical activity to prevent cardiovascular disease events. Many observational studies demonstrate that greater physical activity is associated with lower rates of incident and recurrent myocardial infarction across a spectrum of age.1–3 In clinical trials, physical activity interventions have been shown to slow the progression of coronary artery disease4,5 and can prolong event-free survival in patients undergoing stent placement.6,7 Cardiac rehabilitation trials show reduction in cardiovascular events for patients with recent cardiovascular illness, though most trials include risk factor modification in addition to exercise training, making it difficult to isolate the impact of physical activity. Current guidelines8,9 extend recommendations for 150 minutes of moderate activity per week for middle-aged adults to older adults based on studies to date. However, these trials have not included many frail older adults who are at highest risk for cardiovascular disease and disability, thus many questions remain as to the optimal frequency, intensity and modality of physical activity for older adults. Whether a physical activity intervention can prevent cardiovascular disease events in older, functionally limited individuals is unknown.
The Lifestyle Independence and Interventions for Elders (LIFE) Study compared physical activity to health education and showed that a structured physical activity program can prevent mobility disability in older adults with functional limitations. In the trial, incident major mobility disability occurred in 30.1% of the physical activity group and 35.5% of the health education group [HR 0.82 95% CI, 0.69–0.98)].10 As a tertiary outcome, we compared cardiovascular event rates between the two study groups. The physical activity intervention was conducted over an average of 2.5 years and included moderate aerobic activity, mostly walking, of at least 150 minutes per week, thus the study can be viewed as an evaluation of the benefits of current guidelines for prevention of cardiovascular disease events.8,9
Methods
The LIFE study was a multicenter, single-blinded, randomized trial of physical activity compared with health education conducted at 8 field centers across the U.S. (University of Florida, Gainesville and Jacksonville, Florida; Northwestern University, Chicago, Illinois; Pennington Biomedical Research Center, Baton Rouge, Louisiana; University of Pittsburgh, Pittsburgh, Pennsylvania; Stanford University, Stanford, California; Tufts University, Boston, Massachusetts; Wake Forest School of Medicine, Winston-Salem, North Carolina; and Yale University, New Haven, Connecticut) between February 2010 and December 2013. The Administrative Coordinating Center was located at the University of Florida and the Data Management, Analysis, and Quality Control Center was at Wake Forest School of Medicine. The field centers included rural, suburban and urban communities.
Details of the trial design, recruitment, and primary outcome were published previously.11,12,13 Men and women aged 70–89 years were eligible if they (a) were sedentary, defined as reporting less than 20 min/week in the past month performing regular physical activity and reporting less than 125 min/week of moderate physical activity; (b) were at high risk for mobility disability based on objective lower extremity functional limitations as measured by the Short Physical Performance Battery (SPPB)14 score ≤9 out of a total of 12 (45% of participants were targeted to have a SPPB score <8); (c) could walk 400 m in 15 minutes or less without sitting, leaning, or the help of another person or walker; (d) had no major cognitive impairment (Modified Mini-Mental State Examination15[3MSE] 1.5 standard deviations below education-and race-specific norms); and (e) could safely participate in the intervention as determined by medical history, a practitioner administered physical exam and resting ECG reading. The primary outcome of major mobility disability was defined as the inability to complete a 400 m walk test within 15 min without sitting and without the help of another person or walker.11,12 The study protocol was approved by the institutional review boards at all participating sites. Written informed consent was obtained from all study participants. The trial was monitored by a data safety monitoring board appointed by the National Institute on Aging. The trial is registered at ClinicalsTrials.gov (NCT00116194).
Randomization
Participants were randomized to a physical activity or to a successful aging program, via a secure web-based data management system using a permuted block algorithm (with random block lengths) stratified by field center and gender. Both groups received an initial individual 45-minute face-to-face introductory session by a health educator who described the intervention, communicated expectations, and answered questions.
Interventions
The physical activity intervention involved walking, with a goal of 150 min/week, strength, flexibility, and balance training.11 The intervention included attendance at two center-based visits per week and home-based activity 3–4 times per week for the duration of the study. A protocol was in place to restart the intervention for the participants who suspended the physical activity for medical reasons. The physical activity sessions were individualized and progressed towards a goal of 30 min of walking daily at moderate intensity, 10 min of primarily lower extremity strength training by means of ankle weights (2 sets of 10 repetitions), 10 min of balance training, and large muscle group flexibility exercises. The participants began with lighter intensity and gradually increased intensity over the first 2–3 weeks of the intervention. The Borg’s scale of self-perceived exertion16 (range: 6 to 20), was used to measure intensity of activity. Participants were asked to walk at an intensity of 13 (“somewhat hard”), and lower extremity strengthening exercises were performed at an intensity of 15 to 16. The successful aging group attended weekly workshops of health education during the first 26 weeks, and then monthly sessions thereafter.
Cardiovascular disease assessment
At baseline, prevalent CVD was defined as self-report of myocardial infarction, congestive heart failure, stroke, or MI pattern on ECG. At each 6 month contact, all participants (or a proxy informant if the participant was unavailable) were questioned about all hospitalizations since the last visit. Proxy reports accounted for < 0.1% of the follow-up contacts. Hospital records were obtained to abstract information for study criteria for the primary and secondary outcomes, masked to group assignment. Cardiovascular disease events were a pre-defined tertiary outcome and consisted of a composite of myocardial infarction, hospitalized angina, hospitalized congestive heart failure, revascularization with bypass surgery or percutaneous angioplasty, ruptured abdominal aortic aneurysm, hospitalization for carotid artery disease, hospitalization for peripheral artery disease (PAD) or outpatient revascularization for PAD, any stroke, transient ischemic attack requiring hospitalization, and CVD death. Records and abstraction forms were sent to the administrative coordinating center for central review by two physician-investigators who were also masked to group assignment, with adjudication as definite or probable by two reviewers. If there were differences between these reviewers, cases were discussed by the full committee. Only definite events were included in this report.
Reports of death were tracked through regular surveillance and death certificates were obtained to supplement the hospital record review. Silent MI was assessed by ECGs obtained at baseline, 18, and 36 months and read using standard algorithms at a central reading center.17 The time from randomization date to the first cardiovascular event, fatal or non-fatal, was used to define incidence during the trial. Total CVD events and a limited outcome of MI (excluding silent MI on ECG), stroke or CVD death were examined. Analyses were conducted for the overall sample and by baseline history vs. no prior history of cardiovascular disease.
Measurements
Participants were assessed at baseline and every six months at clinic visits by assessors who were also masked to intervention group assignment. The baseline assessments included the following: self-reported demographic and contact information, medical and hospitalization history, medication inventory, ECG, physical exam, health care utilization, physical activity assessed with the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire,18 and with accelerometry over 7-day periods (Actigraph Inc., Pensacola FL), cognitive testing, 400 m walk test,19 the SPPB, body weight, blood pressure, and pulse rate. These measures were repeated during follow-up at varied intervals.11 The SPPB consisted of 4 m walk at usual pace, a timed repeated chair stand, and three increasingly difficult standing balance tests.14,20 Each measure was assigned a categorical score ranging from 0 (inability to complete the test) to 4 (best performance). A summary score ranging from 0 (worst performers) to 12 (best performers) was calculated by summing the three component scores. Home, telephone, and proxy assessments were attempted if the participants could not come to the clinic.
Statistical Considerations
The collection of cardiovascular events was added as a tertiary outcome after the sample size was fixed for the primary outcome. As such, the power for the combined cardiovascular events outcome was lower than desired. Assuming 8%/year loss to follow-up, a 4%/year event rate in the SA group, and a 30% effect (HR of 0.7), we had only 51% power using a two-sided test at the 5% level. With 80% power, we could detect a minimum effect size of 42% and with 90% power, a 47% effect. Baseline characteristics were summarized by intervention group using mean (SD) or percentages. The effect of the intervention on cardiovascular disease (i.e. time until the initial ascertainment) was tested based on a two-tailed significance of 0.05 using the intention to treat approach in which participants are grouped according to randomization assignment. To compare intervention arms, we used a likelihood ratio test from a Cox regression model, stratified by field center and gender. Interaction terms were entered into these Cox models to assess the consistency of the intervention effect across levels of pre-specified baseline subgroups (ethnicity/race, gender, cardiovascular disease, diabetes, walking speed, and SPPB score). No explicit assessment for multiplicity was made.
Results
The 1635 LIFE study participants were predominantly women (67%), with mean age 78.7 +/− 5.2 years; 20% were African-American, 6% were Hispanic or other race or ethnic group, and 74% were non-Latino white. The overall prevalence of CVD at baseline was 8% for MI, 6.5% for stroke, and 4.2% for heart failure with an overall baseline prevalence of 30%. Hypertension and diabetes were common, 69% and 26% respectively, while only 3% currently smoked cigarettes. Mean BMI was 30 +/− 5.5 kg/m2. Lipid levels, blood pressure LDL and HDL cholesterol varied. Reflecting a high risk of disability, the mean SBBP was 7.4. These characteristics were balanced between the intervention groups (Table 1).
Table 1.
Baseline characteristics of Participants by Intervention Group
| Description | Physical Activity | Successful Aging |
|---|---|---|
| N=818 | N=817 | |
| Age at Randomization, years, mean (SD) | 78.7 (5.2) | 79.1 (5.2) |
| Women, n (%) | 547 (66.9) | 551 (67.4) |
| Race: White, n (%) | 604 (73.8) | 635 (77.7) |
| Education: College or Higher, n (%) | 515 (63.0) | 529 (65.1) |
| Lower Extremity Short Physical | 353 (43.2) | 378 (46.3) |
| Performance Battery (SPPB) <8, n (%) | ||
| 3MSE: Total Score (max=100), mean (SD) | 91.5 (5.5) | 91.6 (5.3) |
| Cardiovascular Disease, n (%) | 236 (28.9) | 254 (31.1) |
| Myocardial Infarction, n (%) | 60 (7.4) | 69 (8.5) |
| Stroke, n (%) | 57 (7.0) | 52 (6.4) |
| Heart Failure, n (%) | 26 (3.2) | 45 (5.6) |
| Other Self-Report CVD, n (%) | 157 (19.2) | 163 (20.0) |
| Framingham Risk Score, mean (SD) | 10.1 (7.3) | 10.1 (7.3) |
| Hypertension, n (%) | 573 (70.5) | 578 (71.5) |
| Diabetes, n (%) | 199 (24.4) | 216 (26.6) |
| Past Smoker, n (%) | 381 (47.2) | 341 (42.7) |
| Current Smoker, n (%) | 26 (3.2) | 24 (3.0) |
| Total Cholesterol, mg/dl mean (SD) | 179.3 (39.6) | 178.5 (39.9) |
| LDL Cholesterol, mg/dl mean (SD) | 93.6 (32.2) | 93.1 (33.1) |
| HDL Cholesterol, mg/dl mean (SD) | 61.1 (18.0) | 61.1 (17.6) |
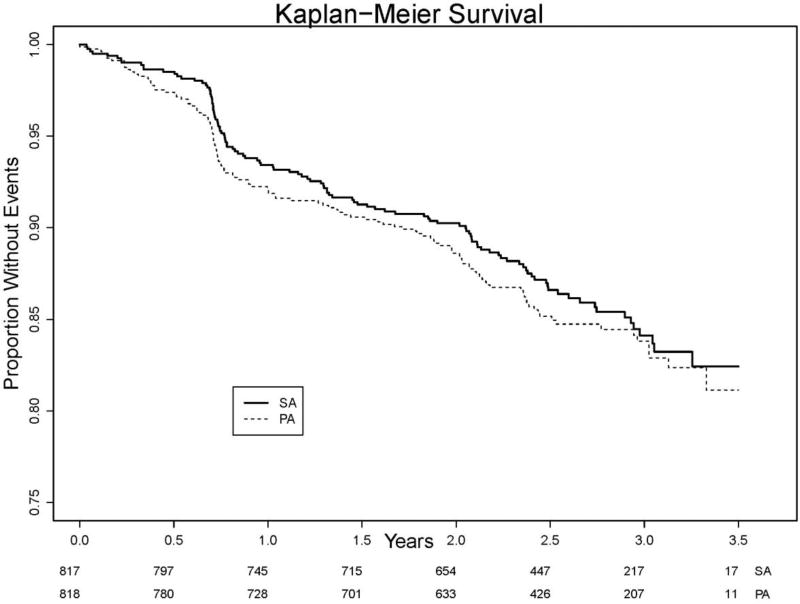
Total incident CVD occurred in 14.3% overall with 14.8% (121/818) of PA and 13.8% (113/817) of SA participants or 6.2 vs. 5.6 events per 100 person-years (HR=1.10, 95%CI: 0.85 to 1.42). For the more limited combined outcome of MI, stroke or cardiovascular death, rates were 4.6% in the PA and 4.5% in the SA group or 1.8 vs. 1.7 events per 100 person-years with HR = 1.05 (95%CI: 0.67 to 1.66). There was no difference in the rate of individual events between intervention groups (Table 2 and Figure 1).
Table 2.
Type of CVD Outcome by Intervention Group
| Physical Activity (N=818) | Successful Aging (N=817) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome | Annualized Incidence Rate per 100py (95% CI)* |
n (%) |
Annualized Incidence Rate per 100py (95% CI)* |
n (%) |
HR (95% CI)** |
P- value |
| Total fatal or non-fatal cardiovascular disease*** | 6.2 | 121 | 5.6 | 113 | 1.10 | 0.49 |
| (5.2, 7.4) | (14.8) | (4.7, 6.8) | (13.8) | (0.85, 1.42) | ||
| Fatal or non-fatal myocardial infarction, stroke or cardiovascular death | 1.8 | 38 | 1.7 | 37 | 1.05 | 0.83 |
| (1.3, 2.5) | (4.6) | (1.3, 2.4) | (4.5) | (0.67, 1.66) | ||
| Cardiovascular death | 0.5 | 10 | 0.7 | 14 | 0.71 | 0.41 |
| (0.3, 0.9) | (1.2) | (0.4, 1.1) | (1.7) | (0.32, 1.60) | ||
| Myocardial infarction | 0.6 | 12 | 0.5 | 10 | 1.22 | 0.64 |
| (0.3, 1.0) | (1.5) | (0.3, 0.9) | (1.2) | (0.53, 2.83) | ||
| Silent Myocardial infarction | 1.7 | 35 | 1.8 | 38 | 0.92 | 0.71 |
| (1.2, 2.4) | (4.3) | (1.3, 2.5) | (4.7) | (0.58, 1.45) | ||
| Angina/symptomatic coronary artery disease | 0.8 | 16 | 0.5 | 10 | 1.64 | 0.22 |
| (0.5, 1.2) | (2.0) | (0.3, 0.9) | (12) | (0.74, 3.61) | ||
| Stroke | 0.9 | 19 | 1.0 | 21 | 0.94 | 0.85 |
| (0.6, 1.4) | (2.3) | (0.6, 1.5) | (2.6) | (0.51, 1.76) | ||
| Coronary Revascularization | 1.0 | 20 | 0.5 | 10 | 2.08 | 0.05 |
| (0.6, 1.5) | (2.4) | (0.3, 0.9) | (1.2) | (0.97, 4.45) | ||
| CHF | 1.3 | 28 | 1.3 | 27 | 1.06 | 0.84 |
| (0.9, 1.9) | (3.4) | (0.9, 1.9) | (3.3) | (0.62, 1.79) | ||
| Abdominal Aortic Aneurysm | 0.1 | 3 | 0.0 | 1 | 2.99 | 0.31 |
| (0.0, 0.4) | (0.4) | (0.0, 0.3) | (0.1) | (0.31, 28.76) | ||
| Peripheral Artery Disease | 0.2 | 5 | 0.3 | 6 | 0.85 | 0.78 |
| (0.1, 0.6) | (0.6) | (0.1, 0.6) | (0.7) | (0.26, 2.78) | ||
| Carotid Revascularization | 0.0 | 0 | 0.1 | 2 | 0.00 | 0.10 |
| (0.0, 0.0) | (0.0) | (0.0, 0.4) | (0.2) | (0.00, 0.0) | ||
| Transient Ischemic Attack | 0.3 | 6 | 0.2 | 5 | 1.24 | 0.72 |
| (0.1, 0.6) | (0.7) | (0.1, 0.6) | (0.6) | (0.38, 4.07) | ||
Incident event rates estimated from an exponential survival model.
HRs from a proportional hazards model stratified by clinical site and gender.
Fatal or nonfatal myocardial infarction, stroke, cardiovascular death, silent myocardial infarction, angina/symptomatic coronary artery disease, peripheral artery disease, abdominal aortic aneurysm (our pre-defined primary CVD endpoint). The total incidence N is the number of participants who develop any of these conditions during the trial and thus will differ from the sum of the disease-specific totals.
Figure 1.
Total CVD event rates by intervention group. Cumulative hazards plot.
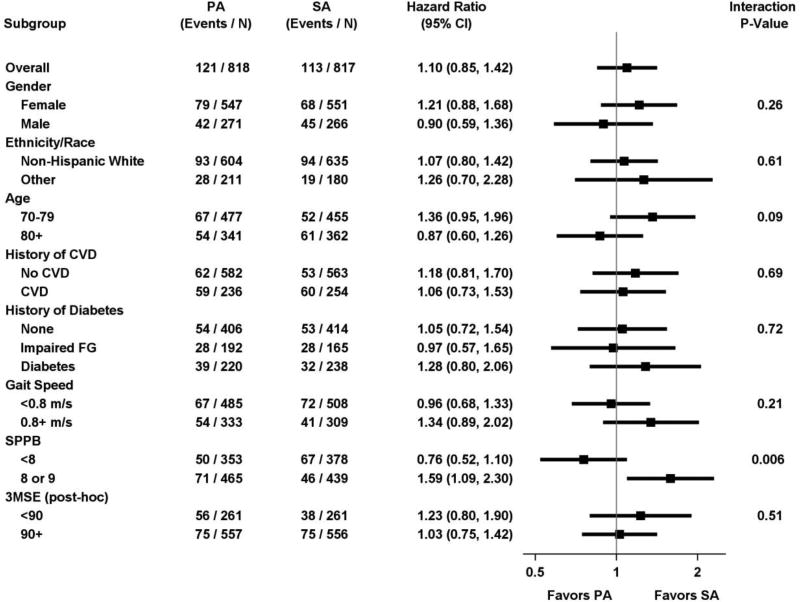
In pre-specified subgroup analyses, there were no differences in rates of incident vs. recurrent CVD (Figure 2). Among participants with SPPB <8, CVD rates were 14.2% in PA vs. 17.7% in SA with HR = 0.76 (95%CI: 0.52 to 1.10), compared to 15.3% vs. 10.5% with HR = 1.59 (95%CI: 1.09 to 2.30) among those with SPPB of 8 or 9 (p for interaction = 0.006). The interaction was not significantly different for the more limited composite endpoint of MI, stroke or CVD death. For the limited outcome, among participants with SPPB <8, CVD rates were 5.4% in PA vs. 5.6% in SA with HR = 0.94 (95%CI: 0.50 to 1.75), compared to 4.1% vs. 3.6% with HR = 1.20 (95%CI: 0.62 to 2.34) among those with SPPB of 8 or 9 (p for interaction = 0.59) (Supplementary Figures 1 and 2).
Figure 2.
Total CVD event rates, hazard ratios and interaction p-values by intervention group and subgroups
Because the observed effect of the intervention on CVD outcomes was marginally significant in lower vs. higher SPPB subgroup, we examined the demographic, health characteristics and types of CVD events by SPPB subgroup. We also examined the level of PA and level of exertion with PA during the trial by intervention group as well as SPPB subgroup (Supplementary Table 1). There were no differences in baseline characteristics by SPPB subgroup such as prevalent CVD, CVD risk factors, physical activity or perceived level of exertion at baseline. There were more silent MI’s by ECG in the higher SPPB subgroup, but not more cardiovascular procedures, and this did not explain the relatively higher risk in the higher SPPB subgroup. Over the course of the study, higher SPPB subgroups had higher measured activity and lower perceived exertion than the lower SBBP subgroups in both the PA and the SA group (Supplementary Figure 3).
Discussion
Among participants in the LIFE Study, an aerobically-based, moderately intensive PA program did not reduce cardiovascular events. LIFE participants had a substantial baseline burden of prevalent cardiovascular disease and cardiovascular risk factors and had a high rate of cardiovascular events (14.3%) during the 2.6 years of follow-up in the LIFE Study. As the only randomized trial of sustained physical activity in functionally limited older adults, the benefits of activity appear to be primarily reduced mobility disability12 and perhaps improved cognition.21 The lack of benefit for cardiovascular disease was similar in most subgroups, including the 1/3 of the cohort with prevalent cardiovascular disease at baseline. Individuals with poorer physical performance at baseline, defined as an SPPB < 8, had a more favorable benefit from physical activity for the outcome of CVD than those with a more moderate level of SPPB as indicated by the statistically significant interaction term. In the LIFE study, a pattern of relatively more favorable benefit for older and more poorly functioning individuals was observed for the primary outcome of major mobility disability12 and for other secondary outcomes.10,22,23 In the case of the CVD outcome, the subgroup with less severe impairment had a higher rate of events in the physical activity group when compared with the successful aging/health education group. Further evaluation of the characteristics and activity of the SPPB 8–9 subgroup did not provide an obvious explanation for the higher event rate with PA in the high SPPB subgroup (8–9) vs. the low SPPB subgroup (≥7). Participants with a higher baseline SPPB score reported a lower level of exertion with activity and had a similar relative increase in activity compared with the control group as did the low SPPB subgroup. Of note, this interaction was not significant when CVD events were restricted to MI, stroke, and CVD death. While pre-specified, these subgroup comparisons were meant to be hypothesis-generating rather than designed to test specific hypotheses.
There are several potential explanations for a lack of CVD reduction in the LIFE study. It is possible that the dose of activity was of suboptimal duration or intensity. Given the high burden of CVD, it is also possible that it was too late for this high-risk group to benefit. We also noted that both the SA and PA groups became more physically active by self-report, though only the PA group by actigraphy.10 Potentially the contrast between the PA and SA groups was less than if a completely sedentary comparator had been used. It is also possible that the more frequent contact biased the PA group to report more events to the masked assessors or that physical activity could have precipitated some events in this vulnerable population. In the analysis stratified by SPPB score, when we excluded the symptomatic outcomes in the limited outcome, the difference by SPPB score was not statistically significant. Most importantly, follow-up was only 2.6 years on average and statistical power was limited to detect small differences in rates.
Previous trials of activity in older adults have focused on intermediate outcomes such as lowering of blood pressure, weight and improving function.24,25 In adults with type 2 diabetes, the Look AHEAD study tested whether a lifestyle intervention that included sustained physical activity with weight loss could reduce cardiovascular disease events. While findings were negative for the primary CVD outcome,26 rates of disability were reduced.25 Together these studies suggest that physical activity should be recommended for improving quality of the remaining years of life. Current guidelines for physical active for older adults include at least 150 minutes per week of moderate intensity aerobic activity with weight training.24 The LIFE intervention meets these guidelines and proved to be safe and efficacious for the prevention of major mobility disability. The lack of benefit for preventing CVD found here should not detract from efforts to promote a program of sustained walking and weight training in frail older adults.
Supplementary Material
Acknowledgments
Grant Funding: The Lifestyle Interventions and Independence for Elders Study is funded by cooperative agreement U01 AG22376 from the National Institutes of Health (NIH) and National Institute on Aging; supplement U01 AG022376-05A2S from the National Heart, Lung, and Blood Institute; and was sponsored in part by the Intramural Research Program. The research is partly supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (P30 AG028740), Wake Forest University (P30 AG21332), Tufts University (P30 AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30 AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744), at University of Florida (U54RR025208) and at Yale University (UL1 TR000142). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (R24 HD065688-01A1) and by the U.S. Department of Agriculture, under agreement No. 58-1950-4-003. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. Dr. Stafford is also supported by an NHLBI mid-career mentoring award (K24 HL086703).
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744),
Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
LIFE investigators are also partially supported by the following:
Dr. Thomas Gill (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging.
Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs.
Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Administrative Coordinating Center, University of Florida, Gainesville, FL
Marco Pahor, MD - Principal Investigator of the LIFE Study
Jack M. Guralnik, MD, PhD - Co-Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD)
Christiaan Leeuwenburgh, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ching-ju Lu, MPH
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston Salem, NC
Michael E. Miller, PhD - DMAQC Principal Investigator
Mark A. Espeland, PhD - DMAQC Co-Investigator
Walter T. Ambrosius, PhD
William Applegate, MD
Daniel P. Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
LIFE -Acknowledgement List13-2014-07-17 2
Delilah Cook, CCRP
Curt D. Furberg, MD, PhD
Lea N. Harvin, BS
Leora Henkin, MPH, Med
John Hepler, MA
Fang-Chi Hsu, PhD
Laura Lovato, MS
Wesley Roberson, BSBA
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
W. Jack Rejeski, PhD
Jeffrey A. Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon L. Mihalko, PhD
Janine M. Jennings, PhD
National Institutes of Health, Bethesda, MD
Evan C. Hadley, MD (National Institute on Aging)
Sergei Romashkan, MD, PhD (National Institute on Aging)
Kushang V. Patel, PhD (National Institute on Aging)
National Heart, Lung and Blood Institute, Bethesda, MD
Denise Bonds, MD, MPH
Field Centers
Northwestern University, Chicago, IL
Mary M. McDermott, MD - Field Center Principal Investigator
Bonnie Spring, PhD - Field Center Co-Investigator
Joshua Hauser, MD - Field Center Co-Investigator
Diana Kerwin, MD - Field Center Co-Investigator
Kathryn Domanchuk, BS
Rex Graff, MS
Alvito Rego, MA
Pennington Biomedical Research Center, Baton Rouge, LA
Timothy S. Church, MD, PhD, MPH - Field Center Principal Investigator
Steven N. Blair, PED (University of South Carolina)
Valerie H. Myers, PhD
Ron Monce, PA-C
Nathan E. Britt, NP
Melissa Nauta Harris, BS
Ami Parks McGucken, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi K. Millet, MPA, BS
Catrine Tudor-Locke, PhD, FACSM
LIFE -Acknowledgement List13-2014-07-17 3
Ben P. Butitta, BS
Sheletta G. Donatto, MS, RD, LDN, CDE
Shannon H. Cocreham, BS
Stanford University, Palo Alto, CA
Abby C. King, PhD - Field Center Principal Investigator
Cynthia M. Castro, PhD William L. Haskell, PhD
Randall S. Stafford, MD, PhD Leslie A. Pruitt, PhD
Kathy Berra, MSN, NP-C, FAAN
Veronica Yank, MD
Tufts University, Boston, MA
Roger A. Fielding, PhD - Field Center Principal Investigator
Miriam E. Nelson, PhD - Field Center Co-Investigator
Sara C. Folta, PhD - Field Center Co-Investigator
Edward M. Phillips, MD
Christine K. Liu, MD
Erica C. McDavitt, MS
Kieran F. Reid, PhD, MPH
Dylan R. Kirn, BS
Evan P. Pasha, BS
Won S. Kim, BS
Vince E. Beard, BS
Eleni X. Tsiroyannis, BS
Cynthia Hau, BS, MPH
University of Florida, Gainesville, FL
Todd M. Manini, PhD - Field Center Principal Investigator
Marco Pahor, MD - Field Center Co-Investigator
Stephen D. Anton, PhD
Susan Nayfield, MD
Thomas W. Buford, PhD
Michael Marsiske, PhD
Bhanuprasad D. Sandesara, MD
Jeffrey D. Knaggs, BS
Megan S. Lorow, BS
William C. Marena, MT, CCRC
Irina Korytov, MD
Holly L. Morris, MSN, RN, CCRC (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Margo Fitch, PT (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Floris F. Singletary, MS, CCC-SLP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jackie Causer, BSH, RN (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Katie A. Radcliff, MA (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
LIFE -Acknowledgement List13-2014-07-17 4
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH - Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH - Field Center Co-Investigator
Bret H. Goodpaster, PhD
Nancy W. Glynn, PhD
Oscar Lopez, MD
Neelesh K. Nadkarni, MD, PhD
Kathy Williams, RN, BSEd, MHSA
Mark A. Newman, PhD
George Grove, MS
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA (deceased)
Diane G. Ives, MPH
Wake Forest University, Winston Salem, NC
Stephen B. Kritchevsky, Ph.D. - Field Center Principal Investigator
Anthony P. Marsh, PhD - Field Center Co-Investigator
Tina E. Brinkley, PhD
Jamehl S. Demons, MD
Kaycee M. Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
Yale University, New Haven, CT
Thomas M. Gill, MD - Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM - Field Center Co-Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, MPH (VA Connecticut Healthcare System)
Nathalie de Rekeneire, MD, MS
Joanne M. McGloin, MDiv, MS, MBA
Karen C. Wu, RN
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Lynne P. Iannone, MS, CCRP
Raeleen Mautner, PhD
Theresa Sweeney Barnett, MS, APRN
Sean N. Halpin, MA
Matthew J. Brennan, MA
Julie A. Bugaj, MS
Maria A. Zenoni, MS
Bridget M. Mignosa, AS
LIFE -Acknowledgement List13-2014-07-17 5
Cognition Coordinating Center, Wake Forest University, Winston Salem, NC
Jeff Williamson, MD, MHS - Center Principal Investigator
Kaycee M Sink, MD, MAS - Center Co-Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Mark Espeland, PhD
Janine Jennings, PhD
Valerie K. Wilson, MD
Electrocardiogram Reading Center, University of Florida, Gainesville, FL
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
Anita Szady, MD
Spirometry Reading Center, Yale University, New Haven, CT
Geoffrey L. Chupp, MD
Gail M. Flynn, RCP, CRFT
Thomas M. Gill, MD
John L. Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
Cost Effectiveness Analysis Center
Erik J. Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System)
Robert M. Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health)
Footnotes
This is an un-copyedited author manuscript that has been accepted for publication in JAMA Cardiology, copyright American Medical Society (AMA). This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the AMA. All research articles published in JAMA Cardiology are made available free online on the day of publication on The JAMA Reader (http://mobile.jamanetwork.com) and 12 months after publication on the JAMA Website (http://jama.jamanetwork.com). The AMA disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Author Contributions:
Daniel Beavers had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsors: The NIH sponsor was a voting member (1 of 12 votes) of the LIFE Steering Committee, which approved design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Study concept and design: Newman, Church, Fielding, Kritchevsky, Pahor.
Acquisition, analysis, or interpretation of data: Newman, Church, Buford, Fielding, Beavers, Pahor, Ambrosius, McDermott.
Drafting of the manuscript: Newman, Church, Fielding, Kritchevsky, Beavers, Pahor, McDermott.
Critical revision of the manuscript for important intellectual content: Newman, Dodson, Church, Buford, Pahor, Fielding, Kritchevsky, Pahor, Stafford, Szady, Ambrosius, McDermott.
Statistical analysis: Ambrosius, Beavers.
Obtained funding: Newman, Church, Fielding, Pahor, McDermott.
Administrative, technical, or material support: Newman, Fielding, Pahor.
Study supervision: Newman, Fielding, Kritchevsky, Pahor, McDermott.
All Authors report no conflict of interest.
Contributor Information
Anne B. Newman, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA; newmana@edc.pitt.edu.
John A. Dodson, New York University School of Medicine, New York, NY, USA; Phone: 646-501-2714; John.Dodson@nyumc.org.
Timothy S. Church, Department of Preventative Medicine, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA; Phone: 225-763-2632; Fax: 225-763-3014; Timothy.Church@pbrc.edu.
Thomas W. Buford, University of Florida, Gainesville, FL, USA; Phone: 352-273-5918; Fax: 352-273-5920; tbuford@ufl.edu.
Roger A. Fielding, Tufts University, Boston, MA, USA; Phone: 617-556-3016; Fax: 617-556-3083; roger.fielding@tufts.edu.
Stephen Kritchevsky, Wake Forest School of Medicine, Winston-Salem, NC, USA; Phone: 336-713-8548; Fax 336-713-8588; skritche@wakehealth.edu.
Daniel Beavers, Wake Forest School of Medicine; Winston-Salem, NC, USA; Phone: 336.713.1616; Fax: 336.716.6427; dbeavers@wakehealth.edu.
Marco Pahor, University of Florida, Gainesville, FL, USA; Phone: 352-265-7227; Fax: 352-294-5836; mpahor@ufl.edu.
Randall S. Stafford, Stanford University, Palo Alto, CA, USA; Phone; 650-724-2400; Fax 650-725-6906; rstafford@stanford.edu.
Anita D. Szady, University of Florida, Gainesville, FL, USA; Phone: 352-273-9078, Fax: 352-273-8889; szadya@medicine.ufl.edu.
Walter T. Ambrosius, Wake Forest School of Medicine, Winston-Salem, NC, USA; Phone: 336-716-6281; Fax: 336-716-6427; wambrosi@wakehealth.edu.
Mary M. McDermott, Northwestern University, Chicago IL, USA, Phone: 312-503-6419; mdm608@northwestern.edu.
References
- 1.Williams PT. Physical fitness and activity as separate heart disease risk factors: a metaanalysis. Med Sci Sports Exerc. 2001;33(5):754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med. 2004;26(5):407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15(3):247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 4.Niebauer J, Hambrecht R, Velich T, et al. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96(8):2534–2541. doi: 10.1161/01.cir.96.8.2534. [DOI] [PubMed] [Google Scholar]
- 5.Hambrecht R, Niebauer J, Marburger C, et al. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol. 1993;22(2):468–477. doi: 10.1016/0735-1097(93)90051-2. [DOI] [PubMed] [Google Scholar]
- 6.Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol. 2001;37(7):1891–1900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- 7.Hambrecht R, Walther C, Mobius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109(11):1371–1378. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 8.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 9.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012).The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33(13):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 10.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: Design and Methods. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A(11):1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The life study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68(12):1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 16.Borg G. Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; US; 1998. [Google Scholar]
- 17.Gregg RE, Deluca DC, Chien CH, Helfenbein ED, Ariet M. Automated serial ECG comparison improves computerized interpretation of 12-lead ECG. Journal of electrocardiology. 2012;45(6):561–565. doi: 10.1016/j.jelectrocard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52(6):972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson JD, Espeland M, Kritchevsky SB, et al. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci. 2009;64(6):688–694. doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sink KM, Espeland MA, Rushing J, et al. The LIFE Cognition Study: design and baseline characteristics. Clin Interv Aging. 2014;9:1425–1436. doi: 10.2147/CIA.S65381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espeland MA, Katula JA, Rushing J, et al. Performance of a computer-based assessment of cognitive function measures in two cohorts of seniors. Int J Geriatr Psychiatry. 2013;28(12):1239–1250. doi: 10.1002/gps.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2(10):801–809. doi: 10.1016/S2213-8587(14)70156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rejeski WJ, Bray GA, Chen SH, et al. Aging and Physical Function in Type 2 Diabetes: 8 Years of an Intensive Lifestyle Intervention. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neiberg RH, Wing RR, Bray GA, et al. Patterns of weight change associated with longterm weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity (Silver Spring) 2012;20(10):2048–2056. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.