Abstract
Background: An ideal peripheral nerve repair construct does not currently exist. Our primary goal was to determine whether fibrin glue adds to the tensile strength of conduit-assisted primary digital nerve repairs. Our secondary goal was to evaluate the impact of varying suture number and location on the tensile strength. Methods: Ninety cadaveric digital nerves were harvested and divided equally into the following repair groups: A (4/4), B (2/2), C (0/2), D (0/1), and E (0/0) with the first number referring to the number of sutures at the coaptation and the second number referring to the number of sutures at each proximal and distal end of the nerve-conduit junction. When fibrin glue was added, the group was labeled prime. The nerve specimens were transected and then repaired with 8-0 nylon suture and conduit. The tensile strength of the repairs was tested, and maximum failure load was determined. The results were analyzed with a 2-way analysis of variance. The Tukey post hoc test compared repair groups if the 2-way analysis of variance showed significance. Results: Both suture group and glue presence significantly affected the maximum failure load. Increasing the number of sutures increased the maximum failure load, and the presence of fibrin glue also increased the failure load. Conclusions: Fibrin glue was found to increase the strength of conduit-assisted primary digital nerve repairs. Furthermore, the number of sutures correlated to the strength of the repair. Fibrin glue may be added to a conduit-assisted primary digital nerve repair to maintain strength and allow fewer sutures at the primary coaptation site.
Keywords: nerve, digital nerve, fibrin glue, conduit, nerve repair
Introduction
The outcomes of nerve repair continue to be suboptimal.9,15 Despite multiple repair methods, 15% of patients are not satisfied with their outcome.2 The exact reasons for poor outcomes are not well understood and appear to be multifactorial. Various theories have been proposed. One theory postulates that mechanical failure at the coaptation site leads to gapping which inhibits nerve regeneration. In this case, primary nerve repair tensile strength is critical and has been previously studied.7 Giddins et al3 found that the strength of the repair correlated with the number of sutures crossing the repair site, and Mukherjee10 showed that nerves repaired with suture in a rabbit model (and with subsequent removal of suture before testing) required 4 weeks to regain the tensile strength of an intact nerve.
Conduit-assisted primary repair is one method of repair that allows nerve regeneration across 2 opposed transected nerve ends without direct coaptation.1 This technique involves placing a nerve wrap around a primary nerve repair site with either no or minimal sutures at the coaptation site, with sutures placed at the proximal and distal ends of the nerve-conduit junction. This repair addresses another theory for poor outcomes, which is the inflammatory response potentially generated by the sutures placed at the coaptation site that may inhibit axonal regeneration.8 Therefore, minimizing or eliminating suture at the coaptation site may improve outcomes. Besides reducing inflammation and potential scar formation, the other hypothetical advantages of this technique are decreasing repair time and maintaining neurotropic factors near the repair site to allow axonal-induced topological reapproximation.6,14
Several sources have reported the benefits of augmentation of repairs with adhesive, including fibrin-based and polyethylene glycol–based products. A 2011 systematic review concluded that the use of fibrin glue decreased inflammation and demonstrated better axonal alignment, regeneration, and recovery of nerve conduction velocities; however, animal and cadaveric studies showed no difference in stiffness and peak load at failure between fibrin glue and suture groups.13 Isaacs et al,5 in particular, found no added strength due to fibrin glue alone but did not study glue in the setting of conduit-assisted repair. In all cases, however, the strength of the repairs in the study by Isaacs et al averaged well below 3 N. To place that value in context, Goldberg et al4 found that the digital nerves had in situ tension up to 4 N.
The purpose of this study is to determine whether the addition of fibrin glue provides additional strength to a conduit-assisted digital nerve repair. We hypothesize that the addition of fibrin glue will increase the tensile strength of the repair, which may decrease the number of sutures needed at the primary coaptation. This study also sought to evaluate the strength of various suture configurations to help guide clinical treatment. We hypothesize that a strong nerve repair construct can be achieved despite minimal number of sutures at the primary coaptation site.
Materials and Methods
Seven fresh-frozen cadaveric hands were thawed to room temperature, and the digital nerves from each finger were dissected and then transected at the proximal bifurcation of the common digital nerve and the distal trifurcation of the proper digital nerve. Saline was used to keep the specimens moist. Lengths of 5 cm of nerve were prepared from the specimens, and the diameter of each was measured with calipers at the midpoint of each length. The nerves were divided into 10 different groups with 9 specimens per group and with the intent to keep the same average diameter in each group. The description of each group included the number of sutures at the coaptation site (first number) and the number of sutures at each end (proximal/distal) of the nerve-conduit junction (second number). Thus, for group B (2/2), there were 2 sutures at the coaptation site and 2 sutures at each end of the nerve-conduit junction, totaling 6 sutures (Figures 1 and 2). The 10 groups were as follows: A (4/4), B (2/2), C (0/2), D (0/1), and E (0/0). For the additional 5 groups, the same suture configuration for each was used but fibrin glue (TISSEEL; Baxter, Deerfield, Illinois) was added in the nerve wrap before suturing the wrap together and then added on the top of the wrap after suturing the wrap together (Figure 3). The fibrin groups were designated with a prime (′) and listed as A′ (4/4), B′ (2/2), C′ (0/2), D′ (0/1), and E′ (0/0). Nerve repairs in group E, with no suture or glue, were held together by friction between the nerve and conduit. The fibrin glue came packaged in pairs of prefilled syringes and was stored frozen until used. One syringe contained a sealer protein solution, and the other contained a thrombin solution. The frozen syringes were thawed in a water bath for 5 minutes and used immediately.
Figure 1.
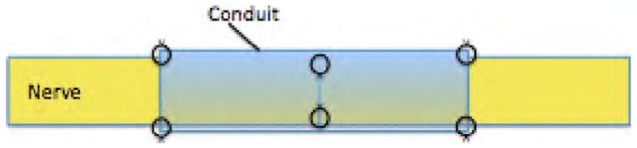
Drawing of repair construct group B (2/2) which has 2 sutures at the primary coaptation and 2 sutures each at the proximal and distal ends of the nerve-conduit junction.
Figure 2.

Picture of nerve repair group B (2/2) without fibrin glue.
Figure 3.

Picture of nerve repair group B′ (2/2) with fibrin glue.
Digital nerve lacerations were created in each specimen with a No. 15 scalpel. For those groups with suture at the primary nerve coaptation, simple epineural sutures using 8-0 nylon (Covidien, Mansfield, Massachusetts) were placed under loupe (2.5×) magnification, 1 mm from the cut end. Knots were tied using 5 single throws lying squarely with the first throw using a surgeon’s knot. When 2 sutures were needed at the coaptation, they were placed 180° from each other on the nerve. When 4 sutures were needed, they were placed 90° to each other on the nerve. The repaired nerves were then wrapped with Nerve Protector (AxoGuard; AxoGen, Alachua, Florida), which was used as described by manufacturer specifications. The nerve protector was cut to 12 mm in length and 10 mm in width and bathed in saline prior to use to provide pliability. The nerve wrap was placed around the repaired nerve and trimmed to match the nerve diameter. For the constructs with fibrin glue, the wrap was held open around the nerve transection and the glue was applied with the technique of alternating 1 drop of sealer protein and 1 drop of thrombin solution until the nerve was covered. The wrap was then folded around the nerve to form a tube, and a simple 8-0 nylon suture was placed at each end of the conduit where the leaflets overlapped; the suture did not include epineurium and was not counted as a nerve-conduit junction suture. If the group required no primary coaptation sutures, the nerve ends were placed end to end in the nerve wrap and the wrap was sutured into a tube as previously described. For those groups with nerve-conduit sutures, 8-0 nylon was placed through the conduit and epineurium; the suture was placed 1 mm from the edge of the conduit and entered and exited the epineurium at a distance of 0.5 mm proximal and distal to the edge of the conduit. When 2 sutures were needed at the nerve-conduit junction, they were placed 180° from each other. When 4 sutures were needed, they were placed 90° to each other. For the fibrin glue groups, fibrin was again added externally for the length of the conduit circumferentially. This was allowed 5 minutes to dry before testing.
Approximately 16 mm of nerves at the free ends were glued to a 4-layer gauze pad with cyanoacrylate and allowed to dry for 30 minutes: 15 minutes with the gauze pads under approximately 230 g of load to ensure a uniform bond and 15 minutes with the gauze pads uncovered to ensure that the cyanoacrylate was fully set. The specimens were loaded into a tensile testing machine (HAAKE MARS II; Thermo Fisher Scientific, Inc, Waltham, Massachusetts; resolution: 0.001 N), and stainless steel clamps gripped the entirety of the gauze pads to secure the nerve from slipping (Figure 4). Specimens were preconditioned 5 times with a ramp load of 0.25 N, followed by loading to failure at 0.33 mm/s. Tension was recorded at 10 Hz.
Figure 4.

Picture of a nerve placed in the tensile testing machine.
A 2-way analysis of variance (ANOVA) with suture group—(4/4), (2/2), (2/0), (1/0), or (0/0)—and glue presence—glue or no glue—was performed to determine whether either factor affected the maximum load to failure. A P value less than .05 was used to determine the validity of 3 null hypotheses: (1) Suture group had no effect on maximum load to failure; (2) glue presence had no effect on maximum load to failure; and (3) there was no interaction between suture group and glue presence that affected the maximum load to failure. If a significant result occurred, the Tukey post hoc test was used to determine where the significant difference existed within the significant factor.
Results
The 2-way ANOVA showed that both suture and glue significantly affected the maximum load to failure (P < .01). The maximum failure load of the nerve repairs increased with either the addition of more suture or the addition of glue (Figure 5). The interaction between suture group and glue presence was not significant. The strongest repair construct was A′ (4/4) which failed at 4.34 N, and the weakest was E (0/0) which failed at 0.06 N (Table 1). The repairs that had more suture failed at higher loads than those with less suture. For the same suture construct, the addition of fibrin glue increased the maximum load to failure. All recorded failures occurred by suture cut-out of the epineurium, first at one end of the nerve-conduit interface and then subsequently at the primary nerve coaptation site.
Figure 5.
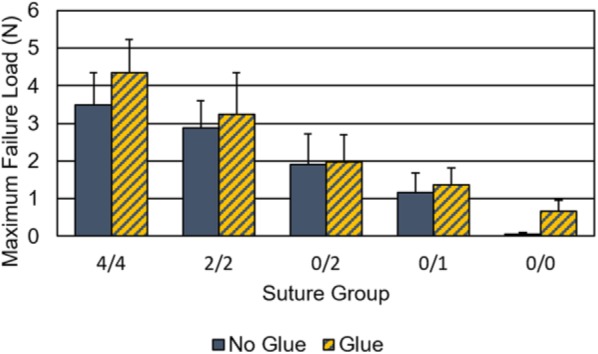
Nerve repair maximum load to failure load as a function of suture group and presence of glue.
Table 1.
Maximum Failure Load During the Load to Failure Testing for Each Repair Group
| Repair group | Primary repair sutures | Conduit sutures per side | Fibrin glue | Maximum failure load (N) |
|---|---|---|---|---|
| A | 4 | 4 | No | 3.50 ± 0.86 |
| A′ | 4 | 4 | Yes | 4.34 ± 0.89 |
| B | 2 | 2 | No | 2.88 ± 0.72 |
| B′ | 2 | 2 | Yes | 3.25 ± 1.10 |
| C | 0 | 2 | No | 1.91 ± 0.81 |
| C′ | 0 | 2 | Yes | 1.98 ± 0.72 |
| D | 0 | 1 | No | 1.16 ± 0.52 |
| D′ | 0 | 1 | Yes | 1.37 ± 0.45 |
| E | 0 | 0 | No | 0.06 ± 0.04 |
| E′ | 0 | 0 | Yes | 0.65 ± 0.31 |
Note. The maximum failure load during the load to failure testing for each repair group is listed. The number of sutures at the primary coaptation and at the nerve-conduit junction for each repair group is listed. Fibrin glue presence is indicated.
Discussion
This study analyzed the effects of suture and fibrin glue on the maximum load to failure of conduit-assisted primary digital nerve repairs. We found that increasing the number of sutures leads to increasing maximum load to failure. Fibrin glue presence was also found to significantly increase maximum failure load.
Isaacs et al5 compared the effects of 4 different glues on nerve repairs performed with two 8-0 nylon sutures and found none of the glues, one of which was fibrin glue, affected maximum failure load. Unlike the present study, however, Isaacs et al did not test conduit-assisted repairs. Temple et al16 showed in a rabbit model that the suturing of nerve lacerations was significantly stronger than fibrin glue (P < .0001) and that the resistance to gapping at the repair site with suture was greater than that with fibrin glue (P < .003). Furthermore, Nishimura et al11 found that suture repair was stronger immediately when compared with glue repair, but they could not demonstrate a statistically significant difference in strength between suture and glue repair from postoperative day 14 to 28. They also suggested that glue may outperform suture repair because fibrin glue’s strength continued to rise on day 28, whereas the suture group was leveling off.
This is the first study to show that fibrin glue is of any benefit in increasing tensile strength of primary nerve repairs. We believe that this outcome was observed because we used the glue in the setting of a conduit-assisted nerve repair. We theorize that the conduit gives the glue a wide surface area to create adhesion between the nerve ends and the conduit. Furthermore, the nerve wrap gives another 2 points of fixation before any disruption of the nerve repair, namely, the proximal or distal nerve-conduit junctions. This is in addition to the primary nerve coaptation.
Although nerve repairs are to be tension free, they have to be able to resist tensile loads or the coaptation will gap and nerve regeneration will be inhibited. The in situ loads that a digital nerve undergoes are not clearly delineated, but Goldberg et al4 determined that the digital nerve is subject to an in situ load of 4 N. Thus, it would be reasonable to strive for nerve repairs that can resist this amount of load. Another benchmark would be to have nerve repairs that are as strong as the common 4 simple suture repair which Goldberg et al determined to be 2.2 N when using 8-0 nylon 1 mm from the nerve edge and 1.6 N when using 9-0 nylon.
In our study, we found that group B′ (2/2) (3.3 N) approached the strength found in group A (4/4) (3.5 N), the second strongest repair, but contained half the number of sutures (6 vs 12). Group B′ was stronger than group B (2.9 N), which had no fibrin glue (Table 1). Thus, group B′ may be a good repair construct option because it is one of the strongest repairs, takes less time to perform, and has less suture at the primary coaptation site, which could decrease inflammation and scar. The addition of fibrin glue strengthens the repair while keeping the coaptation sutures at a minimum. Repair options that have no suture at the primary coaptation include groups C′ (0/2) and C (0/2), which eliminates any possible suture reaction at the primary coaptation. Groups B (2.9 N), C′ (2.0 N), and C (1.9 N) are all still stronger than a 4-suture repair with 9-0 nylon (1.6 N) and nearly as strong as the 8-0 nylon repair (2.2 N).4 One concern with the repair constructs without any suture at the coaptation is gapping at the coaptation during the repair and healing period.
There are some limitations to this study. This is a cadaveric study, so we can only evaluate the strength of the repair at time 0. Furthermore, the effect of biologic healing on the strength of the repair cannot be assessed. No longitudinal tensile studies have been completed for conduit-assisted repair, so we assume the weakest time point is at the initial repair. We also only used 1 preparation of fibrin glue (TISSEEL Duo) in this study. There are numerous commercially available fibrin glues as well as different preparations of the type we used. For example, Povlsen12 found a 20% failure rate in the TISSEEL Kit group and none in the TISSEEL Duo group. The different preparations of fibrin glue may affect the tensile strength of the repair.12
In conclusion, this is the first study to demonstrate that fibrin glue is of any benefit in increasing the tensile strength of conduit-assisted primary digital nerve repair. Suture number is also important in increasing tensile strength. Furthermore, this study adds information regarding various repair strengths of conduit-assisted primary nerve repairs and can give guidance to peripheral nerve surgeons when performing these repairs.
Acknowledgments
AxoGen provided the nerve wraps that were used in this study free of charge. The authors thank Michael P. Smolinski who helped with the nerve strength testing.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: No informed consent was needed for this study.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.T. has received grant money for other projects from AxoGen and is part of their speakers’ bureau.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Alluin O, Wittmann C, Marqueste T, et al. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials. 2009;30(3):363-373. doi: 10.1016/j.biomaterials.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 2. Fakin RM, Calcagni M, Klein HJ, et al. Long-term clinical outcome after epineural coaptation of digital nerves. J Hand Surg Eur Vol. 2016;41(2):148-154. doi: 10.1177/1753193415578986. [DOI] [PubMed] [Google Scholar]
- 3. Giddins GE, Wade PJ, Amis AA. Primary nerve repair: strength of repair with different gauges of nylon suture material. J Hand Surg Br. 1989;14(3):301-302. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg SH, Jobin CM, Hayes AG, et al. Biomechanics and histology of intact and repaired digital nerves: an in vitro study. J Hand Surg Am. 2007;32(4):474-482. doi: 10.1016/j.jhsa.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 5. Isaacs JE, McDaniel CO, Owen JR, et al. Comparative analysis of biomechanical performance of available “nerve glues.” J Hand Surg Am. 2008;33(6):893-899. doi: 10.1016/j.jhsa.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 6. Lee J-Y, Parisi TJ, Friedrich PF, et al. Does the addition of a nerve wrap to a motor nerve repair affect motor outcomes? Microsurgery. 2014;34(7):562-567. doi: 10.1002/micr.22274. [DOI] [PubMed] [Google Scholar]
- 7. Liu CT, Benda CE, Lewey FH. Tensile strength of human nerves; an experimental physical and histologic study. Arch Neurol Psychiatry. 1948;59(3):322-336. [DOI] [PubMed] [Google Scholar]
- 8. Martins RS, Siqueira MG, Da Silva CF, et al. Overall assessment of regeneration in peripheral nerve lesion repair using fibrin glue, suture, or a combination of the 2 techniques in a rat model. Which is the ideal choice? Surg Neurol. 2005;64(suppl 1)(S1):10-16; discussion S1:16. doi: 10.1016/j.surneu.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 9. Millesi H, Zöch G, Reihsner R. Mechanical properties of peripheral nerves. Clin Orthop Relat Res. 1995;(314):76-83. [PubMed] [Google Scholar]
- 10. Mukherjee SR. Tensile strength of nerves during healing. Br J Surg. 1953;41(166):192-195. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura MT, Mazzer N, Barbieri CH, et al. Mechanical resistance of peripheral nerve repair with biological glue and with conventional suture at different postoperative times. J Reconstr Microsurg. 2008;24(5):327-332. doi: 10.1055/s-2008-1080535. [DOI] [PubMed] [Google Scholar]
- 12. Povlsen B. A new fibrin seal in primary repair of peripheral nerves. J Hand Surg Br. 1994;19(1):43-47. [DOI] [PubMed] [Google Scholar]
- 13. Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127(6):2381-2390. doi: 10.1097/PRS.0b013e3182131cf5. [DOI] [PubMed] [Google Scholar]
- 14. Schmidhammer R, Zandieh S, Hopf R, et al. Alleviated tension at the repair site enhances functional regeneration: the effect of full range of motion mobilization on the regeneration of peripheral nerves—histologic, electrophysiologic, and functional results in a rat model. J Trauma. 2004;56(3):571-584. [DOI] [PubMed] [Google Scholar]
- 15. Tarlov IM. How long should an extremity be immobilized after nerve suture? Ann Surg. 1947;126(3):366-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Temple CL, Ross DC, Dunning CE, et al. Resistance to disruption and gapping of peripheral nerve repairs: an in vitro biomechanical assessment of techniques. J Reconstr Microsurg. 2004;20(8):645-650. doi: 10.1055/s-2004-861525. [DOI] [PubMed] [Google Scholar]


