Abstract
Introduction:
Frailty in elderly trauma populations has been correlated with an increased risk of morbidity and mortality. The Score for Trauma Triage in the Geriatric and Middle-Aged (STTGMA) is a validated mortality risk score that evaluates 4 major physiologic criteria: age, comorbidities, vital signs, and anatomic injuries. The aim of this study was to investigate whether the addition of additional frailty variables to the STTGMA tool would improve risk stratification of a middle-aged and elderly trauma population.
Methods:
A total of 1486 patients aged 55 years and older who met the American College of Surgeons Tier 1 to 3 criteria and/or who had orthopedic or neurosurgical traumatic consultations in the emergency department between September 2014 and September 2016 were included. The STTGMAORIGINAL and STTGMAFRAILTY scores were calculated. Additional “frailty variables” included preinjury assistive device use (disability), independent ambulatory status (functional independence), and albumin level (nutrition). The ability of the STTGMAORIGINAL and the STTGMAFRAILTY models to predict inpatient mortality was compared using area under the receiver operating characteristic curves (AUROCs).
Results:
There were 23 high-energy inpatient mortalities (4.7%) and 20 low-energy inpatient mortalities (2.0%). When the STTGMAORIGINAL model was used, the AUROC in the high-energy and low-energy cohorts was 0.926 and 0.896, respectively. The AUROC for STTGMAFRAILTY for the high-energy and low-energy cohorts was 0.905 and 0.937, respectively. There was no significant difference in predictive capacity for inpatient mortality between STTGMAORIGINAL and STTGMAFRAILTY for both the high-energy and low-energy cohorts.
Conclusion:
The original STTGMA tool accounts for important frailty factors including cognition and general health status. These variables combined with other major physiologic variables such as age and anatomic injuries appear to be sufficient to adequately and accurately quantify inpatient mortality risk. The addition of other common frailty factors that account for does not enhance the STTGMA tool’s predictive capabilities.
Keywords: mortality risk, frailty, middle-aged, geriatric, trauma
Introduction
With an aging population, patients aged 65 years and older increasingly comprise the number of annual trauma admissions and mortalities.1 This group is the fastest growing segment of the US population, and its members are enjoying a much more active and independent lifestyle than their predecessors. This increase in longevity and activity has resulted in a greater incidence of traumatic injury.2 National mortality rates reflect these changes as trauma has risen to the seventh leading cause of death among those aged 65 years and older.2
As the trauma population increases in age, these patients are more likely to be characterized as frail. Numerous studies have characterized the association between frailty and morbidity and mortality in the geriatric trauma cohort.3–6 Frailty has been defined as a clinical syndrome resulting in decreased physiologic reserve and increased susceptibility to disability in the presence of stressors such as illness or trauma.7 Although frailty has been shown to be important in the prediction of outcomes in geriatric trauma patients,3 there is no consensus on the best clinical assessment tool to measure frailty.1 A recent systematic review identified 32 unique frailty assessment tools.8 Only 4 tools were deemed objective and feasible, none of which have been validated in the trauma population (Electronic Frailty Model,9 the Fall History,10 the Patel Modified Frailty Index,11 and the National Surgical Quality Improvement Program Frailty Index).12
Given the increase in geriatric trauma, there is a demonstrated need to identify older trauma patients at high risk of morbidity, mortality, and increased resource usage. This group previously demonstrated the predictive ability of patient age, injury severity, level of arousal upon presentation, and comorbidity to detect mortality risk via the Score for Trauma Triage in the Geriatric and Middle-Aged (STTGMA) in a single-level 1 trauma center and subsequently validated the STTGMA tool within the National Trauma Databank (>100 000 patients).13 While it includes frailty factors such as cognition and general health status, it does not include other important frailty factors such as disability, functional independence, or nutritional status. In light of developing literature demonstrating the importance of frailty in the mortality of middle-aged and geriatric trauma patients, we sought to evaluate whether adding these additional frailty variables to the STTGMA score would improve risk stratification of the elderly trauma population.
Methods
In this institutional review board–approved protocol, all patients aged 55 years and older evaluated by orthopedic surgery or trauma surgery within the emergency department for nonpenetrating trauma at an urban level 1 trauma center between October 1, 2014, and September 30, 2016, were prospectively followed. This included but was not limited to all tier 1, 2, and 3 trauma activations as defined by the American College of Surgeons guidelines.14 A total of 1486 consecutive patients met all inclusion criteria. Study variables were obtained by the consulting resident physician at the time of initial patient evaluation and recorded within the medical record. Participating surgical residents were formally educated regarding data collection using an online education module. An Internet-based calculator specifically designed for STTGMA was used to calculate a low-energy STTGMA score (STTGMALE-ORIGINAL) and a high-energy STTGMA score (STTGMAHE-ORIGINAL). Study variables included patient age; Glasgow coma scale (GCS) upon initial evaluation; mechanism of injury; Abbreviated Injury Severity (AIS) subscores for the head and neck (AIS-HN), chest (AIS-CHS), and pelvis and extremity body regions (AIS-EXT); and Charlson comorbidity index score (CCI). Mechanism of injury was dichotomized into low- and high-energy mechanisms. Low-energy mechanism of injury included all ground-level falls less than or equal to 2 stairs. High-energy mechanism of injury included all falls from height (>2 stairs), motor vehicle crashes, motorcycle crashes, and pedestrians struck by vehicles.
Additional variables not routinely collected in trauma registries were also collected. Preinjury functional status was assessed by patient- or family-reported ambulatory status. Patients able to ambulate outside of the home for any period of time without assistance from another person were identified as community ambulators. Patients able to ambulate within the home for any period of time without assistance from another individual were identified as household ambulators. Patients who relied upon another individual for all transfers and ambulation were identified as nonambulatory. Serum albumin was recorded at the time of initial patient evaluation and was used as a surrogate for long-term nutritional status. Use of a gait assistive device was recorded for any patient who reported use of a cane, walker, crutch, or wheelchair for any period of time inside or outside of the home. Preinjury anticoagulation status was also assessed and was defined as any patient presenting to the emergency department currently taking any of the following medications: antiplatelet medications, heparin derivatives, vitamin K antagonists, antifactor Xa inhibitors, and direct thrombin inhibitors. The primary study outcome of inpatient mortality was obtained from the medical record by designated research staff.
Statistical Analyses
All statistical analyses were performed using SPSS software version 22. Descriptive analyses of patients’ characteristics and outcome measures’ summary were first obtained via means (standard deviation [SD]) for continuous variables and n (%) for categorical variables. The predictive capacity of the STTGMAHE-ORIGINAL and STTGMALE-ORIGINAL scores was tested first. The predictive capacity of the model was quantified by calculating the area under the receiver operating characteristic curve (AUROC). The AUROC is a summary measure of the predictive ability of the model, with values between 0.90 and 1 indicating excellent predictive discrimination. An AUC <0.75 was regarded as noncontributory. The AUC values are reported with 95% confidence interval (CI).
To improve upon the original STTGMA model, a backward stepwise logistic regression analysis was used to develop the STTGMAHE-FRAILTY and STTGMALE-FRAILTY. All originally identified study variables (age, AIS subscores, GCS score, and CCI) and additional variables (preinjury ambulatory capacity, assistive device use, albumin level, and anticoagulation status) were considered as initial candidates to model their relationship to mortality status. Multivariate logistic regression models using a backward stepwise variable selection approach were then performed to identify a new prediction model. All of the variables included in the original STTGMA score were included in the final model. For the additional variables, we used an initial significance threshold of P < .20 for inclusion in the model, while the final model included only independent predictors of inhospital mortality with significance level of P < .05. The predictive capacity of the final model was quantified by calculating the AUROC. We compared the AUROC of STTGMAORIGINAL with STTGMAFRAILTY to determine whether there was a difference in predictive capacity for inpatient mortality. To demonstrate the clinical difference between STTGMAORIGINAL and STTGMAFRAILTY, we chose an arbitrary STTGMA score cutoff of 3% to assess ability of the score to predict inpatient mortality.
Results
A total of 1486 patients met the inclusion criteria. Of which 492 (33.1%) patients met criteria for inclusion within the high-energy mechanism of injury cohort and 994 (66.9%) patients met criteria for inclusion within the low-energy mechanism of injury group. The average patient age at initial presentation was 72.2 (11.8) years. Baseline study characteristics of the high-energy and low-energy groups are summarized within Table 1. There were 23 high-energy inpatient mortalities (4.7% mortality rate) and 20 low-energy inpatient mortalities (2.0% mortality rate). The injury distribution of the cohort is shown in Table 2. Application of the STTGMAHE-ORIGINAL mortality risk model prospectively in our patient population produced an AUC of 0.926 (95% CI: 0.875-0.978, P < .001). The STTGMALE-ORIGINAL risk model produced an AUC of 0.896 (95% CI: 0.827-0.965, P < .001).
Table 1.
Population Characteristics.
| Variable | High-Energy Group, n = 492 | Low-Energy Group, n = 994 |
|---|---|---|
| Age, years | 68.05 (10.14) | 74.30 (11.97) |
| Glasgow coma score | 14.02 (2.65) | 14.58 (1.55) |
| Abbreviated Injury Severity subscore | ||
| Head and neck region | 1.08 (147) | 0.45 (1.022) |
| Chest region | 0.39 (0.85) | 0.12 (0.45) |
| Pelvis and extremity region | 1.45 (1.39) | 1.89 (1.21) |
| Serum albumin (g/dL) | 3.94 (0.52) | 3.84 (0.57) |
| Charlson comorbidity index | 0.72 (1.27) | 1. 12 (1.41) |
| Ambulatory status, n (%) | ||
| Community | 468 (95.1%) | 798 (80.3%) |
| Household | 19 (3.9%) | 166 (16.7%) |
| Nonambulatory | 5 (1.0%) | 30 (3%) |
| Assistive device usage | 49 (10%) | 298 (29.1%) |
| Anticoagulant usage | 127 (25.8%) | 335 (33.7%) |
Table 2.
Distribution of Injuries by ICD-10-CM Code for High- and Low-Energy Patients.
| ICD-10-CM Title | High Energy, n = 492 | Low Energy, n = 994 |
|---|---|---|
| Injuries to the abdomen, lower back, lumbar spine, pelvis, and external genitals | 64 (13.0%) | 39 (3.9%) |
| Fracture of lumbar spine and pelvis | 46 (9.5%) | 32 (3.2%) |
| Dislocation and sprain of joints and ligaments of lumbar spine and pelvis | 1 (0.2%) | 0 (0.0%) |
| Injury of lumbar and sacral spinal cord and nerves at abdomen, lower back, and pelvis level | 0 (0.0%) | 1 (0.1%) |
| Injury of blood vessels at abdomen, lower back, and pelvis level | 1 (0.2%) | 1 (0.1%) |
| Injury of intra-abdominal organs | 21 (4.3%) | 6 (0.6%) |
| Injury of urinary and pelvic organs | 4 (0.8%) | 1 (0.1%) |
| Injuries to the ankle and foot | 27 (5.5%) | 22 (2.2%) |
| Injuries to the elbow and forearm | 60 (12.2%) | 142 (14.3%) |
| Injuries to the head and neck | 262 (53.3%) | 233 (23.4%) |
| Injuries to the hip and thigh | 38 (7.7%) | 275 (27.7%) |
| Injuries to the knee and lower leg | 111 (22.6%) | 144 (14.5%) |
| Injuries to the shoulder and upper arm | 54 (11.0%) | 143 (14.4%) |
| Injuries to the thorax | 107 (21.8%) | 51 (5.1%) |
| Injuries to the wrist, hand, and fingers | 32 (6.5%) | 56 (5.6%) |
Abbreviations: ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification.
Both STTGMAHE-FRAILTY and STTGMALE-FRAILTY were generated to evaluate the effect of the newly collected patient variables on mortality prediction. Backward stepwise selection produced a final study cohort-specific prediction model including the following variables for the high-energy group: age; GCS score; AIS subscore for the head and neck, chest, and pelvis and extremity regions; and albumin (Table 3). The AUC of this model was found to be 0.905 (95% CI: 0.862-0.949, P < .001). This AUROC was not significantly different from the AUROCs produced from the STTGMAHE-ORIGINAL model (P = .710). The ROC curves for the 2 high-energy models are shown in Figure 1. In the low-energy mechanism of injury cohort, backward stepwise regression produced a final study cohort-specific model including the following variables: age; GCS score; AIS subscore of the head and neck and chest regions; CCI score; and ambulatory status (Table 4). The AUC of this model was 0.937 (95% CI: 0.888-0.985, P < .001). While this STTGMALE-FRAILTY model produced a greater AUROC, the difference between this and that of the STTGMALE-ORIGINAL was not significant (P = .580). The ROC curves for the low-energy scores are shown in Figure 2.
Table 3.
High-Energy Cohort Analysis.
| Variable | Multivariate Analysis, Odds Ratio (95% CI) | P |
|---|---|---|
| Age | 1.065 (0.997-1.137) | .061 |
| Glasgow coma score | 0.701 (0.591-0.831) | <.001 |
| AIS head and neck subscore | 1.208 (0.792-1.843) | .380 |
| AIS chest subscore | 2.269 (1.405-3.665) | .001 |
| AIS extremity and pelvis subscore | 1.079 (0.684-1.701) | .745 |
| Serum albumin | 0.228 (0.077-0.674) | .008 |
| Charlson comorbidity index | 1.293 (0.892 -1.876) | .175 |
| Ambulatory status | 0.615 (0.075-5.052) | .052 |
| Use of assistive device | 1.016 (0.144-7.150) | .987 |
| Use of anticoagulant | 2.933 (0.638-13.489) | .167 |
Abbreviations: AIS, Abbreviated Injury Severity; CI, confidence interval.
Figure 1.
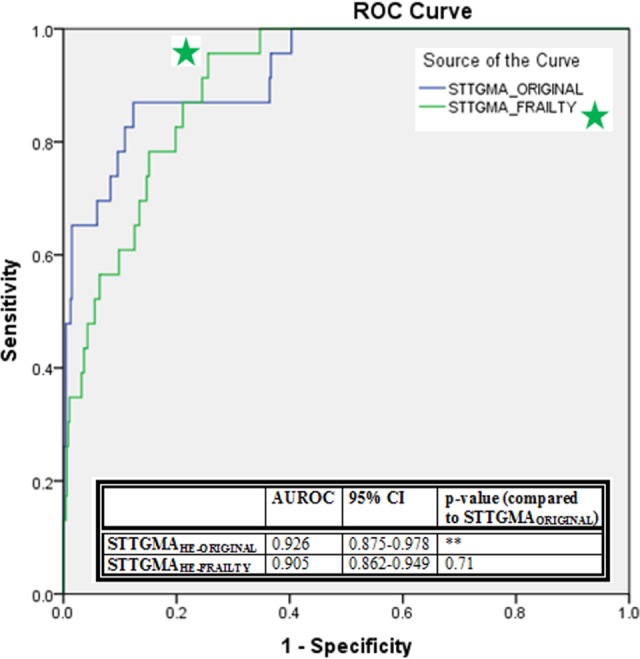
The ROC curves for STTGMAHE-ORIGINAL and STTGMAHE-FRAILTY and comparison of AUROC for 2 models. AUROC indicates area under the receiver operating characteristic curves; STTGMAHE-ORIGINAL, high-energy Score for Trauma Triage in the Geriatric and Middle-Aged; STTGMAHEFRAILTY, high-energy Score for Trauma Triage in the Geriatric and Middle-Aged with additional frailty variables.
Table 4.
Low-Energy Cohort Analysis.
| Variable | Multivariate Analysis, Odds Ratio (95% CI) | P |
|---|---|---|
| Age | 1.018 (0.971-1.068) | .450 |
| Glasgow coma score | 0.721 (0.612-0.851) | <.001 |
| AIS head and neck subscore | 1.925 (1.214-3.048) | .005 |
| AIS chest subscore | 0.954 (0.396-2.300) | .954 |
| AIS extremity and pelvis subscore | 1.072 (0.630-1.824) | .797 |
| Serum albumin | 1.210 (0.493-2.968) | .677 |
| Charlson comorbidity index | 1.704 (1.278-2.273) | <.001 |
| Ambulatory status | 2.763 (1.147-6.657) | .024 |
| Use of assistive device | 1.618 (0.455-5.752) | .457 |
| Use of anticoagulant | 1.391 (0.468-4.128) | .553 |
Abbreviations: AIS, Abbreviated Injury Severity; CI, confidence interval.
Figure 2.
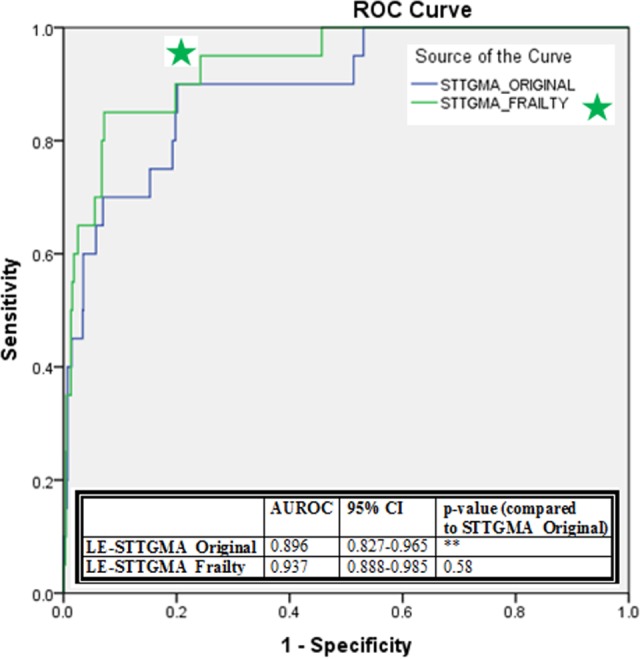
The ROC curves for STTGMALE-ORIGINAL and STTGMALE-FRAILTY and comparison of AUROC for 2 models. AUROC indicates area under the receiver operating characteristic curves; STTGMALE-ORIGINAL, low-energy Score for Trauma Triage in the Geriatric and Middle-Aged; STTGMALEFRAILTY, low-energy Score for Trauma Triage in the Geriatric and Middle-Aged with additional frailty variables.
Of the 23 index hospitalization deaths observed in the high-energy cohort, 20 had a STTGMAHE-ORIGINAL scores of >3%. Using the STTGMAHE-FRAILTY scores, 1 additional patient death would have been identified using the same threshold of 3%. Of the 20 index hospitalization deaths observed in the low-energy cohort, 15 had a >3% STTGMALE-ORIGINAL. Using the STTGMALE-FRAILTY scores, 2 additional patients would have been identified using the same threshold of 3%.
Discussion
The STTGMAORIGINAL accounts for important frailty factors including cognitional and general health status. These variables combined with other major physiologic variables such as age and anatomic injuries appear to be sufficient to adequately and accurately quantify inpatient mortality risk. When additional common frailty factors that account for disability, independent functional ability, and nutritional status were included in the model (STTGMAFRAILTY), several were significant predictors of mortality as shown in Tables 3 and 4. However, the addition of these additional frailty factors does not appear to increase the predictive ability of the model.
Although there is increasing evidence linking frailty to outcomes in trauma patients, quantifying frailty particularly in the trauma setting has proven difficult. The only current clinical tool designed to quantify frailty in the trauma setting is the Trauma-Specific Frailty Index, which is composed of 15 variables including comorbidities, medications, daily activities, health attitude, sexual activity, and nutrition.15 While the purpose of this study was not to design a new clinical tool to measure frailty, the study did seek to determine which “frailty variables” were important in predicting inpatient mortality in middle-aged and geriatric patients. With the growth of electronic medical records, in the future, additional “frailty variables” may be readily available at the time of presentation. Currently, these measures remain lengthy, labor intensive, and are limited by a patient’s ability to provide this information. By utilizing easily collected factors such as ambulatory status and albumin, we aimed to characterize the patient’s functional capacity and health status in a quick and reliable manner within the context of a busy trauma setting. These 2 physiologic characteristics correlate with how “frail” the patient is prior to injury.
This study also demonstrates the ability of the STTGMA tool to be used prospectively to predict inpatient mortality. Previously, the STTGMA tool was validated in a retrospective fashion using the National Trauma Databank, similar to other mortality risk tools.13 To our knowledge, no group has tested a mortality risk model using data collected in real time. We expected the model’s performance to decline using data collected at the time of initial patient presentation; however, the model retained its strong ability to predict inpatient mortality. This demonstrates that resident physicians were able to collect the data needed to calculate a risk score and record the data with adequate fidelity; therefore, the STTGMA tool can be used in real time for clinical decision support.
The STTGMA tool demonstrates ease of variable collection, objectivity in measurement, ease of calculation, portability among settings, and reproducibility. This tool has demonstrated greater predictive ability than other tools within the literature. Bouzat et al reported an AUROC of 0.93 for the Triage-Revised Trauma Score (T-RTS) score and 0.86 for the Trauma Revised Injury Severity Score (TRISS).16 Note, however, that this study as well as all previous studies evaluating the utility of mortality risk scores have combined low- and high-energy trauma which falsely skews the predictive capacity for low-energy trauma.17 The STTGMA tool is unique because it distinguishes between these 2 vastly different mechanisms of injury.
With the advent of large centralized databases, greater emphasis has been placed upon prediction tools to help inform clinician decision-making. Tools such as the Acute Physiology and Chronic Health Evaluation II (APACHE II) have helped clinicians and researchers alike learn to better care for patients. The need for refined care pathways in geriatric trauma care has been well established.1,18 It is the hope that new prediction tools such as the STTGMA score can better identify areas of geriatric care where high-value care can be instituted. Because the STTGMA tool provides a “sickness profile” of the patient that includes comorbidity and injury status, it could allow for triaging of low-risk patients into specific high-value care pathways that standardize and minimize variation in care. Higher risk patients could be triaged into high-value pathways that include early palliative care consultations and goals of care discussions. In addition, these patients may need to be triaged to higher levels of care within the hospital (eg, intensive care unit or step-down unit) or triaged to higher acuity hospitals that can manage these high-risk patients. Other groups have created mortality prediction scores for this geriatric population; however, the STTGMA score is unique in that it allows for mortality prediction at presentation.19 Furthermore, to our knowledge, no such score incorporates a patient’s frailty level, which as highlighted above, plays a significant role in the outcomes of geriatric trauma patients. Finally, this study has confirmed that previously untrained medical providers can generate this score, something no other similar tool has demonstrated.
This project was limited by its sample size. Although there was a large number of patients in our data set as a whole, due to the low incidence of death during the index hospitalization in trauma patients, the number of deaths observed in the population was low. In addition, our sample size was relatively healthy reflected by a low mean CCI score. However, the low CCI score observed in this patient cohort may be artificially low especially in the high-energy trauma population or in those patients with dementia as the complete medical history of these patients is often not available at the time of admission. The measures used to quantify a patient’s injury severity, comorbid conditions, and functional status are imperfect and can be susceptible to the limitations of subjectivity. We sought to limit this subjectivity with a standardized online STTGMA training tool that every resident administering the STTGMA score was required to complete. Future study is needed to assess the ability of the risk score to predict long-term outcomes. As most geriatric trauma patients will survive index hospitalization, information regarding their extended mortality risk and return to baseline function will prove useful. With studies demonstrating that frailty is important not only in predicting mortality but also in determining postinjury functional recovery, further analysis is necessary to determine whether the additional frailty variables used in the STTGMAFRAILTY score improve the tool’s ability to predict functional outcomes compared to the STTGMAORIGINAL score.5 Further study is also warranted to characterize the impact integration of this scoring system into the medical record could have on early intervention in the patient care pathway.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kozar RA, Arbabi S, Stein DM, et al. Injury in the aged: geriatric trauma care at the crossroads. J Trauma Acute Care Surg. 2015;78(6):1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maxwell CA, Miller RS, Dietrich MS, Mion LC, Minnick A. The aging of America: a comprehensive look at over 25,000 geriatric trauma admissions to United States hospitals. Am Surg. 2015;81(6):630–636. [DOI] [PubMed] [Google Scholar]
- 3. Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149(8):766–772. [DOI] [PubMed] [Google Scholar]
- 4. Dwyer JB, Reynoso JF, Seevers GA, et al. Assessing preoperative frailty utilizing validated geriatric mortality calculators and their association with postoperative hip fracture mortality risk. Geriatr Orthop Surg Rehabil. 2014;5(3):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maxwell CA, Mion LC, Mukherjee K, et al. Preinjury physical frailty and cognitive impairment among geriatric trauma patients determine postinjury functional recovery and survival. J Trauma Acute Care Surg. 2016;80(2):195–203. [DOI] [PubMed] [Google Scholar]
- 6. Ensrud KE, Ewing KS, Tyalor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–389. [DOI] [PubMed] [Google Scholar]
- 7. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 8. McDonald VS, Thomson KA, Lewis PR, Sise CB, Sise MJ, Shackford SR. Frailty in trauma: a systematic review of the surgical literature for clinical assessment tools. J Trauma Acute Care Surg. 2016;80(5):824–834. [DOI] [PubMed] [Google Scholar]
- 9. Amrock LG, Neuman MD, Lin HM, Deiner S. Can routine preoperative data predict adverse outcomes in the elderly? Development and validation of a simple risk model incorporating a chart-derived frailty score. J Am Coll Surgeons. 2014;219(4):684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones TS, Dunn CL, Wu DS, Cleveland JC, Jr, Kile D, Robinson TN. Relationship between asking an older adult about falls and surgical outcomes. JAMA Surg. 2013;148(12):1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel KV, Brennan KL, Brennan ML, Jupiter DC, Shar A, Davis ML. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin Orthop Relat Res. 2014;472(3):1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526–1530. [DOI] [PubMed] [Google Scholar]
- 13. Konda SK, Gales JG, Manoli A, Gales J, Karunakar MA; Carolinas Trauma Network Research Group. Development of a middle-age and geriatric trauma mortality risk score: a tool to guide palliative care consultations. Bull Hosp Jt Dis (2013). 2016;74(4):298–305. [PubMed] [Google Scholar]
- 14. American College of Surgeons Committee on Trauma. Resources for Optimal Care of the Injured Patient. Chicago, IL: American College of Surgeon; 1999. [Google Scholar]
- 15. Joseph B, Pandit V, Zangbar B, et al. Validating trauma-specific frailty index for geriatric trauma patients: a prospective analysis. J Am Coll Surg. 2014;219(1):10–17. [DOI] [PubMed] [Google Scholar]
- 16. Bouzat P, Legrand R, Gillois P, et al. Prediction of intra-hospital mortality after severe trauma: which pre-hospital score is the most accurate? Injury. 2016;47(1):14–18. [DOI] [PubMed] [Google Scholar]
- 17. Konda SK, Lack WD, Seymour RB, Karunakar MA. Mechanism of injury differentiates risk factors for mortality in geriatric trauma patients. J Orthop Trauma. 2015;29(7):331–336. [DOI] [PubMed] [Google Scholar]
- 18. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183–189. [DOI] [PubMed] [Google Scholar]
- 19. Cook AC, Joseph B, Inaba K, et al. Multicenter external validation of the geriatric trauma outcome score: a study by the Prognostic Assessment of Life and Limitations After Trauma in the Elderly (PALLIATE) consortium. J Trauma Acute Care Surg. 2016;80(2):204–209. [DOI] [PubMed] [Google Scholar]


