Abstract
Objective
Dementia is a common neurological disease that substantially affects public health. A previous study revealed that dementia occurs when the body’s immune system attacks the cells of the brain, indicating that dementia may be similar to autoimmune rheumatic diseases (ARDs). In the current retrospective cohort study, we focused on middle-aged ARD patients (45 years or older) to investigate the association between ARDs in middle-aged people and dementia by using a nationwide population-based database in Taiwan.
Method
Our study analyzed the medical data of the Taiwanese population from 2001 to 2012, with a follow-up period extending until the end of 2011. We identified middle-aged patients with ARDs by using the Taiwan National Health Insurance Research Database. We selected a comparison cohort from the general population that was randomly frequency-matched by age (in 5-year increments), sex, and index year and further analyzed the dementia risk by using a Cox regression model that considers sex, age, and comorbidities.
Results
The study enrolled 34,660 middle-aged ARD patients (77% female, mean age = 59.8 years) and 138,640 controls. The risk of developing dementia was 1.18 times higher for middle-aged patients with ARDs compared with patients without ARDs after adjustment for age, sex, and comorbidities. Among the patients with ARDs, the subgroups with rheumatoid arthritis, systemic lupus erythematosus, and Sjögren syndrome (SS) were associated with a significantly higher dementia risk (adjusted hazard ratio [HR] 1.14, 95% confidence index [CI] 1.06–1.32; adjusted HR 1.07, 95% CI 0.86–1.34; adjusted HR 1.46, 95% CI 1.32–1.63, respectively). Furthermore, primary SS and secondary SS patients had the highest risks of dementia among all the ADR subgroups (adjusted HR 1.35, 95% CI 1.18–1.54; adjusted HR 1.67, 95% CI 1.43–1.95 respectively).
Conclusion
This nationwide retrospective cohort study demonstrated that dementia risk is significantly higher in middle-aged patients with ARDs compared with the general population.
Introduction
Dementia is a common disorder characterized by a decline in one or more cognitive functions that can impair the performance of daily activities [1]. Alzheimer disease (AD) is the most common type of dementia, accounting for 60% of all dementia cases. Other types of dementia are Parkinson disease dementia, frontotemporal dementia, and Lewy body dementia [2]. All types of neurodegenerative dementia are associated with neuroinflammation, which is characterized by reactive microgliosis, oxidative damage, and mitochondrial dysfunction. Autoimmune rheumatic diseases (ARDs), such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren syndrome (SS), progressive systemic sclerosis, polymyositis, dermatomyositis, vasculitis, and Behçet disease, also result from the dysregulation of the immune system and are characterized by progressive and systemic inflammation. A recent study suggested that dementia may occur when the body’s immune system attacks the cells of the brain, suggesting that some types of dementia may be similar to ARDs [3–4]. Moreover, multiple studies have revealed that ARDs increase the risk of vascular events such as ischemic stroke, acute myocardial infarction, and peripheral arterial occlusive disease [5–10]. Furthermore, several proinflammatory cytokines (IL-1b, IL-6, and TNF-α) participate in and increase the risk of dementia and AD as well as participate in the pathogenesis of ARDs [11–13]. However, most data on the association between ARDs and dementia are from studies with conflicting results that have used a case–control design or are small case series [14–19]. Therefore, the association between ARDs and dementia has not been fully established.
We hypothesize that ARDs predispose patients to the development of dementia. To verify this hypothesis, this cohort study examined the relationship between middle-aged patients (45 years or older) with ARDs and dementia by analyzing a large population-based database.
Methods
Data sources
The National Health Insurance (NHI) program was initiated in 1995 to provide thorough healthcare for citizens and residents of Taiwan. Enrollment in this program is mandatory, resulting in a coverage rate of almost 99% [20]. The Taiwan National Health Insurance Research Database (NHIRD), which is maintained by the Department of Health and the National Health Research Institutes of Taiwan, comprises comprehensive medical care information available for research purposes. This database provides basic information about each person insured by the NHI, including patient characteristics, records of outpatient visits, hospital admissions, drug prescriptions, and disease status and management. The diagnostic codes used are formatted in accordance with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). At the time of this study, the NHIRD was electronic with patients’ personal information being encrypted for privacy protection. The study was approved by the Institutional Review Board of Taipei Medical University (approval number N201509007) and was performed according to the relevant guidelines. Informed consent of the study patients was not required because the dataset used in this study comprised deidentified secondary data released for research purposes. Patient consent was not required to access the NHIRD.
Study patients
Patients diagnosed with ARDs between 2001 and 2012 were identified from the catastrophic illness registry in the NHIRD. In Taiwan, patients with ARDs are eligible for a catastrophic illness certificate after a rheumatology specialist diagnoses them on the basis of clinical manifestations, laboratory data, and the criteria set by the American College of Rheumatology, which is reviewed by rheumatologists commissioned by the NHI. Thus, the catastrophic illness patient data are highly accurate and reliable. Several autoimmune diseases are defined as catastrophic illnesses by the NHI with the related certification requiring precise fulfillment of the following classification criteria: the American College of Rheumatology (ACR) 1997 revised criteria for SLE (ICD-9-CM: 710.0) [21]; the American Rheumatism Association 1987 revised criteria for RA (ICD-9-CM: 714.0) [22]; the ACR criteria for systemic sclerosis (ICD-9-CM: 710.1) [23]; the American–European Consensus Group 2002 revised criteria for SS (ICD-9-CM: 710.2) [24]; the Bohan and Peter 1975 criteria for polymyositis and dermatomyositis (ICD-9-CM: 710.3) [25,26]; the International Study Group 1990 criteria for Behçet disease (ICD-9-CM: 136.1) [27]; and the ACR 1990 criteria for temporal arteritis (ICD-9-CM: 443.1) [28], granulomatosis polyangiitis (ICD-9-CM: 446.4) [29], and Takayasu arteritis (ICD-9-CM: 446.7) [30]. The date of the earliest ARD diagnosis was used as the index date. Patients with a history of dementia or who were younger than 45 years were excluded. Finally, 34,660 patients with ARDs were selected as the study patients and were designated as the ARD cohort. For each ARD patient, four non-ARD patients were randomly selected from the same study period according to the same exclusion criteria and were frequency-matched with the ARD patients according to age and sex to construct the non-ARD cohort, which comprised 138,640 patients.
Outcome measurement and comorbidities
Each study patient was followed until receiving their first diagnosis of any type of dementia by a neurologist during two visits to the outpatient department or one hospital admission. The dementia types included Alzheimer disease (ICD-9-CM: 331.0), arteriosclerotic dementia (ICD-9-CM: 290.4), and unspecified dementia (ICD-9-CM: 290.0–290.3, 294.1, 331.1–331.2, and 331.82). The patients who recorded ICD-9-CM 290.4 in the claims data were classified as having vascular dementia. All remaining patients with dementia who did not belong to the vascular dementia group were defined as having nonvascular dementia. We also differentiated patients with Alzheimer’s disease from those with non-vascular dementia by searching for ICD9-CM code 331.0. The patient was considered lost to follow-up on death, withdrawal from the database, or the end of 2012. At the baseline, major comorbidities such as diabetes (ICD-9-CM 250), hyperlipidemia (ICD-9-CM 272), hypertension (ICD-9-CM 401–405), heart failure (ICD-9-CM 428, I50.2, I50.3), cardiovascular disease (ICD-9 codes 393–398, 410–414, 420–429, 440–449, 451–459), stroke (ICD-9-CM 430–438), major psychosis or a substance-related disorder (ICD-9-CM codes 291–299, 303–305), and traumatic brain injury (ICD-9 CM codes 801–804, 850–854 were considered covariates.
Statistical analyses
This was a nationwide retrospective cohort study. The baseline characteristics were sex, age, and certain comorbidities. The baseline characteristics were matched between the ARD and non-ARD patients according to age and sex and were compared using a chi-square test for categorical variables and a t-test for continuous variables. We observed the time-to-event data in the ARD and non-ARD cases and used a multiple Cox proportional hazards model to explore the association between dementia and the ARDs adjusted for sex, age, and comorbidities. The adjusted hazard ratios (HRs) indicated that after adjusting for covariates, the ARD cases had a higher dementia risk than did the non-ARD cases when HR > 1. The 95% confidence intervals (CIs) of the HRs were also calculated.
To perform a stratified analysis, we separately calculated the incident rate ratio and HRs adjusted for sex, age, comorbidities, and some types of ARDs (including RA, SLE, and SS) for the age groups of less than 65 years and 65 or more years. We used the Kaplan–Meier method to calculate the incidence rates for the ARD, RA, primary SS, secondary SS, and SLE groups over the follow-up period. We then plotted the results in cumulative incidence plots with each comparison group’s cumulative incidence rate. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 34,660 cases of ARDs and 138,640 matched control cases were selected from the NHIRD during the defined period of interest. Of the ARD and the non-ARD patients, 39% were 45–54 years of age and 77% were female (Table 1). The ARD cohort was more likely than the non-ARD cohort to experience diabetes (14.54% vs. 12.98%, p < 0.001), hyperlipidemia (16.66% vs. 14.21%, p < 0.001), psychosis (4.09% vs. 2.4%, p < 0.001), heart failure (4.71% vs. 1.91%, p < 0.001), hypertension (34.99% vs. 27.69%, p < 0.001), stroke (7.18% vs. 4.85%, p < 0.001), traumatic brain injury (1.47% vs. 1.23%, p < 0.001), or cardiovascular disease (34.01% vs. 16.42%, p < 0.001). The mean follow-up period was 5.97 years (standard deviation [SD] 3.05) and 6.37 years (SD 2.95) for the ARD and the non-ARD cohorts, respectively.
Table 1. Baseline characteristics of the ARD patient group and age- and sex-matched comparison group.
| Group | Comparison group | ARD patient group | |||
|---|---|---|---|---|---|
| (N = 138,640) | (N = 34,660) | ||||
| Variable | n | (%) | N | (%) | p value* |
| Sex | 1.000 | ||||
| Male | 31,448 | (22.68) | 7862 | (22.68) | |
| Female | 107,192 | (77.32) | 26,798 | (77.32) | |
| Age, mean (SD) | 59.80 | (10.16) | 59.80 | (10.11) | 0.988 |
| Age group | 0.996 | ||||
| 45–54 | 54,924 | (39.62) | 13,756 | (39.69) | |
| 55–64 | 41,798 | (30.15) | 10,432 | (30.10) | |
| 65–74 | 28,838 | (20.80) | 7203 | (20.78) | |
| ≥75 | 13,080 | (9.43) | 3269 | (9.43) | |
| Comorbidities | |||||
| Diabetes | 17,989 | (12.98) | 5039 | (14.54) | <0.001 |
| Hyperlipidemia | 19,694 | (14.21) | 5575 | (16.66) | <0.001 |
| Psychosis | 3326 | (2.40) | 1419 | (4.09) | <0.001 |
| Heart failure | 2652 | (1.91) | 1634 | (4.71) | <0.001 |
| Hypertension | 38,384 | (27.69) | 12,128 | (34.99) | <0.001 |
| Stroke | 6729 | (4.85) | 2488 | (7.18) | <0.001 |
| Traumatic brain injury | 1712 | (1.23) | 511 | (1.47) | <0.001 |
| Cardiovascular disease | 22,761 | (16.42) | 11,787 | (34.01) | <0.001 |
* p values were calculated using a chi-square test for categorical variables and a t-test for continuous variables
Table 2 lists the dementia incidence densities for the ARD and non-ARD cohorts. During the observation period, 4280 patients in the non-ARD cohort (incidence rate of 48.43 per 10,000 person-years) and 1305 patients in the ARD cohort (incidence rate of 63.08 per 10,000 person-years) developed dementia. When stratified by sex, age, and comorbidities, the patients in the ARD cohort, particularly male patients (HR 1.23, 95% CI 1.09–1.4) and patients aged ≥75 years (HR 1.26, 95% CI 1.13–1.40), were associated with an increased dementia risk.
Table 2. Incidence of dementia and Cox model results for the ARD patient group and the comparison group.
| Comparison group | ARD patient group | |||||
|---|---|---|---|---|---|---|
| Characteristics | Event (%) | Incidence§ | Event (%) | Incidence§ | IRR | Adjusted HRa (95% CI) |
| All | 4280(3.09) | 48.43 | 1305 (3.77) | 63.08 | 1.30 | 1.18 (1.11–1.26)*** |
| Sex | ||||||
| Female | 3211 (3.00) | 46.75 | 969 (3.62) | 59.58 | 1.27 | 1.18 (1.10–1.27)*** |
| Male | 1069 (3.40) | 54.30 | 336 (4.27) | 75.94 | 1.40 | 1.23 (1.09–1.40)** |
| Age | ||||||
| <65 | 828 (0.86) | 12.98 | 259 (1.07) | 16.81 | 1.30 | 1.06 (0.92–1.22) |
| 65–74 | 1894 (6.57) | 107.23 | 567 (7.87) | 145.00 | 1.35 | 1.20 (1.09–1.32)*** |
| ≥75 | 1558 (11.91) | 225.59 | 479 (14.65) | 349.00 | 1.55 | 1.26 (1.13–1.40)*** |
| Diabetes | ||||||
| No | 3164 (2.62) | 40.53 | 985 (3.33) | 54.82 | 1.35 | 1.21 (1.12–1.30)*** |
| Yes | 1116 (6.20) | 108.15 | 320 (6.35) | 117.57 | 1.09 | 1.06 (0.94–1.21) |
| Hyperlipidemia | ||||||
| No | 3408 (2.87) | 44.33 | 1061 (3.67) | 60.96 | 1.38 | 1.23 (1.14–1.32)*** |
| Yes | 872 (4.43) | 75.87 | 244 (4.23) | 74.28 | 0.98 | 1.00 (0.87–1.16) |
| Psychosis | ||||||
| No | 3962 (2.93) | 45.79 | 1164 (3.50) | 58.40 | 1.28 | 1.20 (1.12–1.28)*** |
| Yes | 318 (9.56) | 171.32 | 141 (9.94) | 186.48 | 1.09 | 1.06 (0.87–1.30) |
| Hypertension | ||||||
| No | 1912 (1.91) | 29.10 | 568 (2.52) | 40.55 | 1.39 | 1.32 (1.20–1.45)*** |
| Yes | 2368 (6.17) | 104.50 | 737 (6.08) | 110.31 | 1.06 | 1.05 (0.97–1.14) |
| Stroke | ||||||
| No | 3437 (2.61) | 40.55 | 1050 (3.26) | 53.95 | 1.33 | 1.26 (1.18–1.35)*** |
| Yes | 843 (12.53) | 232.47 | 255 (10.25) | 207.99 | 0.89 | 0.90 (0.78–1.04) |
| Traumatic brain injury | ||||||
| No | 4158 (3.04) | 47.61 | 1247 (3.65) | 61.07 | 1.28 | 1.17 (1.10–1.25)*** |
| Yes | 122 (7.13) | 117.10 | 58 (11.35) | 214.73 | 1.83 | 1.60 (1.16–2.21)** |
| Cardiovascular disease | ||||||
| No | 2731 (2.36) | 36.49 | 649 (2.84) | 46.08 | 1.26 | 1.29 (1.18–1.40)*** |
| Yes | 1549 (6.81) | 114.43 | 656 (5.57) | 99.33 | 0.87 | 1.03 (0.94–1.13) |
§ Incidence per 10,000 person-years
*: p value for HR < 0.05
**: p value for HR < 0.01
***: p value for HR < 0.001
a HR adjusted by age group, sex, and comorbidities
We further divided the ARD cohort into RA, SLE, primary SS, secondary SS, and other ARDs subgroups. An HR of 1.23 (95% CI 1.15–1.31, p < 0.001) was obtained for the ARD cohort (Table 3), and the adjusted HRs for dementia for the RA, SLE, and SS subgroups were 1.14, 1.07, and 1.46, respectively. Furthermore, primary SS and secondary SS patients had the highest dementia risk among all patients with ARDs, with the adjusted HRs for dementia being 1.35 and 1.67, respectively. Specifically, the secondary SS subgroup had the highest dementia risk overall (HR 1.67, 95% CI 1.43–1.95, p < 0.001). In the age group of less than 65 years, the adjusted HRs for dementia for the RA, SLE, primary SS, secondary SS, and other ARDs subgroups were 1.08, 0.88, 1.49, 1.64, and 0.95, respectively. In this age group, secondary SS had the highest risk of dementia (HR 1.64, 95% CI 1.2–2.25, p < 0.01). In the age group of 65 years or older, the adjusted HRs for dementia for the RA, SLE, primary SS, secondary SS, and other ARDs subgroups were 1.15, 1.19, 1.35, 1.66, and 1.33, respectively. In this age group also, secondary SS had the highest risk of dementia (HR 1.66, 95% CI 1.39–1.99, p < 0.001).
Table 3. Incidence of dementia and Cox model results for the ARD patient group and comparison group.
| Characteristics | N | Event (%) | Incidence§ | Adjusted HRa (95%CI) | |
|---|---|---|---|---|---|
| All | |||||
| Comparison group | 138640 | 4280 | (3.09) | 48.43 | 1.00 |
| All ARD patients | 34660 | 1305 | (3.77) | 63.08 | 1.23 (1.15–1.31)*** |
| RA | 19556 | 713 | (3.65) | 58.07 | 1.14 (1.06–1.32)** |
| SLE | 3062 | 82 | (2.68) | 46.32 | 1.07 (0.86–1.34) |
| SS | 8449 | 408 | (4.83) | 87.11 | 1.46 (1.32–1.63)*** |
| Primary SS | 4756 | 238 | (5.00) | 95.96 | 1.35 (1.18–1.54)*** |
| Secondary SS | 3693 | 170 | (4.60) | 77.14 | 1.67 (1.43–1.95)*** |
| Other ARDs | 3593 | 102 | (2.84) | 52.11 | 1.21 (0.99–1.48) |
| Age < 65 | |||||
| Comparison group | 96722 | 828 | (0.86) | 12.98 | 1.00 |
| All ARD patients | 24188 | 259 | (1.07) | 16.81 | 1.16 (1.00–1.33)* |
| RA | 13417 | 142 | (0.79) | 15.78 | 1.08 (0.90–1.29) |
| SLE | 2299 | 17 | (0.74) | 11.70 | 0.88 (0.54–1.42) |
| SS | 5802 | 79 | (1.36) | 23.47 | 1.57(1.24–1.98) *** |
| Primary SS | 3057 | 37 | (1.21) | 22.39 | 1.49(1.07–2.07)* |
| Secondary SS | 2745 | 42 | (1.53) | 24.51 | 1.64 (1.20–2.25)** |
| Other ARDs | 2670 | 21 | (0.79) | 13.23 | 0.95 (0.61–1.47) |
| Age ≥ 65 | |||||
| Comparison group | 41918 | 3452 | (8.24) | 140.50 | 1.00 |
| All ARD patients | 10472 | 1046 | (9.99) | 198.00 | 1.25 (1.16–1.34)*** |
| RA | 6139 | 571 | (9.30) | 174.17 | 1.15 (1.06–1.26)** |
| SLE | 763 | 65 | (8.52) | 205.06 | 1.19 (0.93–1.53) |
| SS | 2647 | 329 | (12.43) | 249.74 | 1.45(1.30–1.63) *** |
| Primary SS | 1699 | 201 | (11.83) | 242.92 | 1.35 (1.17–1.55)*** |
| Secondary SS | 948 | 128 | (13.50) | 261.27 | 1.66 (1.39–1.99)*** |
| Other ARDs | 923 | 81 | (8.52) | 218.88 | 1.33 (1.06–1.66)* |
§ Incidence per 10,000 person-years
*: p value for HR < 0.05
**: p value for HR < 0.01
***: p value for HR < 0.001
a HR adjusted by age group, sex, and comorbidities
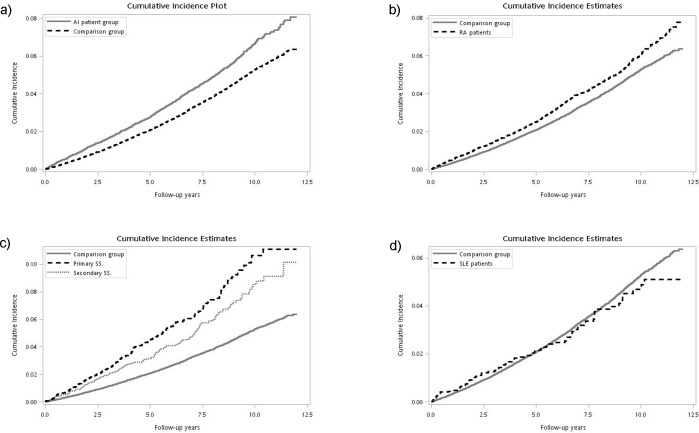
Fig 1(A) presents a comparison of the cumulative incidence of dementia for the ARD and non-ARD patient groups. The incidence of dementia (log rank test, p < 0.001) was significantly higher for patients in the ARD cohort than it was for patients without ARDs. Fig 1(B)–1(D) presents a comparison of the cumulative incidence of dementia for the subgroups of the ARD and non-ARD cohorts. Except for the SLE subgroup, the incidence of dementia (log rank test, p < 0.001) was significantly higher for the patients in the subgroups of the ARD cohort than it was for the non-ARD cohort.
Fig 1. Cumulative incidence plot of dementia among ARD and non-ARD patient group.
(a) presents a comparison of the cumulative incidence of dementia for the ARD and non-ARD patient groups. (b) presents a comparison of the cumulative incidence of dementia for RA and non-ARD cohorts. (c) presents a comparison of the cumulative incidence of dementia for SS (primary SS and secondary SS) and non-ARD cohorts. (d) presents a comparison of the cumulative incidence of dementia for SLE and non-ARD cohorts.
Discussion
To the best of our knowledge, this is the first nationwide population-based study evaluating the relationship between middle-aged patients with ARDs and dementia. In this study, the overall incidence rate of dementia was 30% higher for the ARD cohort than for the non-ARD cohort, with an HR of 1.18 after adjustment for age, sex, and comorbidities. Moreover, ARD subgroups such as the RA and SS subgroups were associated with a significantly higher risk of dementia than was the non-ARD cohort. We therefore postulate that patients with ARDs (except SLE and vasculitis) have an increased risk of dementia.
Young-onset dementia (YOD) is defined as a neurological syndrome that affects the behavior and cognition of patients aged 45–64 years. In the present study, the patients were divided into two age groups, <65 and ≥65 years, for analysis. The age of the patients and follow-up suggested that the association is not restricted to YOD and also involves late-onset dementia. Speculations of a potential association between ARDs and neuropsychiatric affections, including affective disorder, neurotic disorder, personality disorder, dementia, and delirium, have increased [31,32]. The present study highlighted major psychosis or substance-related disorders (ICD-9-CM: 291–299 and 303–305) as covariates to differentiate between psychosis and dementia in SLE. Several substantial interactions between covariate conditions and ARDs have been reported to increase the risk of dementia. Microgial cell activation is a major component of neuroinflammation in degenerative dementia [33–35]. An activated microgial cell can be divided as either M1 (classical phenotype) or M2 (alternatively activated phenotype) [36]. Microglia develop into an M1 phenotype in the presence of interferon and tumor necrosis factor and release massive inflammatory cytokines such as IL1β, IL-12, TNF-α, and inducible nitric oxide synthase. These inflammatory cytokines have also been observed in ARDs. Additionally, the M2 phenotype develops in the presence of IL-4 and IL-13 and has an anti-inflammatory profile. The switch between the M1 and M2 phenotype is a dynamic process and is dependent on the presence of a peripheral inflammation state [37]. However, a specific type of M1 is predominant in ARDs, as reported by Jimenz et al. [35,38], who identified a distinctive shift from M2 to M1 in brains with AD. Therefore, glial cells can be activated by systemic inflammation and consequently deteriorate, presenting clinical symptoms of AD and Parkinson disease [39]. Neuroinflammation results in synaptic impairment, and neuronal death and contributes to neurodegeneration within the brain [40]. Therefore, middle-aged patients with ARDs may have an increased risk of dementia. Some animal models of AD [41,42] and histochemical analyses of human brain serial sections [43] also indicate an aggregation of activated microglia around amyloid plaques in animal [41] and human brains [44–47], respectively. Soluble Aβ may be involved [47] and may trigger neuroinflammation at the BBB level [48], indicating that inflammation is an early process in AD pathogeny.
Kang et al. [49] demonstrated that after adjustment for demographics and comorbidities, the dementia risk did not differ significantly in their ARD and comparison groups. Furthermore, neuroinflammation may have some protective effects against ARD. Additionally, de Simone et al. investigated the phospholipid phosphatidylserine expressed on the surface of apoptotic neurons and reported that its presence may induce a shift in the microglial expression of cytokines from being deleteriously inflammatory (IL-1, TNF-α, NO) to protective (TGF-β and NGF) [50]. Toll-like receptors (TLRs) are believed to promote the proinflammatory pathways. However, studies have reported that TLR2 and TLR4 are present in amyloid plaques [51] and are involved in the uptake of Aβ and other aggregated proteins, promoting their clearance from the central nervous system [52]. Another toll-like receptor, TLR3, can enhance neuronal survival and endothelial cell growth, promoting neuroprotective responses [53]. These findings may be due to neuroinflammation having both neurodestructive and neuroprotective effects. The hypothesis that inflammation leads to dementia implies a predominance of the neurodestructive effects over the neuroprotective effects [54].
Among the ARD subgroups in this study (RA, SLE, primary SS, secondary SS, and other ARDs), the secondary SS subgroup had the highest dementia risk after adjustment for demographics and comorbidities. Conversely, the SLE subgroup had the lowest dementia risk compared with the other ARD subgroups. These findings are consistent with those of Trysberg et al. [47–49], who reported low levels of amyloid β in SLE patients. This may be a consequence of diminished production of the amyloid precursor protein, which is believed to be mediated by heavy anti-inflammatory or immunosuppressive therapy. Therefore, the effects of anti-inflammatory or immunosuppressive therapy on patients deserves consideration.
A strength of our study was the use of a nationwide population-based database with sufficient sample size and statistical power. However, our study had several limitations. First, although the NHIRD includes data on patients that received treatment for ARDs and dementia, in Taiwan, most ARD patients with a catastrophic illness certificate have been treated with anti-inflammatory or immunosuppressive therapy. Additionally, in Taiwan, stopping anti-inflammatory or immunosuppressive therapy in ARD patients with a catastrophic illness certificate is unacceptable. Therefore, we could not adjust for treatment-related effects in our study. Although some studies have revealed that NSAID use reduces the risk of dementia in patients with RA, our data demonstrated an increased risk of dementia (adjusted HR: 1.14) in the RA subgroup. Such conflicting results may have been obtained because the present study did not consider the effects of NSAID use. Second, the NHIRD does not contain parameters such as clinical severity, laboratory data, body mass index, smoking habits, intelligence, and education level, all of which have been also associated with ARDs and dementia [9–10]. Third, dementia is more likely to occur in the elderly; however, patients of varying ages were enrolled to study the different ARDs. Therefore, we did not fully account for the effects of age in our study. Future analysis of each type of ARD may yield more concise results.
In conclusion, this nationwide population-based retrospective cohort study determined that middle-aged patients with ARDs (excluding SLE and vasculitis) have an increased risk of dementia. Among the ARD subgroups, the RA and SS subgroups were associated with a significantly higher risk of dementia, with both the primary and secondary SS subgroups having the highest overall risk of dementia. The underlying mechanism of these results is not fully understood and warrants further research.
Data Availability
The data underlying this study are from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html and stcarolwu@mohw.gov.tw).
Funding Statement
The authors received no specific funding for this work.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatric Association; 2013. [Google Scholar]
- 2.Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509–19. [DOI] [PubMed] [Google Scholar]
- 3.Geschwind MD, Haman A, Miller BL. Rapidly Progressive Dementia. Clinical Neurology and Neurosurgery. 2007;25:783–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayo Clinic Laboratories Test Catalog. Paraneoplastic Autoantibody Evaluation. 2010;Serum Unit Code 83380.
- 5.Kang JH, Keller JJ, Lin YK, Lin HC. A population-based case control study on the association between rheumatoid arthritis and deep vein thrombosis. Journal of Vascular Surgery. 2012;56:1642–48. doi: 10.1016/j.jvs.2012.05.087 [DOI] [PubMed] [Google Scholar]
- 6.Chiang CH, Liu CJ, Huang CC, Chan WL, Huang PH, Chen TJ, et al. Systemic sclerosis and risk of ischaemic stroke: a nationwide cohort study Rheumatology. 2016;52:161–5. [DOI] [PubMed] [Google Scholar]
- 7.Wang IK, Muo CH, Chang YC, Liang CC, Lin SY, Chang CT, et al. Risks, subtypes, and hospitalization costs of stroke among patients with systemic lupus erythematosus: a retrospective cohort study in Taiwan. The Journal of Rheumatology. 2016;39:1611–18. [DOI] [PubMed] [Google Scholar]
- 8.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis and Rheumatism. 2005;52:402–11. doi: 10.1002/art.20853 [DOI] [PubMed] [Google Scholar]
- 9.Querfurth HW, LaFerla FM. Alzheimer’s disease. The New England Journal of Medicine. 2010;362:329–44. doi: 10.1056/NEJMra0909142 [DOI] [PubMed] [Google Scholar]
- 10.Leys D, H'enon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. The Lancet Neurology. 2005;4:752–59. doi: 10.1016/S1474-4422(05)70221-0 [DOI] [PubMed] [Google Scholar]
- 11.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of neurology. 2004;61,668–72. doi: 10.1001/archneur.61.5.668 [DOI] [PubMed] [Google Scholar]
- 12.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68, 1902–08. doi: 10.1212/01.wnl.0000263217.36439.da [DOI] [PubMed] [Google Scholar]
- 13.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology.2009;73,768–74. doi: 10.1212/WNL.0b013e3181b6bb95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broe GA, Henderson AS, Creasey H, McCusker E, Korten AE, Jorm AF, et al. A case-control study of Alzheimer’s disease in Australia. Neurology. 1990;40:1698–707. [DOI] [PubMed] [Google Scholar]
- 15.French LR, Schuman LM, Mortimer JA, Hutton JT, Boatman RA, Christians B. A case control study of dementia of the Alzheimer type. The American Journal of Epidemiology. 1985;121:414–21. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson ML, Bliss MR, Brain AT, Scott DL. Rheumatold arthritis and senile dementia of the alzhelmer’s type. Rheumatology. 1989;28:86–7. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Shen YC, Li YT, Chen CH, Zhau YW, Silverman JM. A case-control study of Alzheimer’s disease in China. Neurology. 1992;42:1481–88. [DOI] [PubMed] [Google Scholar]
- 18.Wallin K, Solomon A, K˚areholt I, Tuomilehto J, Soininen H, Kivipelto M. Midlife rheumatoid arthritis increases the risk of cognitive impairment two decades later: a population-based study. Journal of Alzheimer’s Disease. 2012;31:669–76. doi: 10.3233/JAD-2012-111736 [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa K, Hatate J, Toratani N, Sugiura S, Shimizu Y, Takahash T, et al. Prevalence of Sj¨ogren’s syndrome with dementia in a memory clinic. Journal of the Neurological Sciences. 2012;322:217–21. doi: 10.1016/j.jns.2012.07.060 [DOI] [PubMed] [Google Scholar]
- 20.Cheng TM. Taiwan’s National Health Insurance system: high value for the dollar In: Okma KGH, Crivelli L, eds. Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. Hackensack, New Jersey: World Scientific; 2009;71–204. [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 23.American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 24.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. doi: 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. doi: 10.1056/NEJM197502202920807 [DOI] [PubMed] [Google Scholar]
- 26.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. doi: 10.1056/NEJM197502132920706 [DOI] [PubMed] [Google Scholar]
- 27.International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990;335:1078–80. [PubMed] [Google Scholar]
- 28.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–8. [DOI] [PubMed] [Google Scholar]
- 29.Leavitt RY, Fauci AS, Bloch DA, Michael BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum 1990;33:1101–7. [DOI] [PubMed] [Google Scholar]
- 30.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34 [DOI] [PubMed] [Google Scholar]
- 31.Borowoy AM, Pope JE, Silverman E, Fortin PR, Pineau C, Smith CD, et al. Neuropsychiatric lupus: the prevalence and autoantibody associations depend on the definition: results from the 1000 faces of lupus cohort. Seminars in Arthritis and Rheumatism. 2012;42:179–85. doi: 10.1016/j.semarthrit.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 32.Sundquist K, Li X, Hemminki K, Sundquist J. Subsequent risk of hospitalization for neuropsychiatric disorders in patients with rheumatic diseases: a nationwide study from Sweden. Arch Gen Psychiatry. 2008;65:501–07. doi: 10.1001/archpsyc.65.5.501 [DOI] [PubMed] [Google Scholar]
- 33.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26(6):1049–55. doi: 10.1002/mds.23732 [DOI] [PubMed] [Google Scholar]
- 34.Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, et al. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(1):47–52. doi: 10.1016/j.parkreldis.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 35.Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain.Arch Immunol Ther Exp (Warsz). 2012;60(4):251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73. [DOI] [PubMed] [Google Scholar]
- 37.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 38.Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, et al. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28(45):11650–61. doi: 10.1523/JNEUROSCI.3024-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sastre M, Richardson JC, Gentleman SM, Brooks DJ. Inflammatory risk factors and pathologies associated with Alzheimer’s disease. Curr Alzheimer Res. 2011;8(2):132–41. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. This article is an evidence-based review that reported the most significant articles related to role of neuroinflammation and the progression of chronic neurodegenerative disease. 2013;61(1):71–90. doi: 10.1002/glia.22350 [DOI] [PubMed] [Google Scholar]
- 41.Apelt J, Schliebs R. β-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Research. 2001;894:21–30. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka Y, Picciano M, Malester B, LaFrancois J, Zehr C, Daeschner JM, et al. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. American Journal of Pathology. 2001;158:1345–54. doi: 10.1016/S0002-9440(10)64085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Andrea MR, Reiser PA, Gumula NA, Hertzog BM, Andrade-Gordon P. Application of triple immunohistochemistry to characterize amyloid plaque-associated inflammation in brains with Alzheimer’s disease. Biotechnic and Histochemistry. 2001;76:97–106. [PubMed] [Google Scholar]
- 44.Tooyama I, Kimura H, Akiyama H, McGeer PL. Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer’s disease. Brain Research. 1990;523:273–80. [DOI] [PubMed] [Google Scholar]
- 45.Perlmutter LS, Scott SA, Barron E, Chui HC. MHC class II-positive microglia in human brain: association with Alzheimer lesions. Journal of Neuroscience Research. 1992;33:549–58. doi: 10.1002/jnr.490330407 [DOI] [PubMed] [Google Scholar]
- 46.Uchihara T, Akiyama H, Kondo H, Ikeda K. Activated microglial cells are colocalized with perivascular deposits of amyloid-β protein in Alzheimer’s disease brain. Stroke. 1997;28:1948–50. [DOI] [PubMed] [Google Scholar]
- 47.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. Journal of Neuroinflammation. 2005;2:22 doi: 10.1186/1742-2094-2-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, et al. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiology of Disease. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu K, Wang HK, Yeh CC, Huang CY, Sung PS, Wang LC, et al. Association between Autoimmune Rheumatic Diseases and the Risk of Dementia. BioMed Research International. 2014;2014:861812 doi: 10.1155/2014/861812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simone RD, Ajmone-Cat MA, Tirassa P, Minghetti L. Apoptotic PC12 cells exposing phosphatidylserine promote the production of anti-inflammatory and neuroprotective molecules by microglial cells. Journal of Neuropathology and Experimental Neurology. 2003;62:208–16. [DOI] [PubMed] [Google Scholar]
- 51.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Research Reviews. 2009;59:278–92. doi: 10.1016/j.brainresrev.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69. doi: 10.1111/j.1365-2567.2009.03225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bsibsi M, Persoon-DeRen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. GLIA. 2006;53:688–95. doi: 10.1002/glia.20328 [DOI] [PubMed] [Google Scholar]
- 54.Enciu AM, Popescu BO. Is there a causal link between inflammation and dementia? Biomed Res Int. 2013;2013:316495 doi: 10.1155/2013/316495 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study are from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html and stcarolwu@mohw.gov.tw).