Abstract
Intake of high-fat/high-sucrose (HFS) diet or high fat diet influences bone metabolism in young rodents, but its effects on bone properties of aged rodents still remain unclear. This study aimed to examine the effects of HFS diet intake on trabecular bone architecture (TBA) and cortical bone geometry (CBG) in aged rats. Fifteen male Wistar rats over 1 year were randomly divided into two groups. One group was fed a standard laboratory diet (SLD) and the other group was fed a HFS diet for six months. The femur/tibia, obtained from both groups at the end of experimental period, were scanned by micro-computed tomography for TBA/CBG analyses. Serum biochemical analyses were also conducted. Body weight was significantly higher in the HFS group than in the SLD group. In both femur and tibia, the HFS group showed higher trabecular/cortical bone mass in reference to bone mineral content, volume bone mineral density and TBA/CBG parameters compared with the SLD group. In addition, serum calcium, inorganic phosphorus, total protein, triacylglycerol, HDL and TRACP-5b levels were significantly higher in the HFS group than in the SLD group. There were good correlations between body weight and bone parameters in the femur and tibia. These results suggest that HFS diet intake results in higher bone mass in aged rats. Such effects of HFS diet intake might have been induced by increased body weight.
Abbreviations: ALP, alkaline phosphatase; BMD, bone mineral density; BMC, bone mineral content; BV, bone volume; BV/TV, bone volume fraction; Ca, calcium; CBG, cortical bone geometry; Conn.D, connectivity density; Ct.Ar, cortical bone sectional area; Ct.Th, cortical bone thickness; CV, cortical bone volume; CV/(CV + MV), cortical volume fraction; DXA, dual-energy X-ray absorptiometry; Ec.Pm, endocortical perimeter; HDL, high-density lipoprotein cholesterol; HFD, high fat diet; HFS, high-fat/high-sucrose; IP, inorganic phosphorus; LDL, low-density lipoprotein cholesterol; micro-CT, x-ray micro-computed tomography; MV, medullary volume; OC, osteocalcin; Ps.Pm, periosteal perimeter; VOI, volume of interest; SLD, standard laboratory diet; TBA, trabecular bone architecture; Tb.N, trabecular number; TBPf, trabecular bone pattern factor; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; Tb.W, trabecular width; TC, total cholesterol; TG, triacylglycerol; TMD, tissue mineral density; TP, total protein; TRACP-5b, tartrate-resistant acid phosphatase-5b; TV, tissue volume; vBMD, volume BMD
Keywords: High-fat sucrose diet, Aged rats, Micro-computed tomography, Trabecular bone architecture, Cortical bone geometry
Highlights
-
•
High-fat/high-sucrose (HFS) diet intake resulted in higher trabecular/cortical bone mass in aged rats.
-
•
Enhanced bone metabolism was suggested by bone formation/resorption markers in aged rats fed with HFS diet.
-
•
Serum HDL levels were higher in HFS diet-fed rats than in standard laboratory diet-fed rats.
-
•
There were good correlations between body weight and bone mass or architecture parameters.
-
•
Such positive effects of HFS diet intake on bone mass might have been induced by increased body weight.
1. Introduction
Osteoporosis and obesity are serious and prevalent health issues. Indeed, body weight positively correlated with bone mineral density (BMD), and high body mass index was associated with low fracture risk (Reid, 2008, Reid, 2010). However, there is inconsistency in the literature regarding the effects of obesity on fracture risk. For example, some studies showed that obesity reduces fracture risk and protects against osteoporosis in adults (Reid, 2010, Tang et al., 2013), while others showed that obesity does not protect against fracture in postmenopausal women (Compston et al., 2011, Tanaka et al., 2013). Interestingly, one study reported that obesity is a risk factor of fracture in children, while it is protective against fracture in adults (Dimitri et al., 2012), suggesting that the effects of obesity on bone parameters may differ with age. Thus, the effects of obesity on fracture risk were reported to be inconsistent, although BMD of obese individuals ranged from normal to high levels as compared with non-obese individuals. Therefore, BMD, which generally depends on body weight, may not be predictive of fracture risk in obesity.
Young rodents, fed with a high fat diet (HFD) or a high-fat/high-sucrose (HFS) diet, are often used to assess the effects of obesity on bone parameters. For example, bone mineral content (BMC) and mechanical properties of both the vertebral body (L6) and femoral neck were significantly decreased in rats fed with HFS diet from the age of 4 weeks until the age of 2 years, compared with rats fed with a low-fat, complex-carbohydrate diet (Zernicke et al., 1995). BMC, BMD and skeletal area, assessed by dual-energy X-ray absorptiometry (DXA), were significantly decreased in rats fed with HFD for 10 weeks from the age of 5 weeks, compared with rats fed with a standard diet (Lac et al., 2008). Furthermore, in young mice the HFD intake was reported to induce the deterioration in trabecular bone architecture (TBA) and to increase bone resorption (Cao et al., 2009, Patsch et al., 2011). In addition, bone mechanical strength and fracture toughness were decreased in mice fed with HFD for 19 weeks from the age of 4 weeks (Ionova-Martine et al., 2010). Thus, the HFD/HFS diet intake appears to have negative effects on BMC, BMD, TBA and bone mechanical properties in young rodents.
In opposition to the above findings, there were some studies reporting positive effects of HFD intake on BMC, BMD, TBA, cortical bone geometry (CBG) and bone mechanical properties in young rodents. BMC, BMD and skeletal area, analyzed by DXA, were significantly increased in rats fed with HFD for 9 months from the age of 8–10 weeks, compared with rats fed with a standard diet (Malvi et al., 2014). These HFD-fed rats showed increased levels of serum alkaline phosphatase (ALP) and unchanged levels of serum tartrate-resistant acid phosphatase-5b (TRACP-5b) (Malvi et al., 2014). In young mice fed with HFD, trabecular and cortical bone structure of the tibia were also increased despite a reduction in mineral apposition and bone formation rates (Lecka-Czernik et al., 2015). Moreover, in a rat model of obesity induced by junk food intake for one month from the age of 3 months, femoral ultimate load and energy absorption capacity were significantly higher than in control rats (Brahmabhatt et al., 1998). These results suggest that the HFD intake has positive effects on bone parameters and bone mechanical properties in young rodents. Thus, in young rodents, the effects of HFD intake on bone parameters and bone mechanical properties were reported to be negative or positive, and therefore still remain controversial.
We hypothesized that the effects of HFD/HFS diet intake on bone mass/quality in aged rats may be different from those observed in young rats due to the differences in bone metabolism between growth and aging periods (Lelovas et al., 2008). In contrast to several studies on the effects of HFD/HFS diet intake on bone of young and adult rats (Zernicke et al., 1995, Brahmabhatt et al., 1998, Lac et al., 2008, Malvi et al., 2014), to the best of our knowledge, there was only one study reporting bone properties in male Wistar rats aged 11 months, which were fed with HFS diet for 4 months from 7 months of age (Gerbaix et al., 2012). This HFS diet-fed rats showed an increase in whole body bone mass and BMD compared with standard diet-fed rats, but no change in tibia TBA parameters including bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N), connectivity density (Conn.D) etc. (Gerbaix et al., 2012). However, we have speculated that HFS diet-feeding during the longer period would influence TBA in more aged rats. To examine this speculation, using X-ray micro-computed tomography (micro-CT), we have analyzed trabecular and cortical bone structure in rats fed with HFS diet for 6 months from 12 months of age in the present study.
2. Materials and methods
This study was approved by the Ethics Committee of Research Facilities for Laboratory Animal Sciences in the Kio University, and was performed in accordance with the Guidelines for Animal Experimentation of the University.
2.1. Animal care
Fifteen male Wistar rats over 1 year with mean body weight of 486 g (Japan SLC, Inc., Hamamatsu, Japan) were used in this study. Rats were housed in cages at 23 ± 2 °C temperature, in 50 ± 10% humidity and under a 12-hour day-night cycle. Rats were randomly divided into two groups and were allowed to feed and drink water ad libitum. One group (n = 7) was fed a standard laboratory diet (SLD) containing 4.7% crude fat (CE-2; CLEA, Inc., Hamamatsu, Japan) and the other (n = 8) was fed a HFS diet containing 13.8% crude fat and 25% sucrose (Quick Fat; CLEA, Inc., Hamamatsu, Japan), during the experimental period of 24 weeks. The Quick Fat has been generally used for diabetes mellitus/obesity studies in rodents. Nutritional details of the two diets are provided in Table 1. Body weight and food intake were measured every week throughout the experimental period.
Table 1.
Nutritional content of two experimental diets.
| CE-2 | Quick Fat | |
|---|---|---|
| Moisture (%) | 8.9 | 6.9 |
| Crude protein (%) | 24.2 | 23.7 |
| Crude fat (%) | 4.7 | 13.8 |
| Crude fiber (%) | 4.1 | 2.6 |
| Crude ash (%) | 6.9 | 5.3 |
| Supplemented sucrose (%) | 0 | 25 |
| Ca (%) | 1.1 | 1.0 |
| P (%) | 1.1 | 0.8 |
| Vitamin D (IU/g) | 2.15 | 2.90 |
| Energy (kcal/100 g) | 345.5 | 412.5 |
| Fat energy (%) | 12.2 | 30.1 |
2.2. Analyses of TBA and CBG
The procedures for analyses of TBA and CBG were performed according to the micro-CT guidelines for assessment of rodent bone microstructure (Bouxsein et al., 2010), as previously reported by our group (Minematsu et al., 2016a, Minematsu et al., 2016b, Minematsu et al., 2017). The bilateral femurs and tibias were dissected out, and then soft tissues were removed. The bones were put into the vials filled with 70% ethanol solution (25 °C), and the vials were tightly sealed. In an attempt to prevent the generation of air bubbles/micro-air bubbles in bones, the vials were stored under occasional light vibration in a refrigerator (4 °C) until analyzed. To analyze TBA and CBG using micro-CT (Hitachi Medical Corporation. Tokyo, Japan), the distal femur, proximal tibia and mid-shaft of the tibia were scanned at 65 kVp and 90 μA, with a voxel size of 19.1 μm in the high-definition mode. A BMD phantom was also scanned using micro-CT under the same conditions to calculate tissue mineral density (TMD), BMC and volume BMD (vBMD, i.e., BMC/tissue volume). All micro-CT images were inspected visually to identify possible scanning artifacts, like ring artifact (Bouxsein et al., 2010). Scanned data of images without scanning artifacts were transmitted to a personal computer. After the removal of random image noise was accomplished by median-filtering, bone images were reconstructed by binary coded processing using a discriminative analysis method for global segmentation. TBA and CBG of the volume of interest (VOI) were analyzed using a bone analysis software (TRI BON 3D; Ratoc System Engineering Co., Ltd., Tokyo, Japan). Tissue volume (TV), bone volume (BV), BV/TV, Tb.Th, trabecular width (Tb.W), Tb.N, Tb.Sp, Conn.D and trabecular bone pattern factor (TBPf, i.e., ratio of changed bone surface area to changed bone surface volume in trabecular bone) were assessed as TBA parameters in the distal femur and proximal tibia. Cortical bone volume (CV), medullary volume (MV), cortical volume fraction (CV/(CV + MV)), cortical bone thickness (Ct.Th), cortical bone sectional area (Ct.Ar), periosteal perimeter (Ps.Pm), and endocortical perimeter (Ec.Pm) were assessed as CBG parameters in the tibia. The VOI of TBA in the femur was an area of 2 mm in the femoral metaphysis, with the first slice starting 1 mm away from the distal femoral growth plate physeal-metaphyseal demarcation in the proximal direction. The VOI of TBA in the tibia was an area of 2 mm in the tibial metaphysis, with the first slice starting 1 mm away from the proximal tibial growth plate physeal-metaphyseal demarcation in the distal direction. The VOI of CBG in the tibia was an area of 2 mm in the tibial shaft, with the first slice starting 1 mm away from the inferior tibiofibular junction in the proximal direction.
2.3. Dry bone weight and ash weight measurements
After TBA and CBG analyses, the femur and tibia were dehydrated in 100% ethanol for 48 h and dried at 100 °C for 24 h with a drying machine (Yamato Scientific Co., Ltd. Tokyo, Japan) to measure dry weight of the whole bone. Subsequently, the bones were burned to ash at 600 °C for 24 h with an electric furnace (Nitto Kagaku Co., Ltd., Nagoya, Japan) to obtain ash weight of the whole bone.
2.4. Serum biochemical analyses
At the end of the experiment, blood samples for serum biochemical analyses were obtained from the rats under non-fasting condition in the morning. Serum was separated from blood samples by centrifugation (1000 × g for 20 min). Serum was stored at − 80 °C until analyzed. Measurements of serum calcium (Ca), inorganic phosphorus (IP), alkaline phosphatase (ALP), total protein (TP), total cholesterol (TC), triacylglycerol (TG) and high- and low-density lipoprotein cholesterol (HDL, LDL) concentrations were outsourced to Nagahama Life Science Laboratory (Oriental Yeast Co., Ltd., Tokyo, Japan). In addition, serum concentrations of osteocalcin (OC), a bone formation marker, and TRACP-5b, a bone resorption marker, were measured with an osteocalcin EIA kit (Biomedical Technologies Inc. Stoughton, MA, USA) and a TRACP-5b ELISA kit (Immunodiagnostic Systems Ltd. Boldon, UK), respectively.
2.5. Statistical analysis
Values for all indices are expressed as mean ± standard deviation. Differences in parameters between the SLD and HFS groups were estimated using Welch's t-test. Pearson's correlation coefficients were determined to examine the relationship between bone parameters and body weight. p < 0.05 was considered statistically significant. All statistical analyses were performed using an Excel Statistics software (Excel 2012 ver.1.08 for Windows, Social Survey Research Information Co., Ltd., Tokyo, Japan).
3. Results
3.1. Final body weight, food intake, bone length, dry bone weight and ash weight
Body weight changes in both groups are shown in Fig. 1. From the 3rd week until the last week after the intervention, body weight was significantly higher in the HFS group than in the SLD group. The final body weight in the HFS and SLD groups was 557.2 ± 37.4 g and 460.1 ± 26.5 g, respectively. Daily crude-fat intake was also significantly higher in the HFS group (2.10 ± 0.30 g/day) than in the SLD group (0.77 ± 0.08 g/day), although daily food intake was significantly, albeit slightly, higher in the SLD group than in the HFS group (SLD, 16.4 ± 1.8 g/day vs. HFS, 15.1 ± 2.2 g/day). There was no significant difference in bone lengths of the femur and tibia between two groups (Table 2). Dry and ash weight of the whole bone in the HFS group tended to be heavier, albeit not significantly, compared with the SLD group (Table 2).
Fig. 1.
Body weight changes in the SLD and the HFS groups.
Results are expressed as mean + SD (bar) or mean − SD (bar). *Significantly different from the SLD group (p < 0.05).
Table 2.
Bone length, dry weight of the whole bone, ash weight of the whole bone and trabecular bone mass parameters of metaphysis in femur and tibia.
| SLD (n = 7) | HFS (n = 8) | ||
|---|---|---|---|
| Bone length (mm) | Femur | 41.4 ± 0.8 | 41.5 ± 0.9 |
| Tibia | 44.1 ± 0.8 | 44.5 ± 0.7 | |
| Dry weight of the whole bone (mg) | Femur | 881.6 ± 111.8 | 911.5 ± 64.1 |
| Tibia | 648.4 ± 73.3 | 682.2 ± 47.2 | |
| Ash weight of the whole bone (mg) | Femur | 514.3 ± 60.3 | 528.7 ± 44.0 |
| Tibia | 391.7 ± 36.8 | 411.5 ± 19.9 | |
| TMD (mg/cm3) | Femur | 382.2 ± 19.5 | 389.9 ± 21.9 |
| Tibia | 346.0 ± 23.6 | 385.4 ± 32.3⁎ | |
| BMC (mg) | Femur | 0.562 ± 0.174 | 1.055 ± 0.392⁎ |
| Tibia | 0.340 ± 0.124 | 0.631 ± 0.183⁎ | |
| vBMD (mg/cm3) | Femur | 39.0 ± 11.6 | 70.4 ± 17.1⁎ |
| Tibia | 32.0 ± 10.5 | 56.6 ± 12.8⁎ |
Results are expressed as mean ± SD.
TMD, tissue mineral density; BMC, bone mineral content; vBMD, volume bone mineral density.
Significantly different from the SLD group (p < 0.05).
3.2. TBA and CBG parameters
TBA parameters of the femur and tibia, except for TV, in the HFS group showed a well-developed bone microarchitecture, as compared with the SLD group (Fig. 2). BV, BV/TV, Tb.Th, Tb.W, Tb.N and Conn.D were significantly higher and Tb.Sp and TBPf were significantly lower, in the HFS group than in the SLD group (Fig. 2). Moreover, TMD, BMC and vBMD of trabecular bone in the femur and tibia, except TMD in the femur, were significantly higher in the HFS group than in the SLD group (Table 2). Likewise, some CBG parameters of tibial midshaft in the HFS group showed a well-developed cortical bone structure, as compared with the SLD group (Table 3). CV/(CV + MV) and Ct.Th of the tibial midshaft were significantly higher and MV and Ec.Pm were significantly lower, in the HFS group than in the SLD group (Table 3).
Fig. 2.
Trabecular bone architecture parameters in femoral and tibial metaphysis.
Results are expressed as mean + SD (bar). *Significantly different from the SLD group (p < 0.05).
TV, tissue volume; BV, bone volume; BV/TV, bone volume ratio; Tb.Th, trabecular thickness; Tb.W, trabecular width; Tb.N, trabecular number; Tb.Sp, trabecular separation; Conn.D, connectivity density; TBPf, trabecular bone pattern factor.
Table 3.
Cortical bone geometry parameters of tibial midshaft.
| SLD (n = 7) | HFS (n = 8) | |
|---|---|---|
| TMD (mg/cm3) | 1053.0 ± 40.7 | 1047.9 ± 40.4 |
| BMC (mg) | 6.00 ± 0.40 | 6.14 ± 0.26 |
| CV (mm3) | 5.71 ± 0.48 | 5.86 ± 0.22 |
| MV (mm3) | 2.81 ± 0.27 | 2.20 ± 0.33⁎ |
| CV/(CV + MV) (%) | 67.0 ± 1.7 | 72.9 ± 2.8⁎ |
| Ct.Th (μm) | 464.5 ± 24.7 | 500.0 ± 26.3⁎ |
| Ct.Ar (mm2) | 2.76 ± 0.23 | 2.84 ± 0.11 |
| Ps.Pm (mm) | 7.74 ± 0.35 | 7.56 ± 0.22 |
| Ec. Pm (mm) | 4.56 ± 0.22 | 4.22 ± 0.30⁎ |
Results are expressed as mean ± SD.
TMD, tissue mineral density; BMC, bone mineral content; CV, cortical bone volume; MV, medullary volume; CV/(CV + MV), cortical volume fraction; Ct.Th, cortical bone thickness; Ct.Ar, cortical bone sectional area; Ps.Pm, periosteal perimeter; Ec.Pm, endocortical perimeter.
Significantly different from the SLD group (p < 0.05).
3.3. Serum biochemical analyses
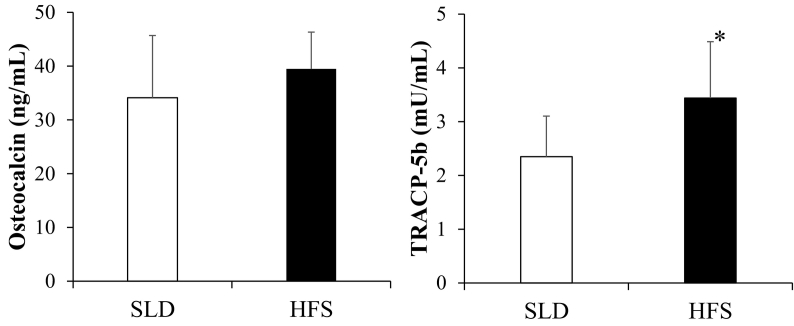
Serum concentrations of Ca, IP, TP, TG and HDL were significantly higher in the HFS group than in the SLD group (Table 4). Mean values of serum OC in the HFS group was increased, but not significantly, as compared with the SLD group. Also, the HFS group showed a significant increase in in serum TRACP-5b levels compared with the SLD group (Fig. 3).
Table 4.
Serum biochemical analyses.
| SLD (n = 7) | HFS (n = 8) | |
|---|---|---|
| Ca (mg/dL) | 9.9 ± 0.6 | 10.5 ± 0.3⁎ |
| IP (mg/dL) | 5.0 ± 0.6 | 6.3 ± 1.0⁎ |
| ALP (IU/L) | 720.4 ± 239.7 | 705.8 ± 168.5 |
| TP (g/dL) | 6.0 ± 0.8 | 6.9 ± 0.4⁎ |
| TC (mg/dL) | 132.3 ± 25.8 | 154.5 ± 24.3 |
| TG (mg/dL) | 181.1 ± 69.3 | 393.8 ± 110.2⁎ |
| HDL (mg/dL) | 35.7 ± 7.4 | 46.5 ± 5.9⁎ |
| LDL (mg/dL) | 15.4 ±5.1 | 15.1 ± 2.2 |
Results are expressed as mean ± SD.
Ca, calcium; IP, inorganic phosphorus; ALP, alkaline phosphatase; TP, total protein; TC, total cholesterol; TG, triacylglycerol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
Significantly different from the SLD group (p < 0.05).
Fig. 3.
Serum concentrations of osteocalcin and TRACP-5b in the SLD and the HFS group.
Results are expressed as mean + SD (bar). *Significantly different from the SLD group (p < 0.05).
TRACP-5b, tartrate-resistant acid phosphatase-5b.
3.4. Correlation between bone parameters and body weight
Finally, we have examined the correlations between body weight and bone parameters in the femur and tibia (Table 5). Good correlations were found not only between body weight and bone mass, TBA or CBG parameters in the tibia, but also between body weight and bone mass or TBA parameters in the femur.
Table 5.
Correlations between body weight and bone parameters.
| Femur | Tibia | |
|---|---|---|
| Dry bone weight | 0.371 | 0.402 |
| Bone ash weight | --- | 0.447 |
| Bone mass parameters | ||
| TMD (metaphysis) | --- | 0.653 |
| BMC (metaphysis) | 0.527 | 0.658 |
| vBMD | 0.627 | 0.684 |
| Trabecular bone architecture parameters | ||
| BV | 0.755 | 0.531 |
| BV/TV | 0.736 | 0.571 |
| Tb.Th | 0.817 | 0.485 |
| Tb.W | 0.788 | 0.567 |
| Tb.N | 0.660 | 0.487 |
| Tb.Sp | − 0.538 | − 0.432 |
| Conn.D | 0.598 | 0.396 |
| TBPf | − 0.766 | − 0.512 |
| Cortical bone geometry parameters | ||
| MV | / | − 0.659 |
| CV/(CV + MV) | / | 0.784 |
| Ct.Th | / | 0.674 |
| Ec.Pm | / | − 0.515 |
Values are Pearson's correlation coefficients (p < 0.05). ---, not significant; /, not measured.
TMD, tissue mineral density; BMC, bone mineral content; vBMD, volume bone mineral density; BV, bone volume; BV/TV, bone volume fraction; Tb.Th, trabecular thickness: Tb.W, trabecular width; Tb.N, trabecular number; Tb.Sp, trabecular separation; Conn.D, connectivity density; TBPf, trabecular bone pattern factor; MV, medullary volume; CV/(CV + MV), cortical volume fraction; Ct.Th, cortical bone thickness; Ec.Pm, endocortical perimeter.
4. Discussion
Previous studies showed that cancellous BMD and BMC of the femoral neck and proximal tibia gradually decrease with age and decrease to about one-third of 9-month-old values at 27 months of age (Wang et al., 2001), although there was a little or no decrease in cortical BMC and BMD of the tibia and femur diaphysis (Banu et al., 2002). In the present study, however, BMC and vBMD of both femur and tibia trabecular bones in HFS-fed rats aged over 1.5 years were about two times as high as those of the control SLD-fed rats. Moreover, almost all the TBA parameters of the femur/tibia and some CBG parameters of the tibia in the HFS group showed a well-developed bone formation as compared with the SLD group. Thus, in aged rats, HFS diet intake resulted in higher levels of bone mass, which was assessed with reference to BMC, vBMD and TBA/CBG parameters.
One factor known to influence bone mass in HFD-/HFS diet-fed rodents appears to be body weight. As mentioned in “Introduction”, body weight positively correlated with BMD (Reid, 2010, Cherif et al., 2017), and the bone parameters changed in HFD-fed rodents with increased body weight (Lac et al., 2008, Patsch et al., 2011, Malvi et al., 2014, Lecka-Czernik et al., 2015). In our HFS group, body weight increased with age after initiating the diet, whereas body weight decreased in the SLD group. Therefore, there were significant differences in body weight between the two groups during week 3–24 of the diet feeding period. Lecka-Czernik et al. have proposed that bone mass acquired in mice with 11 week-diet-induced obesity is a result of a two-phase process; the first phase consists of either the beneficial effect of fat tissue expansion to increase bone mass by increased mechanical loading and/or increased production of bone anabolic adipokines and/or nutritional effect of fatty acids, and the following second phase consists of decreased bone formation/turnover resulting from development of metabolic impairment (Lecka-Czernik et al., 2015). In the present study using aged rats with 24 week-diet induced obesity, the bone mass and structural parameters correlated well with body weight, in accordance with the beneficial effect of fat expansion on bone mass in the above-mentioned first phase. In relation to this, adipose tissue may exert independent effects on bone remodeling by releasing many bioactive substances with hormonal activity, and may contribute to an increase in bone mass (Holecki and Wiecek, 2010). Furthermore, fat mass was found to influence the secretion of insulin, preptin and amylin from the pancreatic beta-cells. Such a co-secretion of pancreatic hormones was reported to lead to an increase in osteoblast activity and a decrease in osteoclast activity, ultimately resulting in the increase of bone mass (Reid, 2010, Reid, 2008). Therefore, in our HFS-fed rats with increased body weight, the higher levels of bone mass may be ascribable to the augmented co-selection of pancreatic hormones. Considering such facts, the relationship among factors, such as aging, bone marrow fat and age-related bone loss, remains to be clarified (Gustavo, 2008).
Another factor that influences bone mass in HFD-/HFS diet-fed rats is the age. In young rodents, the HFD/HFS diet intake had negative effects on bone parameters, such as deterioration of bone mechanical strength (Zernicke et al., 1995, Lac et al., 2008, Cao et al., 2009, Ionova-Martine et al., 2010, Patsch et al., 2011). In rats fed with HFS diet for two years, bone mechanical strength was found to be deteriorated despite normal levels of bone mineral content and geometry in femoral neck except the cortical shell, suggesting that the HFS diet intake may affect bone quality, like bone collagen and bone turnover (Zernicke et al., 1995). To the contrary, in adult rodent rats as well as in our aged rats, the HFD intake had positive effects on bone mass and structure (Lecka-Czernik et al., 2015, Malvi et al., 2014). For example, in 8–10-week-old male Wistar rats, the HFD intake for 7 months brought forth a significantly increase in BMD, BMC and skeletal area and subsequently the HFD intake for 9 months induced a rise in BV/TV and Tb.N and a decline in Tb.Sp and TBPf as compared with control rats (Malvi et al., 2014). Likewise, 12-week-old male mice fed with HFD for 11 weeks showed an increase in BMD, BMC and trabecular/cortical bone structural parameters as compared with control mice (Lecka-Czernik et al., 2015). On the other hand, in human, the effects of obesity/fat mass on bone mass and fracture risk were dependent upon age and sex (Freitas et al., 2016, Kim et al., 2016, Dimitri et al., 2012). In addition, HFD-induced trabecular bone loss was reported to be greater in male than in female young mice (Gautam et al., 2014). Therefore, future studies are required to assess whether the effects of HFD/HFS diet intake on bone properties of aged rats are influenced by sex.
In rats fed with HFD, circulating levels of bone formation/resorption markers remain controversial; one study reporting the unchanged levels of serum TRACP-5b and increased levels of serum ALP (Malvi et al., 2014), and another study reporting the increased levels of serum TRACP-5b and the decreased levels of serum bone-specific alkaline phosphatase (Lecka-Czernik et al., 2015). In the present study, the HFS-fed rats showed significantly increased TRACP-5b levels and increased, but not significantly, mean OC value, as compared with the SLD-fed rats, suggesting that the bone metabolism may be so enhanced that the bone mass is well preserved in our HFS diet-fed rats with about two-fold increase in BMC and vBMD.
In the present study, serum HDL levels were higher in the HFS group than in the SLD group. Recently, it has been appreciated that HDL can interact directly with both osteoblasts and osteoclasts (Ackert-Bicknell, 2012), and also that HDL can facilitate osteoblastogenesis and bone synthesis and most probably affect osteoclastogenesis and osteoclast bone resorption (Papachristou and Blair, 2016). In addition, HDL may play a protective role in the development of degenerative and metabolic conditions such as osteoarthritis and osteoporosis (Papachristou and Blair, 2016). Thus, HDL may be involved in the molecular events of bone metabolism or bone/cartilage homeostasis via the HDL metabolic pathways. In our HFS-fed rats, therefore, the enhanced bone metabolism, which was suggested by bone formation/resorption markers, may be attributable to the high HDL levels.
5. Limitations
In the current study using aged rats, we have examined the effects of HFS diet intake on bone properties in femur and tibia, but not in vertebral body. Likewise, most of the previous studies used femur and/or tibia to clarify HFD-/HFS diet-induced bone alterations in young and/or mature rats (Zernicke et al., 1995, Brahmabhatt et al., 1998, Lac et al., 2008, Malvi et al., 2014, Tian and Yu, 2017), but, to the best of our knowledge, using micro-CT or DXA, only two papers reported BMD and/or bone microarchitecture in vertebral body of mature rats fed with HFS diet (Lavet et al., 2016, Cherif et al., 2017). We have preferred femur and tibia to vertebral body in order to compare our data and several previous data. As mentioned above, there seems to be several factors to influence bone mass in HFS diet-fed rats, besides high fat and/or high sucrose, e.g., body weight, adipokines, pancreatic hormones and HDL. Although body weight-induced bone mass increase is suggested by good correlations between body weight and bone parameters in our aged rats, comparing the studies of weight-bearing femur/tibia and of non-weight-bearing vertebra may elucidate the question whether positive effects of HFS diet intake on bone parameters are due directly to body weight or not.
In the present study, ash/dry weight of the whole femur/tibia bones were slightly, but not significantly, higher in the HFS group than in the SLD group, while BMC, BV and BV/TV of the femoral/tibial trabecular bones were significantly higher in the HFS group than in the SLD group. Moreover, in the tibial cortical bones, CV/(CV + MV) and Ct.Th were significantly higher in the HFS group than in the SLD group, but BMC and CV of both groups were similar to each other. Thus, we could not find any significant differences in ash/dry bone weight between both groups, though increased bone mass in the HFS group was suggested by these bone parameters, which were obtained by analyzing bone CT images of 2 mm-VOI using micro-CT with a voxel size of 19.1 μm, similar to the voxel size used in other studies (18.0 μm, Malvi et al., 2014; 16.1/21.3 μm, Minematsu et al., 2017). In view of these facts, inconsistency observed between whole ash/dry bone weight and BMC/bone microarchitecture may be explainable by the following three reasons. First, trabecular bone of the HFS group showed about two-fold increase in BMC, BV and BV/TV compared with those of the SLD group, while cortical bone of the HFS group showed no increase in BMC and CV. Because the proportion of trabecular bone weight to whole bone weight is very small, increase in trabecular bone mass would not have led to significant increase in whole bone weight. Second, 2 mm-VOI corresponds to about 1/20 of femur length or about 1/22 of tibia length. Therefore, bone changes in VOI may not have reflected the overall bone changes. Finally, no significant difference in whole bone weight between both groups may have resulted from the wide range of whole bone weight in each group. Analysis of expanded VOI by micro-CT with smaller voxel size, i.e., with higher resolution capability, will bring forth more exact data as to bone parameters. In addition, DXA measurements of bones obtained from aged rats fed with HFS diet are needed in order to further examine our observations.
Age-related bone loss was assessed in male Sprague-Dawley (SD) rats (Wang et al., 2001) and male F344 rats (Banu et al., 2002) by scanning bones using peripheral quantitative computed tomography densitometry. In the proximal tibia, cancellous BMC decreased after 9 months of age in SD rats (Wang et al., 2001), while, in F344 rats, it increased up to 18 months of age and thereafter decreased (Banu et al., 2002). Thus, there was a considerable difference in the age-related BMC changes between two different strains of rats. In this study, bone parameters have been measured in male Wistar rats aged 18 months, which were fed with HFS diet for 6 months from 12 months of age. Since we have no data of base line group, i.e., 12-month-old rats, it remains unknown whether the HFS diet-increased bone mass is due to enhanced bone formation or preservation of the age-related bone loss. Therefore, studies including not only experimental but also base line groups are indispensable for solving this important problem.
For a better understanding of the regulation between dietary nutrition and bone health, bone properties, including BV, BMC, BMD, microarchitecture, mechanical strength, etc., were examined in rodents fed with various diets such as HFD, HFS diet, glucose diet, sucrose diet and fructose-rich diet (Tian and Yu, 2017). Our HFS diet, which was a commercially available diet for experimental rodents, contained 25% supplemented sucrose and a slightly higher vitamin D content, in addition to a higher fat content (13.8% fat), compared with the control SLD. In agreement with our results, HFS diet (23.4% fat + 22% sucrose) intake for 16–27 weeks was reported to improve whole body bone mass and BMD (Gerbaix et al., 2012) and also increase trabecular bone mass, i.e., BV/TV (Lavet et al., 2016) in male Wistar rats. To the best of our knowledge, there was only one study reporting the effects of high sucrose diet on bone properties; the densities, bone calcium concentrations and bone breaking strengths of both femur and tibia were significantly lower in Wistar rats, which were fed with high sucrose diet (43% sucrose) for 5 weeks from 3 weeks of age (Tjäderhane and Larmas, 1998). In association with such deleterious effects of high sucrose diet, glucose administration was reported to augment urinary calcium excretion by reducing the renal tubular reabsorption of calcium (Lennon and Piering, 1970), and also high glucose concentration was found to suppress the differentiation and proliferation of cultured osteoblasts (Terada et al., 1998). Therefore, excess sucrose intake, by which high blood glucose is induced, appears to attenuate bone formation via bone metabolism disturbance and/or malfunction of osteoblasts and eventually lose bone mass. Actually, we have recently reported that bone properties, including bone mass, TBA/CBG parameters and bone mechanical strength, deteriorate in the femur/tibia of diabetic Otsuka Long-Evans Tokushima Fatty rats with high blood glucose and high HbA1c values (Minematsu et al., 2016b, Minematsu et al., 2017). In view of these facts, HFS diet-induced trabecular/cortical bone mass increase, reported herein and by Lavet et al., might have been ascribable to high fat content rather than high sucrose content in the diet; in other words, high fat content in the HFS diet might have hidden the deleterious action of sucrose on bone health. Further studies using HFD without sucrose are needed to reveal positive effects of high fat content on bone mass in aged rats.
Since vitamin D is well-known to be responsible for increasing intestinal absorption of calcium and indispensable for promoting the healthy growth and bone remodeling, there is a possibility that higher vitamin D content in our HFS diet might have contributed to higher bone mass in aged rats. Recently, vitamin D diet (2 IU/g) intake for 8 weeks was found to cause no significant difference in femur/tibia bone properties, including BMC, vBMD, BV/TV, Tb.Th, Tb.N, Ct.Ar, Ct.Th, etc., between the rats fed with vitamin D diet and those fed with a control diet (Bianchini et al., 2015). Therefore, it seems unlikely that HFS diet-induced high bone mass would have been caused by a small difference in vitamin D content (0.75 IU/g) between the HFS diet (vitamin D, 2.90 IU/g) and the SLD (vitamin D, 2.15 IU/g). Our HFS diet contained 13.8% crude fat. To investigate, in more detail, the effects of fat on bone properties, further studies should be carried out using fat diets with the different quantities of fat and/or the rigid quality of fat, e.g., HFD composed of known amounts of various fatty acids, like saturated, monounsaturated and polyunsaturated fatty acids.
In this study, we have not measured bone mechanical strength which reflects both bone quantity and bone quality, but have examined bone mass, TBA/CBG parameters and bone formation/resorption markers. In view of our results reported herein, it seems likely that bone mechanical strength is well preserved in our aged rats fed with HFS. Nonetheless, the direct measurements of bone mechanical strength would be required for better understanding the role of HFD/HFS diet intake in bone physiology.
6. Conclusions
In aged rats, HFS diet intake resulted in higher trabecular/cortical bone mass in reference to BMC, vBMD and TBA/CBG parameters, which correlated well with body weight, as compared with SLD intake. Therefore, such effects of HFS diet intake might have been induced by increased body weight.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Ackert-Bicknell C.L. HDL cholesterol and bone mineral density: is there a genetic link? Bone. 2012;50:525–533. doi: 10.1016/j.bone.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu J., Wang L., Kalu D.N. Age-related changes in bone mineral content and density in intact male F344 rats. Bone. 2002;30:125–130. doi: 10.1016/s8756-3282(01)00636-6. [DOI] [PubMed] [Google Scholar]
- Bianchini C., Lavery P., Agellon S., Weiler H.A. The generation of C-3α epimer of 25-hydroxyvitamin D and its biological effects on bone mineral density in adult rodents. Calcif. Tissue Int. 2015;96:453–464. doi: 10.1007/s00223-015-9973-9. [DOI] [PubMed] [Google Scholar]
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Brahmabhatt V., Rho J., Bernardis L., Gillespie R., Ziv I. The effects of dietary-induced obesity on the biomechanical properties of femora in male rats. Int. J. Obes. Relat. Metab. Disord. 1998;22:813–818. doi: 10.1038/sj.ijo.0800668. [DOI] [PubMed] [Google Scholar]
- Cao J.J., Gregoire B.R., Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44:1097–1104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Cherif R., Vico L., Laroche N., Sakly M., Attia N., Lavet C. Dual-energy X-ray absorptiometry underestimates in vivo lumbar spine bone mineral density in overweight rats. J. Bone Miner. Metab. 2017 doi: 10.1007/s00774-017-0813-z. [DOI] [PubMed] [Google Scholar]
- Compston J.E., Watts N.B., Chapurlat R., Cooper C., Boonen S., Greenspan S., Pfeilschifter J., Silverman S., Díez-Pérez A., Lindsay R., Saag K.G., Netelenbos J.C., Gehlbach S., Hooven F.H., Flahive J., Adachi J.D., Rossini M., LaCroix A.Z., Roux C., Sambrook P.N., Siris E.S., Glow Investigators Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011;124:1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitri P., Bishop N., Walsh J.S., Eastell R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone. 2012;50:457–466. doi: 10.1016/j.bone.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Freitas P.M., Garcia Rosa M.L., Gomes A.M., Wahrlich V., Di Luca D.G., da Cruz Filho R.A., da Silva Correia D.M., Faria C.A., Yokoo E.M. Central and peripheral fat body mass have a protective effect on osteopenia or osteoporosis in adults and elderly? Osteoporos. Int. 2016;27:1659–1663. doi: 10.1007/s00198-015-3414-5. [DOI] [PubMed] [Google Scholar]
- Gautam J., Choudhary D., Khedgika V., Kushwaha P., Singh R.S., Shingh D., Tiwari S., Trivedi R. Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br. J. Nutr. 2014;111:1811–1821. doi: 10.1017/S0007114514000051. [DOI] [PubMed] [Google Scholar]
- Gerbaix M., Metz L., Mac-Way F., Lavet C., Guillet C., Walrand S., Masgrau A., Linossier M.-T., Vico L., Daniel C. Impact of an obesogenic diet program on bone densitometry, micro architecture and metabolism in male rat. Lipids Health Dis. 2012;11:91. doi: 10.1186/1476-511X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavo D. Bone and fat connection in aging bone. Curr. Opin. Rheumatol. 2008;20:429–434. doi: 10.1097/BOR.0b013e3283025e9c. [DOI] [PubMed] [Google Scholar]
- Holecki M., Wiecek A. Relationship between body fat mass and bone metabolism. Pol. Arch. Med. Wewn. 2010;120:361–367. [PubMed] [Google Scholar]
- Ionova-Martine S.S., Do S.H., Barth H.D., Szadkowska M., Porter A.E., Ager J.W., III, Ager J.W., Jr., Alliston T., Vaisse C., Ritchie R.O. Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone. 2010;46:217–225. doi: 10.1016/j.bone.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.M., Kim S.H., Kim S., Yoo J.S., Choe E.Y., Won Y.J. Variations in fat mass contribution to bone mineral density by gender, age, and body mass index: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2011. Osteoporos. Int. 2016;27:2543–2554. doi: 10.1007/s00198-016-3566-y. [DOI] [PubMed] [Google Scholar]
- Lac G., Cavalie H., Ebal E., Michaux O. Effect of a high fat diet on bone of growing rats. Correlations between visceral fat, adiponectin and bone mass density. Lipids Health Dis. 2008;7:16. doi: 10.1186/1476-511X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavet C., Martin A., Linossier M.-T., Bossche A.V., Laroche N., Thomas M., Gerbaix M., Ammann P., Fraissenon A., Lafage-Proust M.-H., Courteix D., Vico L. Fat and sucrose intake induces obesity-related bone metabolism disturbances: kinetic and reversibility studies in growing and adult rats. J. Bone Miner. Res. 2016;31:98–115. doi: 10.1002/jbmr.2596. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B., Stechschulte L.A., Czernik P.J., Dowling A.R. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol. Cell. Endocrinol. 2015;410:35–41. doi: 10.1016/j.mce.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Lelovas P.P., Xanthos T.T., Thoma S.E., Lyritis G.P., Dontas I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- Lennon E.J., Piering W.F. A comparison of the effects of glucose ingestion and NH4Cl acidosis on urinary calcium and magnesium excretion in man. J. Clin. Invest. 1970;49:1458–1465. doi: 10.1172/JCI106363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvi P., Piprode V., Chaube B., Pote S.T., Mittal M., Chattopadhyay N., Wani M.R., Bhat M.K. High fat diet promotes achievement of peak bone mass in young rats. Biochem. Biophys. Res. Commun. 2014;455:133–138. doi: 10.1016/j.bbrc.2014.10.131. [DOI] [PubMed] [Google Scholar]
- Minematsu A., Nishii Y., Imagita H., Takeshita D., Sakata S. Whole-body vibration can attenuate the deterioration of bone mass and trabecular bone microstructure in rats with spinal cord injury. Spinal Cord. 2016;54:1169–1175. doi: 10.1038/sc.2015.220. [DOI] [PubMed] [Google Scholar]
- Minematsu A., Hanaoka T., Takada Y., Okuda S., Imagita H., Sakata S. Femoral bone structure in Otsuka Long-Evans Tokushima Fatty rats. Osteoporos. Sarcopen. 2016;2:25–29. doi: 10.1016/j.afos.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minematsu A., Hanaoka T., Takeshita D., Takada Y., Okuda S., Imagita H., Sakata S. Long-term wheel-running can prevent deterioration of bone properties in diabetes mellitus model rats. J. Musculoskelet. Neuronal Interact. 2017;17:433–443. [PMC free article] [PubMed] [Google Scholar]
- Papachristou D.J., Blair H.C. Bone and high-density lipoprotein: the beginning of a beautiful friendship. World J. Orthop. 2016;7(2):74–77. doi: 10.5312/wjo.v7.i2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch J.M., Kiefer F.W., Verga P., Paila P., Raunera M., Stupphanna D., Reschf H., Moserg D., Zyssetd P.K., Stulnigc T.M., Pietschmann P. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism. 2011;60:243–249. doi: 10.1016/j.metabol.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I.R. Relationships between fat and bone. Osteoporos. Int. 2008;19:595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- Reid I.R. Fat and bone. Arch. Biochem. Biophys. 2010;503:20–27. doi: 10.1016/j.abb.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Kuroda T., Saito M., Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporos. Int. 2013;24:69–76. doi: 10.1007/s00198-012-2209-1. [DOI] [PubMed] [Google Scholar]
- Tang X., Liu G., Kang J., Hou Y., Jiang F., Yuan W., Shi J. Obesity and risk of hip fracture in adults: a meta-analysis of prospective cohort studies. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Inaba M., Yano Y., Hasuma T., Nishizawa Y., Morii H., Otani S. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone. 1998;22:17–23. doi: 10.1016/s8756-3282(97)00220-2. [DOI] [PubMed] [Google Scholar]
- Tian L., Yu X. Fat, sugar, and bone health: a complex relationship. Nutrients. 2017;9:506. doi: 10.3390/nu9050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjäderhane L., Larmas M. A high sucrose diet decreases the mechanical strength of bones in growing rats. J. Nutr. 1998;128:1807–1810. doi: 10.1093/jn/128.10.1807. [DOI] [PubMed] [Google Scholar]
- Wang L., Banu J., McMahan C.A., Kalu D.N. Male rodent model of age-related bone loss in men. Bone. 2001;29:141–148. doi: 10.1016/s8756-3282(01)00483-5. [DOI] [PubMed] [Google Scholar]
- Zernicke R.F., Salem G.J., Barnard R.J., Schramm E. Long-term, high-fat-sucrose diet alters rat femoral neck and vertebral morphology, bone mineral content, and mechanical properties. Bone. 1995;16:25–31. doi: 10.1016/s8756-3282(00)80007-1. [DOI] [PubMed] [Google Scholar]