Abstract
Background: We evaluated the effectiveness of robot-assisted motion and activity in additional to physiotherapy (PT) and occupational therapy (OT) on stroke patients with hand paralysis. Methods: A randomized controlled trial was conducted. Thirty-two patients, 34.4% female (mean ± SD age: 68.9 ± 11.6 years), with hand paralysis after stroke participated. The experimental group received 30 minutes of passive mobilization of the hand through the robotic device Gloreha (Brescia, Italy), and the control group received an additional 30 minutes of PT and OT for 3 consecutive weeks (3 d/wk) in addition to traditional rehabilitation. Outcomes included the National Institutes of Health Stroke Scale (NIHSS), Modified Ashworth Scale, Barthel Index (BI), Motricity Index (MI), short version of the Disabilities of the Arm, Shoulder and Hand (QuickDASH), and the visual analog scale (VAS) measurements. All measures were collected at baseline and end of the intervention (3 weeks). Results: A significant effect of time interaction existed for NIHSS, BI, MI, and QuickDASH, after stroke immediately after the interventions (all, P < .001). The experimental group had a greater reduction in pain compared with the control group at the end of the intervention, a reduction of 11.3 mm compared with 3.7 mm, using the 100-mm VAS scale. Conclusions: In the treatment of pain and spasticity in hand paralysis after stroke, robot-assisted mobilization performed in conjunction with traditional PT and OT is as effective as traditional rehabilitation.
Keywords: hand, stroke, rehabilitation, robotic, wearable
Introduction
Stroke (or cerebrovascular accident) is a sudden ischemic or hemorrhagic episode which causes a disturbed generation and integration of neural commands from the sensorimotor31 areas of the cortex. As a consequence, the ability to selectively activate muscle tissues for performing movement is reduced.26 Sixty percent of those individuals who survive a stroke exhibit a sensorimotor deficit of one or both hands and may benefit from rehabilitation to maximize recovery of the upper extremity.23,25 Restoration of arm and hand motility is essential for the independent performance of daily activities.23,26 A prompt and effective rehabilitation approach is essential28 to obtain recovery of an impaired limb to prevent tendon shortening, spasticity, and pain.2
Recent technologies have facilitated the use of robots as tools to assist patients in the rehabilitation process, thus maximizing patient outcomes.4 Several groups have developed robotic tools for upper limb rehabilitation of the shoulder and elbow.27 These robotic tools assist the patient with carrying out exercise protocols and may help restore upper limb mobility.22,26 The complexity of wrist and finger articulations had delayed the development of dedicated rehabilitation robots until 2003 when the first tool based on continuous passive motion (CPM) was presented followed by several other solutions, with various levels of complexity and functionality.3
A recent review on the mechanisms for motor relearning reported factors such as attention and stimuli (reinforcement) are crucial during learning which indicates that motor relearning can take place with patients with neurological disorders even when only the sensorial passive stimulation is applied.30 In addition, another review reported the benefits of CPM for stretching and upper limb passive mobilization for patients with stroke but that CPM treatment requires further research.40
Among robotic devices, Gloreha (Figure 1),5,10 with its compliant mechanical transmission, may represent an easily applied innovative solution to rehabilitation, because the hand can perform grasp and release activities wearing the device by mean of a flexible and light orthosis. Our objective of this study was to determine the efficacy of robot-assisted motion in addition to traditional physiotherapy (PT) and occupational therapy (OT) compared with additional time spent in PT and OT on stroke patients with hand paralysis on function, motor strength, spasticity, and pain.
Figure 1.

Wearable glove/orthosis.
Methods
Study Design
We conducted a double blind randomized clinical trial. Informed consent was obtained from all participants, and procedures were conducted according to the Declaration of Helsinki. This research protocol has been approved by the Local Ethical Committee of “IRCCS Regione Lombardia,” Italy, and was registered with ClinicalTrials.gov (NCT02711787). The study has been registered at Trial registration Current Controlled Trials website.
Patients
We screened 32 patients and enrolled 21 men and 11 women, aged 50 to 90 years, from July 2014 to February 2015. All participants were typical rehabilitation patients in the acute phase following stroke (between 0.5 and 12 months post onset). All patients had self-reported functional impairments of their upper extremities after stroke. A neurologist established the diagnosis of the acute phase of stroke. All patients had been admitted to 1 of the 3 participating rehabilitation hospitals. Each patient underwent both subjective and physical examination performed by a physician experienced in neurologic conditions and rehabilitation to evaluate the patients in regard to the inclusion and exclusion criteria. To be included in the study, the patients needed to have a history of acute phase of stroke,41 first stroke episode, no history of peripheral nerve injury or musculoskeletal disease (eg, arthritis, musculotendinous injury, or bone fracture) in the affected upper extremity, no contracture of the affected wrist or fingers (Modified Ashworth < 3),6 and no history of any invasive procedure (Botulinum toxin type A) for the treatment of spasticity for at least 6 months prior to the start of this study,17,36 and paralysis of the wrist and fingers and absence in voluntarily initiating and controlling finger extension movements. The exclusion criteria included unstable medical disorders, active complex regional pain syndrome, severe spatial neglect, aphasia, or cognitive problems.17 Patients were excluded if they scored greater than 4 points on the Beck Depression Inventory37 or more than 30 points in the State Trait Anxiety Inventory.35
None of the individuals in this study had received prior interventions post stroke. Therefore, they were naive to the treatment they received. We also excluded patients who did not sign the informed consent.
Instruments
Gloreha9 is a robotic device for the rehabilitation of the hand, in the shape of a glove. The power generators of the robot1 are not on the hand of the patient, as common in rehabilitation devices, but it is physically separated from the glove. The mechanical power is transmitted to the glove through a flexible beam.8 In this way, the weight of the device does not act on the hand of the patient during the rehabilitation session. The transmission from the compliant beam to the fingertip of the glove is realized with a compliant transmission7 on the back of the glove. This is a lightweight solution and represents an element of mechanical safety, because it limits the transmission of mechanical power from the robot to the hand and from the hand to the robot. Gloreha is able to move the fingers of the patients independently in different range of motions and different speeds. It is also endowed with a virtual reality interface to stimulate the patient visually during the rehabilitation session.
Protocol
All patients underwent a standard rehabilitation approach consisting of 1-hour sessions 5 days per week of both PT and OT. Patients in both groups received the same number of treatment sessions and for a similar duration of time, including both dexterity and gait training, according to an individually tailored exercise program determined by the treating therapist, who was blinded to the treatment allocation. In addition to usual rehabilitation, eligible patients also received a 30-minute session for 3 day per week of either the experimental treatment using the hand Gloreha or the control treatment of 30 minutes of additional PT and OT executed by a trained physical and occupational therapist. The PT and OT were blinded to all data that were collected for the study. The patients were assigned to experimental (n = 16) and control (n = 16) treatment groups with simple randomization.
Experimental group
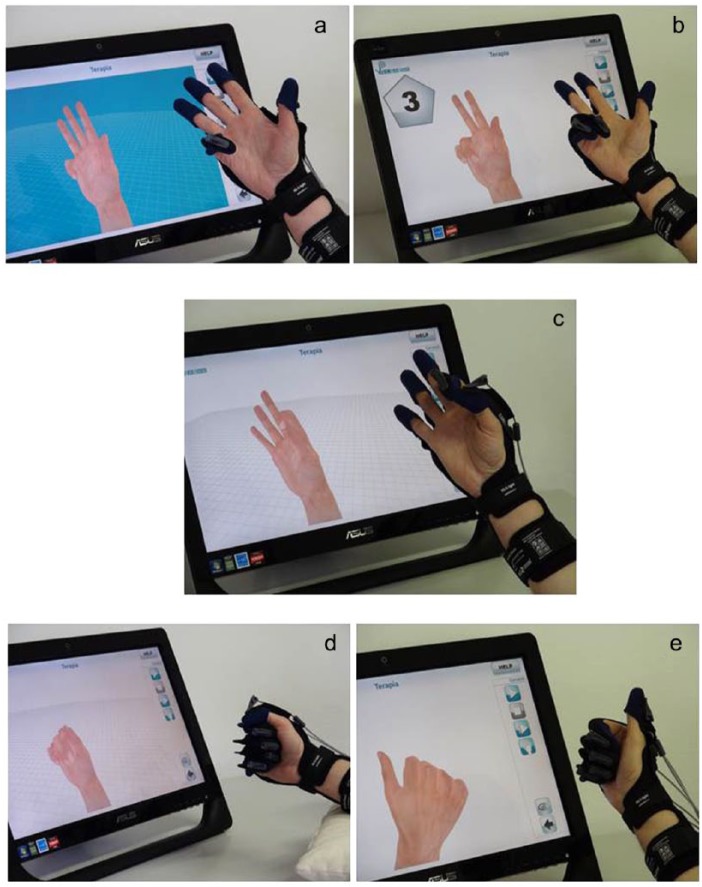
Fifteen half-hour treatments were performed during the morning with the following protocol (each finger was mobilized individually), “number,” “fist,” “pinch,” (thumb-index) and “synchronous” (II-III-IV-V finger are mobilized simultaneously, the thumb individually) in presence of visual feedback (Figure 2). The fingers of the patients were hooked to individual thimbles connectable through a nylon thread to a device fixed on the glove that interfaced with a hybrid system (compressed air and oil) performing the passive movement of flexion-extension of the fingers.
Figure 2.
Hand exercises. Each finger was mobilized individually: a and b, number; c, pinch (thumb-index); d, fist; and e, synchronous, (II-III-IV-V finger are mobilized simultaneously, the thumb individually) in the presence of visual feedback.
Thanks to the presence of these flexible cables, the Gloreha can gradually moderate the intensity of forces applied on patient’s hand and fingers (Figure 1). The length of a single cable can be adjusted in the aim to guarantee a progressive and specific adaptation of each single finger according to the aim of the treatment, the type of exercise, and the clinical condition of the patient (level of spasticity, pain, etc).
Control group
Patients in the control group received the same number of treatment sessions17 of a similar duration as those in the experimental group, but they only received additional traditional rehabilitation for 30 minutes, such as assisted stretching, shoulder and arm exercises, and functional reaching tasks.19 A physiotherapist or occupational therapist with more than 5 years in neurological rehabilitation applied the techniques. The therapists were blinded to the participants’ pretreatment measurements.
Outcomes measures
An assessor blinded to the participants’ intervention assignment collected the pretreatment measurements. The pretreatment clinician-reported outcome measures traditionally used in stroke clinical trials: National Institutes of Health Stroke Scale (NIHSS),16 Barthel Index (BI)29 were used to measure functional ability, Modified Ashworth Scale (MAS) for Grading Spasticity18,20 were used to measure spasticity, Motricity Index (MI)24 was used to measure motor strength, the short version of the Disabilities of the Arm, Shoulder and Hand (QuickDASH),13 and the intensity of hand pain assessed with a 100-mm visual analog scale (VAS).38 After pretreatment measurements, participants were assigned by arrival order, into 1 of the 2 groups. The same assessor who took the pretreatment measurements remained blinded to the treatment allocation of the patients and performed the posttreatment assessments 5 minutes after the application of the last procedure. The testing protocol and assessment protocol was prepared according to the editorial form of medical publishing and Consolidated Standards of Reporting Trials (CONSORT) publishing rules.39
Statistical Analysis
Sample size and power calculations were performed prior to undertaking the study to determine the number of participant’s needed in each group with the ENE 3.0 software (GlaxoSmithKline, Universidad Autónoma, Barcelona). The calculations were based on detecting a mean difference of 20-mm minimally clinically important difference on a 100-mm VAS assuming a standard deviation of 20 mm, a 2-tailed test, an alpha level of 0.05, and a desired power of 80%. The estimated desired sample size was 16 individuals per group. Data were analyzed using SPSS version 21.0 (SPSS Inc, Chicago, Illinois) and conducted following an intention-to-treat analysis using the last value forward method. Group data were summarized using means and standard deviations. The Kolmogorov-Smirnov test was used to confirm the normality of the distribution of the data. Comparison of baseline characteristics and outcome variables was performed with use of a 2-tailed independent Student t test for the continuous variable of age and baseline scores. Paired Student t tests were used to determine the level of significance of the differences between the pretreatment and posttreatment measurements of the individual groups. A 2 × 2 repeated measures analysis of variance was used to determine the differences in time (preintervention and postintervention) as the within-participants factor and group (experimental or control) as the between-participants factor. The main hypothesis of interest was Group × Time interaction. Between-group differences were expressed as mean differences with 95% confidence intervals. Between-groups effect sizes were calculated using Cohen’s d coefficient. An effect size greater than 0.8 was considered large, 0.5 moderate, and less than 0.2 small. In all analyses, P < .05 was considered statistically significant.
Results
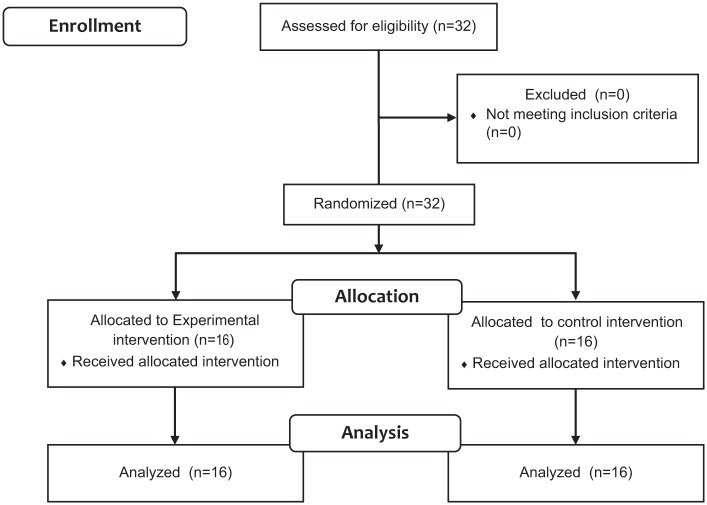
Thirty-two patients (n = 32) (mean age: 68.9 ± 11.6 years; 34.4% female) satisfied all eligibility criteria and agreed to participate; all of the patients had the onset of acute stroke not more than 3 months prior the beginning of the study. Sixteen patients were assigned to the experimental group and the other 16 patients to the control group. Figure 3 provides a flow diagram of participant recruitment and retention through the study. None of the participants had modified the regular pharmacologic therapy during the study. The anthropometric characteristics were similar between groups (Table 1). Also those parameters did not show significant statistical differences between the control and intervention group (Table 1).
Figure 3.
Flow diagram of the study.
Table 1.
Baseline Demographics for Both Groups.
| Experimental group (n = 16) |
Control group (n = 16) |
|
|---|---|---|
| Age, years Male gender, n (%) |
67 (11) 11 (68.8) |
70 (12) 10 (62.5) |
| Ischemic stroke, n (%) | 12 (75.0) | 12 (75.0) |
| Right hemiplegia, n (%) | 7 (43.8) | 8 (50.0) |
| Right-handed, n (%) | 15 (93.8) | 16 (100) |
| MAS | 0.1 (0.3) | 0.1 (0.3) |
| VAS | 11.9 (17.2) | 10.6 (21.7) |
| STAI | 26.6 (2.6) | 26.7 (3.0) |
| BDI | 3.1 (1.9) | 2.9 (2.2) |
Note. Data are expressed as mean ± SD. MAS = Modified Ashworth Scale; VAS = visual analog scale; STAI = State Trait Anxiety Inventory; BDI = Beck Depression Inventory.
Response to Treatment
Outcomes for NIHSS, BI, MI, and QuickDASH demonstrated a significant time factor (F1.0 = 94.675; P < .001, F1.0 = 169.731; P = .001, F1.0 = 111.383; P = .001, and F1.0 = 50.063; P = .001, respectively) but not for group-by-time interaction. The post hoc analysis revealed both clinically and statistically significant differences between the baseline and 3 week for outcome scores, with the exception of the MAS scores, for both the experimental and control group (all, P <.001). The between-groups effect sizes for the change scores were small at posttreatment period for the QuickDASH, moderate for the BI, and large for the MI and MAS (Table 2). For spasticity measured over the MAS, there was no significance for time (F1.0 = 11.791; P = .002), or group-by-time (F1.0 = 0.628; P = .4), interactions (Table 2).
Table 2.
Mean (SD) for Outcome at All Study Visits for Each Group, Mean (SD) Difference Within Groups, and Mean (95% Confidence Interval) Difference Between Groups.
| Groups |
Difference within groups |
Effect size |
Difference between groups |
|||||
|---|---|---|---|---|---|---|---|---|
| Week 0 |
Week 3 |
Week 3 minus Week 0 |
Week 3 minus Week 0 |
Week 3 |
||||
| Experimental group |
Control group |
Experimental group |
Control group |
Experimental group |
Control group |
|||
| Outcome | (n = 16) | (n = 16) | (n = 16) | (n = 16) | (n = 16) | (n = 16) | Cohen’s d | Experimental group minus Control group |
| NIHSS (Score, 0 to 42) |
8.0 (3.1) |
7.5 (2.6) |
4.6 (2.7) |
4.1 (2.5) |
−3.4a
(0.5) |
−3.4a
(0.5) |
0 | 0.5 (1.4 to −2.4) |
| MAS (Score, 0 to 4) |
0.1 (0.3) |
0.1 (0.3) |
0.6 (0.8) |
0.4 (0.7) |
0.5 (0.2) |
0.3 (0.2) |
1.0 | 0.2 (0.4 to −0.7) |
| BI (Score, 0 to 100) |
36.6 (21.0) |
35.3 (23.6) |
59.4 (24.0) |
56.9 (24.3) |
22.8a
(2.4) |
21.6a
(2.4) |
0.5 | 2.5 (15.0 to −20.0) |
| MI (Score, 0 to 100) |
30.6 (21.2) |
36.3 (37.4) |
55.0 (19.6) |
51.1 (36.6) |
24.4a
(2.6) |
14.9a
(2.6) |
3.65 | 3.9 (−17.3 to −25.1) |
| QuickDASH (Score, 0 to 100) |
68.0 (11.0) |
61.2 (15.3) |
58.1 (14.1) |
52.1 (20.4) |
−9.9a
(1.9) |
−9.1a
(1.9) |
0.42 | 5.9 (−6.7 to −18.6) |
| VAS (100 mm) |
11.9 (17.2) |
10.6 (21.7) |
0.6 (3.0) |
6.9 (13.0) |
−11.3a
(4.4) |
−3.7 (4.4) |
1.73 | −7.6 (−13.0 to −0.3) |
Note. NIHSS = National Institutes of Health Stroke Scale; MAS = Modified Ashworth Scale; BI = Barthel Index; MI = Motricity Index; QuickDASH = short version of the Disabilities of the Arm, Shoulder and Hand; VAS = visual analog scale.
Significantly different within group, P < .05 (95% confidence interval).
Hand Pain Intensity (VAS)
VAS revealed a significant effect of time (F1.0 = 5.775; P = .02), but not for the group-by-time interaction (F1.0 = 1.444; P = .2) for pain intensity. The post hoc analysis revealed significant within-group differences for the experimental group (P = .02), but not for the control group (P = .4). Between-groups effect sizes for change scores from pre treatment to post treatment were large when interpreted using Cohen’s d at posttreatment period (Table 2).
Discussion
Our study combined the robot-assisted or traditional rehabilitation as an adjunctive therapy to PT and OT on acute stroke patients, with upper limb hemiplegia. We found that both interventions produced either statistical or clinically relevant changes in the outcomes of function, motor ability, and pain. These findings are consistent with the studies that found benefits of robot-assisted rehabilitation provided in conjunction with PT and OT.12,19,21,22,26,33 Robotic devices are able to provide force feedback for sensorimotor-type rehabilitative training and assist patients by passively moving the limb.21 It has been reported that the repetitive training of isolated movements and robot training can have a greater effect on stroke-related motor impairments than increased therapy time alone.11,14 A benefit of the Gloreha intervention is in the fact that once the therapist has set up the Gloreha, the patient can be left alone with the glove and thereby reduces the need for one-on-one skilled intervention for sole passive range of motion exercises of the hand and it provides visual feedback to the participant while the exercise is performed. Like robotic devices, the Gloreha is a safe treatment intervention that can be performed in the patient’s room while the patient is supine in bed or sitting in a chair.21 The high intensity of sensorimotor robot-aided exercises, in which the stroke patient repeatedly performs a well-defined motor task, is hypothesized to produce plastic changes in the cerebral cortex.14,15,32 Gloreha implemented different safety approaches: a compliant transmission that does not transfer mechanical overloads, an electronic safety circuit, a safety software structure, and finally a manual command for the patient that can be actioned in presence of excessive pain. The Gloreha glove, which provides repetitive movement of the hand, may also be beneficial in reorganizing the somatosensory and motor cortexes. The use of Gloreha introduces some disadvantages with respect to the manual therapy. The wearing time can be quantified in 10 minutes, and this time cannot be used for the therapy, but it is compensated by a reduction of the personnel rehabilitation costs (70% with respect to manual therapy).34 The second disadvantage is associated to the cost of the investment, that must be calibrated with a proper amortization plan equal or less than the reduction costs to avoid a negative economic impact. The third disadvantage is associated with the cleaning of the glove; a new demand associated an artificial device, despite the use of an under glove.
Limitations
The incorporation of OT and PT into the treatment makes it difficult to quantify the sole effect of Gloreha on the outcomes. Perhaps the same findings would have occurred whether or not the additional intervention was performed. However, it is often common standard practice for stroke patients to receive more than 1 session of therapy during the acute phase of stroke rehabilitation.12,19,21,22,26 In addition, the follow-up period was not long, and future studies are needed with a longer follow-up time.
Despite our positive results, many questions regarding Gloreha-assisted passive manipulation of the hand remain to be quantified and studied further. The dose and length of training time are as yet undefined. Further randomized controlled studies implementing this therapy in a large number of patients and extending the duration of the study are needed to confirm these results and the long-term benefits of the Gloreha intervention. We recommend further large, high-quality, randomized controlled studies of such techniques to demonstrate their validity.
Conclusions
These results provide further support to the generalized therapeutic impact of intensive robot-assisted treatment on hand recovery functions in individuals with acute stroke. The robot-assisted treatment may contribute toward the recovery of hand motor function in acute stroke patients. The positive results obtained through the safe and reliable robotic rehabilitation treatment reinforce the recommendation to extend it to a larger clinical practice as traditional rehabilitation.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all participants, and all procedures were conducted according to the Declaration of Helsinki.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Aggogeri F, Borboni A, Faglia R, et al. Precision positioning systems: an overview of the state of art. Appl Mech Mater. 2013;336-338:1170-1173. [Google Scholar]
- 2. Andringa AS, Van de, Port IG, Meijer JW. Tolerance and effectiveness of a new dynamic hand-wrist orthosis in chronic stroke patients. NeuroRehabilitation. 2013;33:225-231. [DOI] [PubMed] [Google Scholar]
- 3. Balasubramanian S, Klein J, Burdet E. Robot-assisted rehabilitation of hand function. Curr Opin Neurol. 2010;23:661-670. [DOI] [PubMed] [Google Scholar]
- 4. Bishop L, Stein J. Three upper limb robotic devices for stroke rehabilitation: a review and clinical perspective. NeuroRehabilitation. 2013;33:3-11. [DOI] [PubMed] [Google Scholar]
- 5. Bissolotti L, Villafane JH, Gaffurini P, et al. Changes in skeletal muscle perfusion and spasticity in patients with poststroke hemiparesis treated by robotic assistance (Gloreha) of the hand. J Phys Ther Sci. 2016;28:769-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bohannon RW, Smith MB. Interrater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206-207. [DOI] [PubMed] [Google Scholar]
- 7. Borboni A, Aggogeri F, Faglia R, eds. Fast kinematic model of a seven-bar linkage with a single compliant link. ASME 2014 12th Biennial Conference on Engineering Systems Design and Analysis, ESDA 2014; 2014. Copenhagen (1300), Denmark. [Google Scholar]
- 8. Borboni A, De Santis D. Large deflection of a non-linear, elastic, asymmetric Ludwick cantilever beam subjected to horizontal force, vertical force and bending torque at the free end. Meccanica. 2014;49:1327-1336. [Google Scholar]
- 9. Borboni A, Villafañe JH, Mullè C, Valdes K, et al. Robot-Assisted Rehabilitation of Hand Paralysis After Stroke Reduces Wrist Edema and Pain: A Prospective Clinical Trial. J Manipulative Physiol Ther. 2017;40:21-30. [DOI] [PubMed] [Google Scholar]
- 10. Borboni A, Mor M, Faglia R. Gloreha-hand robotic rehabilitation: design, mechanical model, and experiments. J Dyn Sys Meas Control. 2016;138:111003. [Google Scholar]
- 11. Butefisch C, Hummelsheim H, Denzler P, Mauritz KH. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci. 1995;130:59-68. [DOI] [PubMed] [Google Scholar]
- 12. Chang JJ, Tung WL, Wu WL, Huang MH, Su FC. Effects of robot-aided bilateral force-induced isokinetic arm training combined with conventional rehabilitation on arm motor function in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88:1332-1338. [DOI] [PubMed] [Google Scholar]
- 13. Gabel CP, Yelland M, Melloh M, Burkett B. A modified QuickDASH-9 provides a valid outcome instrument for upper limb function. BMC Musculoskel Dis. 2009;10:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halder P, Sterr A, Brem S, et al. Electrophysiological evidence for cortical plasticity with movement repetition. Eur J Neurosci. 2005;21:2271-2277. [DOI] [PubMed] [Google Scholar]
- 15. Hayashi S, Hasegawa Y, Kasai T. Transcranial magnetic stimulation study of plastic changes of human motor cortex after repetitive simple muscle contractions. Percept Mot Skills. 2002;95:699-705. [DOI] [PubMed] [Google Scholar]
- 16. Kelly ML, Rosenbaum BP, Kshettry VR, et al. Comparing clinician- and patient-reported outcome measures after hemicraniectomy for ischemic stroke. Clin Neurol Neurosur. 2014;126:24-29. [DOI] [PubMed] [Google Scholar]
- 17. Kim EH, Chang MC, Seo JP, et al. The effect of a hand-stretching device during the management of spasticity in chronic hemiparetic stroke patients. Ann Rehabil Med. 2013;37:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kong KH, Chua KS, Lee J. Symptomatic upper limb spasticity in patients with chronic stroke attending a rehabilitation clinic: frequency, clinical correlates and predictors. J Rehabil Med. 2010;42:453-457. [DOI] [PubMed] [Google Scholar]
- 19. Lum PS, Burgar CG, Van der Loos M, et al. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: a follow-up study. J Rehabil Res Dev. 2006;43:631-642. [DOI] [PubMed] [Google Scholar]
- 20. Lundstrom E, Smits A, Terent A, et al. Time-course and determinants of spasticity during the first six months following first-ever stroke. J Rehabil Med. 2010;42:296-301. [DOI] [PubMed] [Google Scholar]
- 21. Masiero S, Celia A, Rosati G, et al. Robotic-assisted rehabilitation of the upper limb after acute stroke. Arch Phys Med Rehabil. 2007;88:142-149. [DOI] [PubMed] [Google Scholar]
- 22. Mazzoleni S, Sale P, Franceschini M, et al. Effects of proximal and distal robot-assisted upper limb rehabilitation on chronic stroke recovery. NeuroRehabilitation. 2013;33:33-39. [DOI] [PubMed] [Google Scholar]
- 23. Mukherjee M, Koutakis P, Siu KC, et al. Stroke survivors control the temporal structure of variability during reaching in dynamic environments. Ann Biomed Eng. 2013;41:366-376. [DOI] [PubMed] [Google Scholar]
- 24. Nijboer TC, Kollen BJ, Kwakkel G. The impact of recovery of visuo-spatial neglect on motor recovery of the upper paretic limb after stroke. PLoS ONE. 2014;9:e100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nowak DA. The impact of stroke on the performance of grasping: usefulness of kinetic and kinematic motion analysis. Neurosci Biobehav Rev. 2008;32:1439-1450. [DOI] [PubMed] [Google Scholar]
- 26. Prange GB, Jannink MJA, Groothuis-Oudshoorn CGM, et al. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Dev. 2006;43:171-183. [DOI] [PubMed] [Google Scholar]
- 27. Reinkensmeyer DJ, Schmit BD, Rymer WZ. Assessment of active and passive restraint during guided reaching after chronic brain injury. Ann Biomed Eng. 1999;27:805-814. [DOI] [PubMed] [Google Scholar]
- 28. Salter RB. Continuous passive motion: from origination to research to clinical applications. J Rheumatol. 2004;31:2104-2105. [PubMed] [Google Scholar]
- 29. Sanchez-Sanchez ML, Belda-Lois JM, Mena-Del Horno S, et al. Functional principal component analysis as a new methodology for the analysis of the impact of two rehabilitation protocols in functional recovery after stroke. J Neuroeng Rehabil. 2014;11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seitz AR, Dinse HR. A common framework for perceptual learning. Curr Opin Neurobiol. 2007;17:148-153. [DOI] [PubMed] [Google Scholar]
- 31. Song D, Lan N, Loeb GE, et al. Model-based sensorimotor integration for multi-joint control: development of a virtual arm model. Ann Biomed Eng. 2008;36:1033-1048. [DOI] [PubMed] [Google Scholar]
- 32. Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. J Clin Neurophysiol. 2004;21:124-131. [DOI] [PubMed] [Google Scholar]
- 33. Taveggia G, Borboni A, Salvi L, et al. Efficacy of robot-assisted rehabilitation for the functional recovery of the upper limb in post-stroke patients: a randomized controlled study. Eur J Phys Rehabil Med. 2016;52:767-773. [PubMed] [Google Scholar]
- 34. Vanoglio F, Luisa A, Garofali F, et al. Fattibilita’ ed Efficacia Riabilitativa di un Apparecchio di Mobilizzazione Passiva Continua della Mano nel Recupero di Destrezza e Forza nel Paziente Emiplegico: Studio Pilota della Mano nel Recupero di Destrezza e Forza nel Paziente Emiplegico: Studio Pilota. SIMFER (Societa Italiana di Medicina Fisica e Riabilitativa) Congress; 2013; Bari, Italy. [Google Scholar]
- 35. Villafane JH, Bishop MD, Fernandez-de-Las-Penas C, et al. Radial nerve mobilisation had bilateral sensory effects in people with thumb carpometacarpal osteoarthritis: a randomised trial. J Physiother. 2013;59:25-30. [DOI] [PubMed] [Google Scholar]
- 36. Villafane JH. Botulinum toxin type A combined with neurodynamic mobilization for lower limb spasticity: a case report. J Chiropr Med. 2013;12:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villafane JH, Silva GB, Chiarotto A. Effects of passive upper extremity joint mobilization on pain sensitivity and function in participants with secondary carpometacarpal osteoarthritis: a case series. J Manipulative Physiol Ther. 2012;35:735-742. [DOI] [PubMed] [Google Scholar]
- 38. Villafane JH, Valdes K. Combined thumb abduction and index finger extension strength: a comparison of older adults with and without thumb carpometacarpal osteoarthritis. J Manipulative Physiol Ther. 2013;36:238-244. [DOI] [PubMed] [Google Scholar]
- 39. von Elm E, Altman DG, Egger M, et al. [The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting of observational studies]. Internist. 2008;49:688-693. [DOI] [PubMed] [Google Scholar]
- 40. Winter J, Hunter S, Sim J, et al. Hands-on therapy interventions for upper limb motor dysfunction following stroke. Cochrane Database Syst Rev. 2011;(6):CD006609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamaguchi T, Tanabe S, Muraoka Y, et al. Effects of integrated volitional control electrical stimulation (IVES) on upper extremity function in chronic stroke. Keio J Med. 2011;60:90-95. [DOI] [PubMed] [Google Scholar]