Abstract
Experience-driven synaptic plasticity in the lateral amygdala (LA) is thought to underlie the formation of associations between sensory stimuli and an ensuing threat. However, how the central amygdala (CeA) participates in such learning process remains unclear. Here we show that PKC-δ-expressing CeA neurons are essential for the synaptic plasticity underlying learning in the LA, as they convey information about unconditioned stimulus to LA neurons during fear conditioning.
Adaptive behavioral responses to a threat are dependent on memories linking the threat with its associated environmental cues. Extensive evidence indicates that such memories are formed in the LA, in which the convergence of information about a neutral environmental cue (also known as the conditioned stimulus (CS)) and a threatening event (also known as the unconditioned stimulus (US)), as exemplified in Pavlovian auditory fear conditioning (FC) by pairing of a sound with electrical shock, induces Hebbian plasticity1. This plasticity, expressed as strengthening of the synapses onto LA neurons driven by CS inputs, is considered as a cellular substrate of aversive memory1.
Recent studies demonstrate that the CeA is another amygdala nucleus indispensable for learning during fear conditioning2–8. Nevertheless, how the CeA contributes to the learning process remains unclear. In traditional views, the LA and the CeA are the main input and output, respectively, nuclei of the amygdala, so that information flows from the LA to the CeA1,6. Surprisingly, direct evidence for such serial information processing in FC has been lacking. On the other hand, previous studies have described functions of the CeA – including its involvement in attention or alerting processes – that are independent of the LA9,10, suggesting that the two nuclei are not simply organized in series.
We reasoned that, if information indeed flows serially from the LA to the CeA, then inhibiting the CeA should leave the FC-induced LA synaptic plasticity intact. To test this hypothesis, we inhibited the major classes of CeA neurons in mice with the tetanus toxin light chain (TeLC), which blocks neurotransmitter release (see Methods). We first targeted somatostatin-expressing (SOM+) neurons in the lateral CeA (CeL) by bilaterally injecting the CeL of Som-Cre mice, in which the Cre recombinase is expressed under the endogenous Som promoter2,4, with an adeno-associated virus (AAV) expressing the TeLC-GFP or GFP (as a control) in a Cre-dependent manner (see Methods) (Supplementary Fig. 1a–f). In the same mice we also injected the auditory thalamus (the medial geniculate nucleus, MGN), which transmits CS information in auditory FC, with an AAV expressing the light-gated cation channel channelrhodopsin (ChR2) (see Methods) (Supplementary Fig. 1a, f).
These mice were subsequently subjected to auditory FC followed by preparation of acute brain slices, in which we recorded from LA neurons the AMPA receptor (A) and the NMDA receptor (N) mediated components of synaptic responses evoked by light-stimulation of MGN axons (Supplementary Fig. 1a–c). Inhibition of SOM+ CeL neurons did not affect the FC-induced synaptic strengthening, measured as an increase in A/N ratio11, onto LA neurons (Supplementary Fig. 1b, c). This manipulation did, however, cause impairment in conditioned freezing behavior (Supplementary Fig. 1d), an effect that is consistent with our previous findings2,4.
We next examined the effects of inhibiting protein kinase C-δ-expressing (PKC-δ+) CeL neurons, another major population in the CeL2, with the TeLC in Prkcd-Cre mice that express Cre in PKC-δ+ CeL neurons12 (Fig. 1a–d, Supplementary Fig. 2, Supplementary Fig. 3a–d). To our surprise, inhibiting PKC-δ+ CeL neurons completely abolished the FC-induced synaptic strengthening onto LA neurons (Fig. 1a–c). Notably, unilateral inhibition of PKC-δ+ CeL neurons was sufficient to abolish the synaptic strengthening in the ipsilateral LA, while leaving that in the contralateral LA intact (Fig. 1b, c). Furthermore, bilateral inhibition of PKC-δ+ CeL neurons drastically impaired conditioned freezing (Fig. 1d; Supplementary Fig. 2, Supplementary Fig. 3a, b), while unilateral inhibition of these neurons was less effective (Supplementary Fig. 3a). Bilateral inhibition of PKC-δ+ CeL neurons also completely abolished conditioned lick-suppression7 (Supplementary Fig. 4). Such conditioned suppression of action, like the conditioned freezing, has been shown to depend on both LA plasticity11 and CeL function7.
Fig. 1. PKC-δ+ CeL neurons are required for plasticity underlying learning in the LA.
(a) A schematic of the experimental configuration. Pyramidal neurons in the dorsal LA were chosen for recording. (b) Example traces of the excitatory postsynaptic currents (EPSCs). (c) Quantification of A/N (from left to right: n = 20 cells/3 mice, 22 cells/3 mice, 9 cells/2 mice, 29 cells/4 mice, 30 cells/4 mice; F(4,105) = 15.28, P < 0.0001; ****P < 0.0001, n.s., not significant (P > 0.05), compared with the control naïve group; one-way ANOVA followed by Bonferroni’s test). (d) Quantification of freezing behaviour (GFP, n = 11, TeLC, n = 11, F(1,20) = 57.88, P < 0.0001; ****P < 0.0001, ***P < 0.001, two-way RM-ANOVA followed by Bonferroni’s test). (e) A schematic of the experimental configuration. (f) Quantification of freezing behaviour (GFP, n = 3, Arch, n = 4, F(1,5) = 52.41, P < 0.001; *P < 0.05, **P < 0.01, ****P < 0.0001, n.s., not significant (P > 0.05); two-way RM-ANOVA followed by Bonferroni’s test). Note that the light illumination period coincided with the duration of CS and US presentations in each trial. Data are presented as mean ± s.e.m. in c, d, and f.
The behavioral effect of PKC-δ+ CeL neuron inhibition is likely caused by impairment in learning rather than expression of the defensive responses, as suggested by the impaired LA synaptic plasticity. To verify this possibility we used optogenetics, for which we bilaterally injected the CeL of the Prkcd-Cre mice with a Cre-dependent AAV expressing the light-sensitive proton pump archaerhodopsin (Arch) (see Methods), and subsequently implanted optical fibres above the CeL for light delivery (Fig. 1e, Supplementary Fig. 3e, f). Optogenetic inhibition of PKC-δ+ CeL neurons during conditioning, but not during memory recall, significantly reduced conditioned freezing behavior (Fig. 1f). Of note, we found that optogenetic inhibition of PKC-δ+ CeL neurons in naïve mice did not induce freezing behavior or other aversive responses (Supplementary Fig. 5). Together, these results indicate that the activity of PKC-δ+ CeL neurons is required for both the FC-induced LA synaptic plasticity and learning.
Why are neurons in the CeL required for synaptic plasticity and learning in the LA? In auditory FC, the convergence of sound (CS) and shock (US) onto LA neurons is thought to be a prerequisite for these neurons to undergo synaptic strengthening underlying learning. While sound can reach the LA via the MGN and auditory cortex, the route through which shock is transmitted to the LA remains elusive1. Interestingly, it has recently been shown that CeL neurons, including PKC-δ+ neurons, are the direct postsynaptic targets of the parabrachial nucleus (PBN)13, a brainstem structure that provides nociceptive signals, and that activation of the PBN–CeL pathway is sufficient to drive aversive learning13,14. These findings raise the possibility that PKC-δ+ CeL neurons may participate in relaying US information from the PBN during FC.
As a first step to test this possibility, we performed another optogenetic inhibition experiment, in which we restricted the inhibition of PKC-δ+ CeL neurons to the period of US presentation during conditioning. This manipulation was sufficient to impair the formation of fear memory (Supplementary Fig. 6), supporting a critical role of the PKC-δ+ CeL neurons in processing US.
Next, we examined whether and how PKC-δ+ CeL neurons might respond to US. We delivered GCaMP6, the genetically encoded calcium indicator15, into PKC-δ+ neurons by injecting the CeL of the Prkcd-Cre;Som-Flp mice with an intersectional AAV-Con/Foff-GCaMP6m16. This strategy ensures specific infection of PKC-δ+ neurons and avoids infection of a small fraction of CeL neurons expressing both PKC-δ and SOM2. The same mice were implanted in the CeL with gradient-index (GRIN) lenses, through which the calcium signals could be recorded using a miniature integrated fluorescence microscope (Fig. 2a)17. We subsequently trained these mice in the conditioned lick-suppression task (Supplementary Fig. 4)7 while imaging PKC-δ+ CeL neuron calcium responses (Fig. 2a; Supplementary Fig. 7).
Fig. 2. The CS and US responses in PKC-δ+ CeL neurons during fear conditioning.
(a) A schematic of the experimental configuration. (b) Heat-maps of the average temporal calcium activities of all CS-responsive PKC-δ+ CeL neurons (data from 3 mice) for each trial during habituation, conditioning, and recall. Dashed lines indicate the timing of CS or US. (c) Average CS-induced responses in the same neurons as those in (b) for each trial. Shaded areas represent s.e.m. (d) CS responses of the same neurons as those in (c), averaged for all trials during habituation, conditioning, or recall (F(2,100) = 17.97, P < 0.0001; ***P < 0.001, ****P < 0.0001, n.s., not significant (P > 0.05); one-way ANOVA followed by Bonferroni’s test). (e) A heat-map of the average temporal calcium activities of all US-responsive PKC-δ+ CeL neurons for each trial during conditioning. Dashed lines indicate the timing of CS or US. (f) The time course (left) and peak amplitude (right) of US-evoked responses in the same neurons as those in (e), averaged for the trials in different stages of conditioning (n = 93 cells, 3 mice; F(1.5,140.2) = 26.41, P < 0.0001; **P < 0.01, ****P < 0.0001; one-way RM-ANOVA followed by Bonferroni’s test; shaded areas in (f) represent s.e.m.). Data are presented as mean ± s.e.m. in c, d, and f.
We identified active PKC-δ+ CeL neurons based on their spontaneous activities and CS or US responses (Supplementary Fig. 7 & 8; Video 1). The fractions of neurons showing CS-evoked responses were 10.2% (16/157), 20.0% (38/190), and 26.5% (49/185) during habituation, conditioning, and recall, respectively (P < 0.05, χ2 test with Bonferroni’s correction, comparing habituation with other groups) (Fig. 2b–d). On average, the CS responses of these neurons desensitized during habituation, but recovered quickly following US presentations during conditioning, and were enhanced during recall (Fig. 2b–d). These results indicate that PKC-δ+ CeL neurons acquire CS responses following FC.
During conditioning, 48.9% (93/190) of PKC-δ+ CeL neurons showed prominent US-evoked responses (Fig. 2b, e, f; Supplementary Fig. 8). Interestingly, these responses decreased as conditioning progressed (Fig. 2b, e, f; 39/93 cells (42%) showed significant decrease, P < 0.05), consistent with theories and evidence that instructive US signals are suppressed when they become expected9,18–20. To specifically test the effect of expectation, we tracked the responses of each PKC-δ+ CeL neuron to a series of US presentations, some of which were signaled by the CS while the others were delivered unexpectedly (Supplementary Fig. 9a, b). We found that, among all the US-responsive neurons, 21.1% had stronger responses, while 8.8% showed weaker responses to unsignaled than to signaled US (Supplementary Fig. 9b–e). These results demonstrate that about half of PKC-δ+ CeL neurons show robust US responses, with a subpopulation of these neurons having US responses suppressed by expectation, the property of a teaching signal9,18–20.
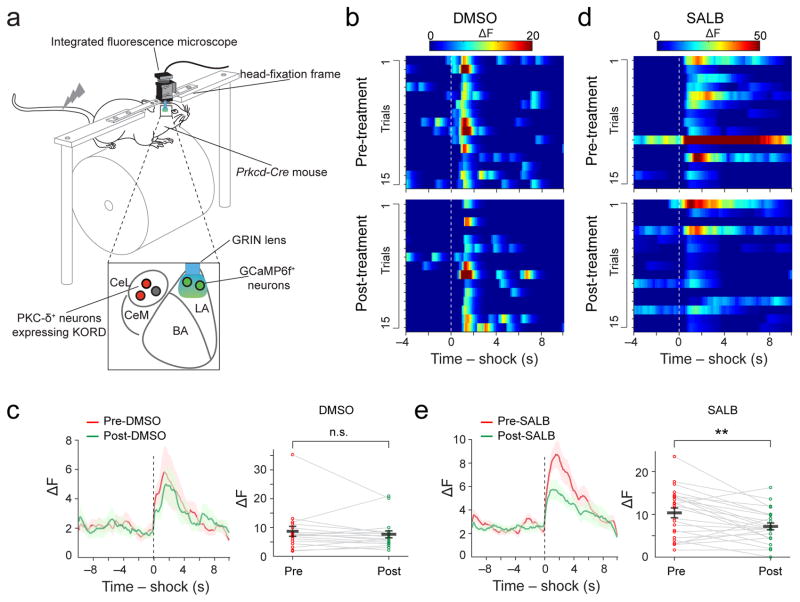
To examine the role of PKC-δ+ CeL neurons in conveying the US to the LA, we implanted GRIN lenses in the LA of Prkcd-Cre mice, in which the GCaMP6 was expressed in LA neurons and in which an inhibitory DREADD (Designer Receptor Exclusively Activated by Designer Drug) derived from the kappa-opioid receptor (KORD) (see Methods) (Supplementary Fig. 10) was selectively expressed in PKC-δ+ CeL neurons (Fig. 3A, Supplementary Fig. 11) by AAVs. This strategy allowed us to track the US responses of the same LA neurons (Supplementary Fig. 12a–e; Video 2) before and after transiently inhibiting PKC-δ+ CeL neurons with systemically applied salvinorin B (SALB), the agonist of KORD (see Methods) (Fig. 3). We found that LA neurons had more delayed shock responses than PKC-δ+ CeL neurons (Supplementary Fig. 12f). Notably, inhibition of PKC-δ+ CeL neurons suppressed shock-evoked responses of LA neurons (Fig. 3b–e). These results indicate that PKC-δ+ CeL neurons play an important role in conveying US signal to the LA.
Fig. 3. PKC-δ+ CeL neurons are required for the US responses of LA neurons.
(a). A schematic of the experimental configuration. (b) Heat-maps of the temporal calcium activities of a representative LA neuron, before (top panel) and after (bottom panel) DMSO application. The dashed line indicates the onset of US. (c) Left: average temporal activities of all shock-responsive LA neurons aligned to shock onset (dashed line), before and after DMSO treatment. Shaded areas represent s.e.m. Right: scatter plot of the peak shock responses of each of the neurons before and after DMSO treatment (T(17) = 0.93, n.s., P > 0.05; paired t test; n = 18 neurons/4 mice; 18/123 (15%) of LA neurons showed shock responses). (d & e) Same as in (b & c), except that SALB was applied instead of DMSO to the same mice in different imaging sessions (T(23) = 3.5, **P < 0.01; paired t test; n = 24 neurons/4 mice; 24/143 (17%) of LA neurons showed shock responses). Data are presented as mean ± s.e.m. in c and e.
If PKC-δ+ CeL neurons convey US during FC, then they may also carry negative emotional valence and, moreover, be able to instruct learning. Indeed, we found that inhibition of these neurons with the TeLC reduced animals’ reactions to electrical shocks (Supplementary Fig. 13a), suggesting that these neurons are important for processing the affective component of the US. Conversely, optogenetic activation of PKC-δ+ CeL neurons induced aversion in mice in a real-time place aversion task (Supplementary Fig. 13b, c; Supplementary Fig. 14a). Furthermore, in a conditioned place aversion task, pairing optogenetic activation of PKC-δ+ CeL neurons with one side of a chamber caused mice to avoid that side when tested in the following day (Supplementary Fig. 13d; Supplementary Fig. 14b). Together, these results indicate that PKC-δ+ CeL neurons convey aversive information and are sufficient to drive aversive learning.
To identify the potential routes through which PKC-δ+ CeL neurons may convey US information to the LA, we conducted anatomic tracing experiments. We first employed our recently developed anterograde transsynaptic herpes simplex virus type 1 strain 129 that expresses four copies of GFP (H129-G4) (so that it is bright enough for direct visualization of labeled neurons) (see Methods), and injected it into the CeL. This resulted in the labeling of cells in a number of brain regions downstream of CeL neurons (Supplementary Fig. 15). We next specifically infected PKC-δ+ CeL neurons with an AAV expressing the red fluorescent protein mRuby, and screened for areas innervated by axon fibers originating from these neurons (Supplementary Fig. 16). The regions identified by both methods, including BNST, SI, CeM, SNc, RRF, and PBN, are the potential postsynaptic targets of PKC-δ+ CeL neurons (Supplementary Fig. 15 & 16). Finally, by using retrograde tracing methods we revealed that, among these regions, the SI, SNc, and RRF send direct projections to the BLA (Supplementary Fig. 17a–h), and that most of those BLA-projecting SNc neurons are dopaminergic (Supplementary Fig. 17f–h). Thus, neurons in these areas are good candidates that can relay US information from PKC-δ+ CeL neurons to the LA. Interestingly, it has been shown that midbrain dopamine neurons, including those in the SNc (Supplementary Fig. 17f–h), play an important role in fear learning, and that CeA neurons preferentially innervate GABAergic neurons over dopamine neurons in the SNc/VTA (see Supplementary Fig. 18). Therefore, it is likely that PKC-δ+ CeL neurons drive disinhibition of dopamine neurons in response to US, thereby instructing learning in the LA (Supplementary Fig. 18). Alternatively, or additionally, neurons in the SI or the RRF may also mediate the function of PKC-δ+ CeL neurons.
Altogether, our results indicate that PKC-δ+ CeL neurons play an important role in conveying information about US to the LA during FC, hence uncovering a previously unknown amygdala functional organization (Supplementary Fig. 18). Our findings also revise a prevailing model for the function of PKC-δ+ CeL neurons, which posits that these neurons are “fear-off” neurons (a CeL population that shows inhibitory CS responses following fear conditioning8) that act to tonically suppress fear responses through inhibition of amygdala output12. In fact, we show that a substantial population of PKC-δ+ CeL neurons are essentially “fear-on” neurons as they convey aversive US signals, drive aversive learning, and are activated by the CS predicting the US. Furthermore, optogenetic silencing of PKC-δ+ CeL neurons did not induce any freezing behavior or aversive responses (Supplementary Fig. 5), which would be expected if these neurons were “fear-off” neurons12. Nevertheless, it is still possible that some of the “fear-off” neurons in the CeL may indeed be PKC-δ+ neurons that evaded detection in our experiments. It is certainly possible that PKC-δ+ CeL neurons are heterogeneous, subserving aversive learning (this study), regulation of feeding and other distinct functions that can be determined, at least in part, by the divergent projections of these neurons (Supplementary Fig. 15 & 16; and see Supplementary Fig. 18) together with the various inputs that they may receive2,4,13.
Methods
Please also see relevant information in the Life Sciences Reporting Summary.
Animals
Before surgery, mice were housed under a 12-h light-dark cycle (7 a.m. to 7 p.m. light) in groups of 2–5 animals, with food and water freely available. Animals with implants were housed singly. All behavioral experiments were performed during the light cycle. The Som-cre21, Prkcd-Cre12, Som-Flp4, Ai32 and Ai3522, and lox-stop-lox-H2B23 mice have all been described elsewhere. The Som-Cre and Som-Flp mice were provided by Dr. Z. Josh Huang. The Prkcd-Cre mice were purchased from the Mutant Mouse Regional Resource Centers (MMRRC) as cryo-preserved spermatozoa (Donor: Dr. Nathaniel Heintz). Other mice were purchased from the Jackson Laboratory. All mice were bred onto C57BL/6J genetic background. The Prkcd-Cre;Som-Flp mice were bred by crossing the Prkcd-Cre mice with Som-Flp mice. Male and female mice of 40–60 d of age were used for all the experiments. All procedures involving animals were approved by the Institute Animal Care and Use Committees of Cold Spring Harbor Laboratory and Wuhan Institute of Virology, Chinese Academy of Sciences.
Viral vectors
The AAV-DIO-TeLC-GFP (the AAV expressing the tetanus toxin light chain (TeLC), which blocks neurotransmitter release24 in a Cre-dependent manner; DIO, double-floxed inverse open reading frame), AAV-DIO-GFP, AAV-CAG-ChR2(H134R)-eYFP, AAV-DIO-ArchT-GFP, AAV-DIO-ChR2(H134R)-eYFP, and AAV-hSyn-GCaMP6f viruses (all serotype 2/9) were made by the Penn Vector Core (Philadelphia, PA). The AAV-hSyn-DIO-HA-KORD-IRES-mCitrine (2/8) virus was made by the University of North Carolina Vector Core (Chapel Hill, NC). The AAVdj-hSyn-Con/Foff-GCaMP6m and AAVdj-hSyn-Con/Foff-hChR2-mCherry viruses were made by the Stanford Vector Core (Stanford, CA). The H129-G4 virus was produced at Wuhan Institute of Virology, Chinese Academy of Sciences, as previously described25. The retrograde canine adenovirus expressing Cre recombinase (CAV2-Cre)26 was purchased from Montpellier vector platform (Plateforme de Vectorologie de Montpellier (PVM), Biocampus Montpellier, Montpellier, France). All viruses were stored in aliquots at –80 °C until use. For AAVs, we waited for at least 5 weeks after injection for optimal viral expression; for the H129-G4, we waited for 3 days before examining the tracing results; for the CAV2-Cre, we waited for 4 weeks.
Stereotaxic surgery
Standard surgical procedures were followed for stereotaxic injection2,4. Briefly, mice were either anesthetized with ketamine (100 mg per kg of body weight) supplemented with dexmedetomidine hydrochloride (0.4 mg per kg), or anesthetized with isoflurane (using 2% at the beginning and 0.5–1% for the rest of the surgery procedure). Mice were positioned in a stereotaxic injection frame and laid on a heating pad maintained at 35°C. A digital mouse brain atlas was linked to the injection frame to guide the identification and targeting (Angle Two Stereotaxic System, myNeuroLab.com).
Viruses (~0.3 μl) were delivered with a glass micropipette (tip diameter, ~5 μm) through a skull window (1–2 mm2) by pressure applications (5–20 psi, 5–20 ms at 0.5 Hz) controlled by a Picrospritzer III (General Valve) and a pulse generator (Agilent). The injection was performed using the following stereotaxic coordinates for the CeL: −1.22 mm from Bregma, 2.9 mm lateral from the midline, and 4.6 mm vertical from skull surface; for the LA: −1.55 mm from Bregma, 3.2 mm lateral from the midline, and 4.2 mm vertical from skull surface; for the MGN: −3.16 mm from Bregma, 1.90 mm lateral from the midline, and 3.20 mm vertical from skull surface.
For the in vivo photostimulation experiments, immediately after viral injection, mice were bilaterally implanted with optical fibers (core diameter, 105 μm; Thorlabs, Catalog number FG105UCA) that were placed above the CeL (coordinates of the fiber tip: −1.22 mm from Bregma, 2.9 mm lateral from the midline, and 4.3 mm vertical from skull surface). The optical fiber together with the ferrule (Thorlabs) was secured to the skull with C&B-Metabond Quick adhesive luting cement (Parkell Prod), followed by dental cement (Lang Dental Manufacturing).
For the in vivo imaging experiments, immediately after viral injection, a GRIN lens (diameter, 500 μm; ~8.4mm length, part ID 130-000152; Inscopix) was implanted 200 μm above the center of injection.
For animals used in experiments under head fixation, following the above procedures, a small piece of metal bar was mounted on the skull of each mouse, which was used to hold the mouse in the head fixation frame during experiments.
Immunohistochemistry
Immunohistochemistry experiments were performed following standard procedures. Briefly, mice were anesthetized with Euthasol (0.4 ml; Virbac, Fort Worth, Texas, USA) and transcardially perfused with 40 ml of PBS, followed by 40 ml of 4% paraformaldehyde in PBS. Brains were extracted and further fixed in 4% PFA overnight followed by cryoprotection in a 30% PBS-buffered sucrose solution for 36 h at 4 °C. Coronal sections (40 or 50 μm thickness) were cut using a freezing microtome (Leica SM 2010R, Leica). Sections were first washed in PBS (3 × 5 min), incubated in PBST (0.3% Triton X-100 in PBS) for 30 min at room temperature (RT) and then washed with PBS (3 × 5 min). Next, sections were blocked in 5% normal goat serum in PBST for 30 min at RT and then incubated with primary antibodies overnight at 4 °C. Sections were washed with PBS (5 × 15 min) and incubated with fluorescent secondary antibodies at RT for 1 h. After washing with PBS (5 × 15 min), sections were mounted onto slides with Fluoromount-G (eBioscience, San Diego, California, USA). Images were taken using a LSM 780 laser-scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). The primary antibodies used were: mouse anti-PKC-δ (BD Biosciences, NJ, USA, cat. # 610398); rabbit anti-HA-Tag (C29F4, Cell Signaling, Danvers, MA, USA, cat. # 3724S); rabbit anti-TH (tyrosine hydroxylase) antibody (Millipore, Billerica, MA, USA, cat. # AB152). The fluorophore-conjugated secondary antibody used was Alexa Fluor® 594 donkey anti-rabbit IgG (H+L) or Alexa Fluor® 488 goat anti-rabbit IgG (H+L) (Life Technologies, Carlsbad, California, USA; catalogue number A21207 or A11008, respectively), depending on the desired fluorescence color. All antibodies used in this study have been validated by previous studies2–4,27,28.
In vitro electrophysiology
To assess the synaptic plasticity in LA neurons induced by auditory fear conditioning, we specifically examined the synaptic transmission onto these neurons driven by the auditory thalamus – the medial geniculate nucleus (MGN) – that conveys the conditioned stimulus to the LA. To this end, we used mice in which the MGN was injected with the AAV-CAG-ChR2(H134R)-YFP, so that the MGN–LA pathway can be optogenetically stimulated in acute slices. Patch clamp recording was performed as previously described2,4. Briefly, 24 h following fear conditioning and immediately after the recall test (or 24 h following habituation for the naïve group), mice were anesthetized with isoflurane before they were decapitated; their brains were then dissected out and placed in ice chilled dissection buffer (110 mM choline chloride, 25 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM KCl, 0.5 mM CaCl2, 7.0 mM MgCl2, 25.0 mM glucose, 11.6 mM ascorbic acid and 3.1 mM pyruvic acid, gassed with 95% O2 and 5% CO2). An HM650 Vibrating-blade Microtome (Thermo Fisher Scientific) was then used to cut 300 μm thick coronal sections that contained the amygdala. These slices were subsequently transferred to a storage chamber that contained oxygenated artificial cerebrospinal fluid (ACSF) (118 mM NaCl, 2.5 mM KCl, 26.2 mM NaHCO3, 1 mM NaH2PO4, 20 mM glucose, 2 mM MgCl2 and 2 mM CaCl2, at 34 °C, pH 7.4, gassed with 95% O2 and 5% CO2). Following 40 min of recovery, slices were transferred to RT (20–24 °C), where they were continuously bathed in the ACSF.
Visually guided whole-cell patch clamp recording from LA neurons was obtained with Multiclamp 700B amplifiers and pCLAMP 10 software (Molecular Devices, Sunnyvale, California, USA), and was guided using an Olympus BX51 microscope equipped with both transmitted and epifluorescence light sources (Olympus Corporation, Shinjuku, Tokyo, Japan). LA pyramidal neurons were identified for patching. Light-stimulation was used to evoke excitatory postsynaptic currents (EPSCs) driven by the ChR2-expressing axons originating from the MGN. The light source was a single-wavelength LED system (λ = 470 nm; CoolLED.com) connected to the epifluorescence port of the Olympus BX51 microscope. Light pulses of 0.2–0.5 ms, triggered by a TTL signal from the Clampex software (Molecular Devices), were used to evoke synaptic transmission. Synaptic responses were low-pass filtered at 1 KHz and recorded at holding potentials of –70 mV (for AMPA-receptor-mediated responses) and +40 mV (for NMDA-receptor-mediated responses). The AMPA/NMDA (A/N) ratio was calculated as the ratio of peak current at –70 mV to the current at 100 ms after light-stimulation onset at +40 mV29. Recordings were made in the ACSF with picrotoxin (100 μM) added. The internal solution contained 115 mM caesium methanesulphonate, 20 mM CsCl, 10 mM HEPES, 2.5 mM MgCl2, 4 mM Na2ATP, 0.4 mM Na3GTP, 10 mM sodium phosphocreatine and 0.6 mM EGTA (pH 7.2). The EPSCs were analysed using pCLAMP10 software (Molecular Devices).
All cells that met the standard criteria (leak current < 50 pA, access resistance < 30 mΩ, input resistance > 10 x access resistance) were selected for analysis. We typically recorded up to 6 cells/slice and 2–3 slices/mouse.
Behavioral tasks
Fear conditioning measuring conditioned freezing
We followed standard procedures for conventional auditory fear conditioning2–4. Briefly, mice were initially handled and habituated to a conditioning cage, which was a mouse test cage (18 cm × 18 cm × 30 cm) with an electrifiable floor connected to a H13–15 shock generator (Coulbourn Instruments). The test cage was located inside a sound attenuated cabinet (H10–24A; Coulbourn Instruments). Before each conditioning session the test cage was wiped clean with 70% ethanol. During conditioning the cabinet was illuminated and the behaviour was captured with a monochrome CCD-camera (Panasonic WV-BP334) at 3.7 Hz and stored on a personal computer. The FreezeFrame software (Coulbourn Instruments) was used to control the delivery of both tones and foot shocks. For habituation, five 4-kHz, 75-dB tones (conditioned stimulus), each of which was 30 s in duration, were delivered at variable intervals. During conditioning, mice received five presentations of the conditioned stimulus, each of which co-terminated with a 2-s, 0.7-mA foot shock (unconditioned stimulus). The recall of fear memory was tested 24 h following conditioning in a novel illuminated context, where mice were exposed to two presentations of unreinforced conditioned stimulus (120 s inter-stimulus interval). The novel context was a cage with a different shape (22 cm × 22 cm × 21 cm) and floor texture compared with the conditioning cage. Prior to each use the floor and walls of the cage were wiped clean with 0.5% acetic acid to make the scent distinct from that of the conditioning cage. Freezing responses to the conditioned stimuli were analyzed with FreezeFrame software (Coulbourn Instruments). The average of the freezing responses to the two conditioned stimuli during recall was used as an index of the conditioned fear.
Fear conditioning measuring conditioned lick-suppression
As previously described7, water deprivation started 23 hours before training. Mice were trained to stay on a movable wheel under head fixation for 30 minutes in the first day, and 10 minutes daily afterwards. A metal spout was placed in front of animal mouth for water delivery. The spout also served as part of a custom “lickometer” circuit, which registered a lick event each time a mouse completed the circuit by licking the spout. The lick events were recorded by a computer through a custom software written in LabView (National Instruments). Each lick triggered a single opening of a water valve calibrated to deliver 0.3 μl water. It took mice 4–7 days to achieve stable licking, the criterion for which was 10-minute continuous lick without any gap longer than 10 s.
Mice with stable licking behavior were first subjected to sound habituation sessions (1 session/day for two days), during which auditory stimuli were presented through a computer speaker in each trial. Each stimulus was composed of 5 pips of pure tone (8 kHz, 70 dB). Pip duration was 250 ms, and inter-pip-interval was 750 ms. Each of the habituation sessions contained 15 trials with variable inter-trial-intervals (30–50 s). 24 h following habituation, mice were conditioned for 15 trials with variable inter-trial-intervals (30–50 s). In each of these trials, the auditory stimulus (CS) was presented and followed immediately by a tail shock (US; 100 μA for 500 ms), which was generated from an isolator (ISO-Flex, A.M.P. Instruments LTD, Israel) and delivered through a pair of wires secured to the tail with silicone tubing. The shock was shortened to 50 ms in the imaging experiments to minimize motion artifact.
We used a lick suppression index to quantify animals’ performance in this task: Lick suppression index = (LPRE – LCS)/(LPRE + LCS), where LPRE is the number of licks in the 5 s period before CS onset, and LCS is the number of licks in the 5 s CS period7.
Real time place aversion (RTPA)
As previously described27, one side of a custom chamber (23 × 33 × 25 cm; made from plexiglass) was assigned as the stimulation zone, counterbalanced among mice. Mice were placed individually in the middle of the chamber at the onset of the experiment, the duration of which was 20 min. Laser stimulation (5-ms pulses delivered at 5, 10, or 30 Hz) was triggered when mice entered the stimulation zone, and lasted until mice exited the stimulation zone. Mice were videotaped with a CCD camera interfaced with the Ethovision software (Noldus Information Technologies), which was used to control the laser stimulation and extract the behavioral parameters (position, time, distance, and velocity).
Conditioned place aversion
The same chamber as that for the RTPA was used for the conditioned place aversion test. To make the two sides of the chamber distinct from each other, each side was decorated with a unique visual pattern (dotted vs striped) and scented with a unique odor (cherry vs. blueberry). The test consisted four sessions, each per day for four consecutive days. In session 1, the habituation session, mice were individually placed in the center of the chamber and allowed to freely explore both sides. In session 2 and 3, the conditioning sessions, the mice received 10 trials of laser stimulation, each consisting of a10-s train of 30-Hz 5-ms pulses, in one side of the chamber that was chosen as the stimulation side (counterbalanced among mice). The exit of the stimulation side was blocked during the conditioning. In session 4, the recall session, the mice were allowed to freely explore both sides of the chamber. The mice were videotaped in session 1 and 4 with a CCD camera interfaced with the Ethovision software (Noldus Information Technologies), which was used to control the laser stimulation and extract the behavioral parameters (position, time, distance, and velocity)27.
In vivo optogenetics
We used the light-gated cation channel channelrhodopsin (ChR2)30 and the light-sensitive proton pump archaerhodopsin (Arch)31 for optogenetic activation and inhibition, respectively, of neuronal activities. For bilateral optogenetic stimulation in the CeL in behaving mice, a rotary joint (Doric Lenses, Inc., Quebec, Canada, Catalog number FRJ_1x2i_FC-2M3_0.22) was used in the light delivery path, with one end of the rotary joint connected to a laser source (λ = 473 or 532 nm, OEM Laser Systems) and the other end, which has two terminals, to two optical fibers (for bilateral stimulation) through sleeves (Thorlabs). This configuration allows the mice carrying fiber-optic implants to freely move during optogenetic stimulation. The stimulation was typically composed of 5-ms 30-Hz light pulses delivered for various durations, unless otherwise specified. Laser intensity was 10 mW measured at the end of optical fibers.
In vivo calcium imaging and analysis
We followed a recently described procedure for the in vivo imaging experiments17. All imaging experiments were conducted on awake behaving mice under head fixation in a dark, sound attenuated box. GCaMP6 fluorescence signals were imaged using a miniature, integrated fluorescence microscope system (Inscopix, Palo Alto, CA) with GRIN lenses implanted in the target areas (CeL and LA). We imaged PKC-δ+ CeL neuron activities while subjecting the mice to sound habituation, conditioning and recall sessions in the conditioned lick suppression task. Each session contained 15 trials, with random inter-trial-intervals (10–30 s). The same mice were subsequently used to image PKC-δ+ CeL responses to signaled and unsignaled shocks. A total of 16 shocks, 8 signaled and 8 unsignaled, were delivered in a randomly interleaved manner. In addition, the assignment of the first trial as having a signaled or unsignaled shock was counterbalanced among the mice.
We also imaged LA neuron responses to shocks before and after transient inhibition of PKC-δ+ CeL neurons with chemogenetics, in which we used an inhibitory DREADD (Designer Receptor Exclusively Activated by Designer Drug) derived from the kappa-opioid receptor (KORD)32, and applied salvinorin B (SALB) subcutaneously (s.c.) (10 mg/kg of body weight) to activate KORD32. As a control experiment, we imaged LA neuron responses to shocks before and after systemic application (s.c.) of DMSO (the vehicle of SALB). Each session contained 15 trials.
For the experiments in which PKC-δ+ CeL neurons were imaged, we installed a baseplate on top of the GRIN lens for each mouse, as described previously17. Before imaging, the miniature microscope was attached to the baseplate. The microscope was adjusted such that the best dynamic fluorescence signals were at the focal plane, which was subsequently kept constant across imaging sessions. For the experiments in which LA neurons were imaged, the microscope was mounted on top of, and aligned with the GRIN lens with a custom adjustable micromanipulator that allows movement in all 3 axes. The focus of the microscope was adjusted through the micromanipulator to get the best focal plane as described above.
During imaging, the Data Acquisition Box of the nVista Imaging System (Inscopix, Palo Alto, CA) was triggered by an NI data acquisition device (USB6008, National Instruments, CA). Compressed gray scale images were then recorded with nVistaHDV2 (Inscopix) at 10 frames per second. The analog gain (1 to 5) and LED output power (8% to 30% of the maximum) of the microscope were set to be constant for the same subject across imaging sessions. During imaging, the time stamps of different events, including the trigger signals sent to the microscope, the auditory stimuli, the electrical shocks, and the licks, were recorded with a custom program written in LabView (National Instruments, CA).
For imaging data processing and analysis, we began by importing the compressed video files into Mosaic (version 1.0.0b; Inscopix, Palo Alto, CA), in which we trimmed the first frame of the video for each trial to minimize the influence of the flash associated with LED light onset. We subsequently used Mosaic to combine all the trimmed video files into a single .tiff stack, apply a 4-pixel bin to the .tiff stack, and correct the motion artifact. A new .tiff stack was then saved for further processing.
Next, to address the problem of high levels of background fluorescence signals intrinsic to one-photon imaging, we applied our newly developed imaging analysis method, the extended Constrained Nonnegative Matrix Factorization (CNMF-E)33, in which we model the background with two realistic components: (1) one models the constant baseline and slow trends of each pixel, and (2) the other models the fast fluctuations from out-of-focus signals and is therefore constrained to have low spatial-frequency structure. This decomposition avoids cellular signals being absorbed into the background term. After subtracting the background approximated with this model, we used Constrained Nonnegative Matrix Factorization (CNMF)34 to demix neural signals and get their denoised and deconvolved temporal activity, termed as ΔF.
Code availability
The CNMF-E method was carried out using a custom Matlab algorithm (for a detailed description and availability of this method, see33).
Once the temporal activity of the neurons was extracted, we characterized the CS (sound) or US (shock) responses of each neuron using auROC (area under the receiver-operating characteristic curve) analysis, in which we compared the average ΔF value during the baseline period (2 s immediately prior to the delivery of CS or US) with that during CS or US presentations (2 s immediately after the onset of CS or US) in each trial by moving a criterion from zero to the maximum ΔF value. We then plotted the probability that the ΔF values during CS or US presentations was greater than the criteria against the probability that the baseline ΔF values was greater than the criteria. The area under this curve quantifies the degree of overlap between the two ΔF distributions (i.e., the discriminability of the two). A permutation test (iteration 5000 times) was used to determine whether the average ΔF values during CS or US presentations were significantly (P < 0.05) higher than during baseline, and thus classify a neuron as being CS- responsive or US-responsive, respectively. The peak CS or US response amplitude in each trial was determined by searching the maximum value within a 3.75 or 5-s, respectively, time window immediately after stimulus onset.
Anterograde transsynaptic tracing with H129-G4 and serial two-photon tomography (STPT)
H129-G4 viral injection and brain sample preparation were performed at Wuhan Institute of Virology, Chinese Academy of Sciences. STPT imaging was performed at Fudan University. Mice were transcardially perfused with saline and 4% paraformaldehyde (PFA) at 72 h after injecting the CeL with the H129-G4. The brains were further fixed in 4% PFA at 4°C overnight, followed by 2–4 days in 0.1 M phosphate buffer (PB) with 30% sucrose at 4°C for dehydration. The brains were subsequently stored in PB at –20°C until imaging. Detailed information about STPT imaging and related analysis procedures is described previously35,36. Briefly, brain was embedded in 4% oxidized agarose and crosslinked by sodium borohydrate. The embedded brain was placed on the motorized stage in tissuecyte 1000 (Tissuevision) and the whole-brain was imaged at a resolution of 1 μm at the x–y plane for a series of 280 z sections with 50 μm inter-section-interval. Both the signal from the green channel (GFP signal) and that from the red channel (background) were simultaneously acquired, and the latter was used to subtract background from the green channel to enhance signal to noise ratio.
Retrograde tracing
For retrograde tracing, the CAV2-Cre (~0.25 μl) or cholera toxin subunit B (CTB) (~0.1 μl) was injected into the LA of lox-stop-lox-H2B or wild-type mice, respectively. We waited for 4 weeks (for CAV2-Cre) or 1 week (for CTB) before examining the tracing results.
Statistics and data presentation
All statistics are indicated where used. Statistic analyses were performed with Origin8 Software (OriginLab Corporation, Northampton, MA), GraphPad Prism Software (GraphPad Software, Inc., La Jolla, CA), or Matlab (MathWorks, Natick, MA). No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications2–4,7. Normality was tested by D’Agostino-Pearson or Shapiro-Wilk normality test. All tests are two-sided. No randomization was used to assign experimental groups, but mice were assigned to specific experimental groups without bias. Data collection and analysis were not performed blind to the conditions of the experiments. All experiments were controlled by computer systems, and data were collected and analyzed in an automated and unbiased way. No mice or data points were excluded.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
We thank Dr. Garret D. Stuber for helping with the in vivo imaging experiments. We thank Drs. Garret D. Stuber and Joshua Johansen for critically reading an earlier version of the manuscript, Ga-Ram Hwang for technical assistance, and members of the Li laboratory for helpful discussions. This work was supported by grants from the National Institutes of Health (NIH) (R01MH101214, B.L.), Human Frontier Science Program (RGP0015/2016, B.L.), NARSAD (23169, B.L.; 21227, S.A.), National Natural Science Foundation of China (81428010, B.L. and M.H.; 91432106, M.H.), China Postdoctoral Science Foundation (2016M590316, L.G.), Louis Feil Trust (B.L.), the Stanley Family Foundation (B.L.), Simons Foundation (344904, B.L.), and Wodecroft Foundation (to B.L.).
Footnotes
AUTHOR CONTRIBUTIONS
K.Y. and B.L. designed the study. K.Y., S.A., X.Z., and H.S. conducted experiments (X.Z. performed the experiments in which LA neurons were imaged; S.A. and H.S. performed the experiments in which synaptic plasticity in LA neurons was examined; K.Y. conducted all the rest of the experiments). K.Y., X.Z., S.A., and H.S. analyzed data. C.R., L.F. and K.D. developed the intersectional viral strategy and provided critical reagents. F.Z. and M.H.L. developed the H129-G4 viral system and performed the anterograde tracing with it. L.G. and M.H. performed imaging with the STPT. P.Z. and L.P. developed and assisted with the imaging analysis methods (CNMF and CNMF-E). B.L. wrote the paper with inputs from all authors.
References
- 1.Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- 2.Li H, et al. Experience-dependent modification of a central amygdala fear circuit. Nature Neuroscience. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:2432–2437. doi: 10.1523/JNEUROSCI.4166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penzo MA, et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519:455–459. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- 6.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu K, Garcia da Silva P, Albeanu DF, Li B. Central Amygdala Somatostatin Neurons Gate Passive and Active Defensive Behaviors. J Neurosci. 2016;36:6488–6496. doi: 10.1523/JNEUROSCI.4419-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 9.Roesch MR, Esber GR, Li J, Daw ND, Schoenbaum G. Surprise! Neural correlates of Pearce-Hall and Rescorla-Wagner coexist within the brain. Eur J Neurosci. 2012;35:1190–1200. doi: 10.1111/j.1460-9568.2011.07986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends in Neurosciences. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Nabavi S, et al. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haubensak W, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell. 2015;162:363–374. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M, et al. The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Mol Brain. 2015;8:22. doi: 10.1186/s13041-015-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenno LE, et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resendez SL, et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat Protoc. 2016;11:566–597. doi: 10.1038/nprot.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends in Neurosciences. 2011;34:283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature Neuroscience. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He M, et al. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron. 2012;73:35–48. doi: 10.1016/j.neuron.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray AJ, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng WB, et al. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol Neurodegener. 2017;12:38. doi: 10.1186/s13024-017-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bru T, Salinas S, Kremer EJ. An update on canine adenovirus type 2 and its vectors. Viruses. 2010;2:2134–2153. doi: 10.3390/v2092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson-Jones M, et al. A basal ganglia circuit for evaluating action outcomes. Nature. 2016;539:289–293. doi: 10.1038/nature19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beier KT, et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 31.Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vardy E, et al. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron. 2015;86:936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P, Resendez SL, Stuber GD, Kass RE, Paninski L. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. 2016 doi: 10.7554/eLife.28728. arXiv:1605.07266 [q-bio.NC] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pnevmatikakis EA, et al. Simultaneous Denoising, Deconvolution, and Demixing of Calcium Imaging Data. Neuron. 2016;89:285–299. doi: 10.1016/j.neuron.2015.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ragan T, et al. Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat Methods. 2012;9:255–258. doi: 10.1038/nmeth.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, et al. Whole-Brain Mapping of Neuronal Activity in the Learned Helplessness Model of Depression. Front Neural Circuits. 2016;10:3. doi: 10.3389/fncir.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.