Abstract
Long-term storage of viable mammalian cells is important for applications ranging from in vitro fertilization to cell therapy. Cryopreservation is currently the most common approach, but storage in liquid nitrogen is relatively costly and the requirement for low temperatures during shipping is inconvenient. Desiccation is an alternative strategy with the potential to enable viable cell preservation at more convenient storage temperatures without the need for liquid nitrogen. To achieve stability during storage in the dried state it is necessary to remove enough water that the remaining matrix forms a non-crystalline glassy solid. Thus, the glass transition temperature is a key parameter for design of cell desiccation procedures. In this study, we have investigated the effects of moisture content on the glass transition temperature (Tg) of mixtures of sugars (trehalose or raffinose), polymers (polyvinylpyrrolidone or Ficoll), penetrating cryoprotectants (ethylene glycol, propylene glycol, or dimethyl sulfoxide), and phosphate buffered saline (PBS) solutes. Aqueous solutions were dried to different moisture contents by equilibration with saturated salt solutions, or by baking at 95°C. The glass transition temperatures of the dehydrated samples were then measured by differential scanning calorimetry. As expected, Tg increased with decreasing moisture content. For example, in a desiccation medium containing 0.1 M trehalose in PBS, Tg ranged from about 360 K for a completely dry sample to about 220 K at a water mass fraction of 0.4. Addition of polymers to the solutions increased Tg, while addition of penetrating cryoprotectants decreased Tg. Our results provide insight into the relationship between relative humidity, moisture content and glass transition temperature for cell desiccation solutions containing sugars, polymers and penetrating cryoprotectants.
Introduction
Maintaining the viability mammalian cells during long term storage is important in many areas, including embryo preservation for breeding of domestic animals [1], species conservation [2] and assisted human reproduction [3], as well as preservation of cell-based therapeutic products such as stem cells [4]. Currently, the leading method for long-term storage of mammalian cells is cryopreservation, which requires that cells be kept at temperatures near the boiling point of nitrogen (-196°C). The requirement for storage in liquid nitrogen increases costs and makes it more challenging to ship the cells from the storage facility to the end user. To address these drawbacks, several researchers have recently begun to investigate the potential for cell desiccation to enable storage at higher temperatures [5–11]. While results have been encouraging in some cases (e.g., blood platelets [12]), mammalian cells have generally proven to be difficult to preserve in the dry state.
In contrast to mammalian cells, several other organisms (including yeast, tardigrades and even larger organisms such as resurrection plants) can survive nearly complete dehydration [13]. These organisms have developed adaptations that provide protection at low water content, most notably the accumulation of sugars such as trehalose and sucrose [13]. The study of these organisms has resulted in a developing consensus about the physical mechanisms of desiccation protection. Sugars provide protection by directly interacting with polar groups on biomolecules such as lipids and proteins, stabilizing them at low water content. In essence, the sugars are thought to replace the hydrogen bonds that would normally be made by water. For this reason, this protective mechanism is known as the water replacement hypothesis [14]. Another key mechanism of desiccation protection is the formation of aqueous glass, which embeds cellular components in a noncrystalline solid matrix and slows down degradative chemical reactions [14].
Sugars such as trehalose and sucrose are commonly used in studies of mammalian cell desiccation [5–11]. For effective protection, sugars must be present on both sides of the biological membranes [15–18]. This presents a challenge for mammalian cells, which lack membrane transporters for sugars such as trehalose and sucrose. While various strategies have been devised for getting trehalose across the plasma membrane [16, 19–23], it remains a challenge to ensure distribution into intracellular organelles.
Penetrating cryoprotectant additives (CPAs), such as dimethyl sulfoxide (DMSO), ethylene glycol (EG), and propylene glycol (PG), are membrane permeable and may offer protection against dehydration stresses in the cytoplasm as well as within organelles. Such CPAs have been shown to mitigate damage from cellular dehydration during cryopreservation [24, 25]. When used in combination with intracellular sugars, small amounts of DMSO have been shown to enable successful cryopreservation of mouse oocytes [18, 26]. While penetrating CPAs are generally poor glass formers compared with sugars, they may interact with and stabilize membrane phospholipids and other biomolecules at low water content. Thus, the combination of sugars and penetrating CPAs may be useful for cell desiccation.
Polymers such as dextran and polyvinylpyrrolidone (PVP) have also been studied as additives during cell desiccation [27, 28]. Polymers are known to be good glass formers, but are relatively difficult to get into cells and generally do not form stabilizing interactions with biomolecules [14]. Thus, they are typically used in combination with a sugar such as trehalose to help promote formation of an extracellular glass.
One of the most important performance characteristics of a desiccation solution is its ability to form a glass as water is removed during drying. There have been numerous studies of the glass formation properties of binary aqueous solutions, especially the water-trehalose system [29]. However, glass transition data for more complex multi-component solutions is relatively scarce. The glass transition temperature (Tg), which refers to a narrow temperature range in which the material undergoes a second-order phase transition (i.e., liquid to glass or vice versa), ultimately determines the appropriate biospecimen storage temperature. Consequently, different methods such as differential scanning calorimetry (DSC) and thermomechanical analysis have been developed to measure Tg [30]. DSC is the most widely used technique to determine Tg of biopreservation mixtures based on a change in the heat capacity [5, 14, 31]. In this study, we used DSC to examine the glass transition properties of aqueous solutions containing various combinations of sugars (trehalose or raffinose), polymers (PVP or Ficoll), penetrating CPAs (DMSO, EG, or PG), and phosphate buffered saline (PBS). The resulting data will be useful for selecting promising compositions for cell desiccation media.
Materials and methods
Sample preparation
Mixtures with the compositions shown in Table 1 were investigated. All aqueous solutions were prepared using purified water (ACS grade, Ricca Chemical Company, Arlington, TX) and filtered using a 0.45-μm filter. Trehalose dihydrate (≥ 99% purity) was obtained from Sigma Aldrich (St. Louis, MO). Raffinose (99% purity) was obtained from Alfa Aesar (Ward Hill, MA). Polyvinylpyrrolidone (99% purity, ~40 kDa) was obtained from Amresco (Solon, OH). Ficoll PM 70 (~70 kDa) was obtained from GE Healthcare (Pittsburg, PA). Dimethyl sulfoxide (≥ 99.9% purity) and ethylene glycol (≥ 99% purity) were obtained from Avantor Performance Materials (Center Valley, PA). Propylene glycol (≥ 99.5% purity) was obtained from VWR (Radnor, PA). Phosphate buffered saline was prepared by mixing 8 g of sodium chloride (EMD Millipore, Burlington, MA), 0.2 g of potassium chloride (Avantor Performance Materials), 2.16 g of sodium monohydrogen phosphate heptahydrate (VWR), and 0.2 g of potassium dihydrogen phosphate (Mallinckrodt Chemicals, St. Louis, MO) in purified water raised to a total volume of 1 L volume then corrected to pH 7.4 using ~1 mL of 1 M HCl. PBS containing calcium and magnesium (PBS Mg/Ca) was obtained from Quality Biological (Gaithersburg, MD).
Table 1. Mixture compositions for glass transition studies.
| Composition of Desiccation Solution | Corresponding Solute Mass Percentages |
|---|---|
| 0.1 M trehalose in water | trehalose (100%) |
| 0.1 M trehalose in PBS a | trehalose (78.2%), PBS salts (21.8%) |
| 0.1M trehalose, 10% PVP in water | trehalose (25.5%), PVP (74.5%) |
| 0.1 M trehalose, 10% Ficoll in PBS | trehalose (23.6%), PBS salts (7.3%), Ficoll (69.1%) |
| 0.1 M trehalose, 10% PVP in PBS | trehalose (23.6%), PBS salts (7.3%), PVP (69.1%) |
| 0.1 M trehalose, 10% PVP in PBS Mg/Ca b | trehalose (23.6%), PBS Mg/Ca salts (7.3%) PVP (69.1%) |
| 0.1 M raffinose, 10% PVP in PBS | raffinose (31.5%), PBS salts (6.0%), PVP (62.5%) |
| 0.1 M trehalose, 10% PVP, 0.25 M DMSO in PBS | trehalose (20.8%), PBS salts (6.4%), PVP (60.9%), DMSO (11.9%) |
| 0.1 M trehalose, 10% PVP, 0.25 M EG in PBS | trehalose (21.3%), PBS salts (6.6%), PVP (62.4%), EG (9.7%) |
| 0.1 M trehalose, 10% PVP, 0.25 M PG in PBS | trehalose (20.9%), PBS salts (6.4%), PVP (61.1%), PG (11.6%) |
| 0.1 M trehalose, 10% PVP, 0.5 M DMSO in PBS | trehalose (18.6%), PBS salts (5.8%), PVP (54.4%), DMSO (21.2%) |
| 0.1 M trehalose, 10% PVP, 0.5 M EG in PBS | trehalose (19.5%), PBS salts (6%), PVP (56.9%), EG (17.6%) |
| 0.1 M trehalose, 10% PVP, 0.5 M PG in PBS | trehalose (18.7%), PBS salts (5.8%), PVP (54.7%), PG (20.8%) |
a PBS is phosphate buffered saline without calcium and magnesium (see text for details).
b PBS Mg/Ca is phosphate buffered saline with calcium and magnesium.
To prepare samples with different moisture contents, mixtures containing sugar (trehalose or raffinose), PBS salts, and polymers (PVP or Ficoll) in various combinations were equilibrated in defined relative humidity environments at room temperature (~21°C). Four different relative humidities, ranging from 6.5% to 75%, were established using saturated salt solutions sealed in mason jars (Table 2). Samples were placed on stacked 3D printed platforms (acrylonitrile butadiene styrene filament) within the mason jars, to hold the specimens above the saturated salt solutions. Two different approaches were used to prepare the samples to be placed in the mason jars. The first involved placing aqueous solution into the sample containers within the sealed mason jars, thus allowing equilibration of the samples by loss of water to the controlled relative humidity environment. These samples were weighed at 2-day intervals until the mass stabilized, which occurred after approximately 3 weeks. For the second approach, the samples were first lyophilized overnight in a benchtop freeze dryer with a condenser temperature of -56°C (Virtis Lyo-Centre: 3.5L DBT ES-55) and then placed in sample pans within the controlled relative humidity environments. In this case, samples typically equilibrated by absorbing moisture from the air. These samples were equilibrated for 2–3 weeks, until their mass had stabilized.
Table 2. Relative humidity values at room temperature for saturated salt solutions [32].
| Salt Solution | Relative Humidity (%) |
|---|---|
| Lithium Bromide, LiBr | 6.5 |
| Potassium Acetate, KCH3COO | 23 |
| Magnesium Nitrate, Mg(NO3)2 | 54 |
| Sodium Chloride, NaCl | 75 |
Two different types of sample containers were used depending on the intended use of the sample. For moisture content determination, samples were equilibrated in mason jars within disposable aluminum pans (2.8 cm diameter, 1 cm depth, VWR). Samples destined for differential scanning calorimetry (DSC) studies were equilibrated in Tzero DSC sample pans (part number 901683.901, TA Instruments, New Castle, DE). Samples were prepared in triplicate and each replicate was equilibrated in a separate relative humidity chamber.
To prepare samples containing any of the penetrating CPAs, it was not possible to equilibrate mixtures in controlled relative humidity environments as described above. This is because these CPAs are volatile and can be lost from the sample during the equilibration process. Therefore, any mixtures containing DMSO, EG, or PG (see Table 1) were created by equilibrating a CPA-free mixture in mason jars to achieve a range of moisture contents, and then adding an appropriate volume of CPA to the sample to achieve the desired CPA content.
Completely dry samples for DSC experiments were prepared by placing the mixture of interest in a DSC pan and baking it in an oven at 95°C for 6–7 days. The DSC pan was then hermetically sealed and used in DSC experiments. By testing samples of 0.5 M trehalose-water that had dried for 3, 5, 6, 7, and 9 days, it was determined that 6–7 days were sufficient to dry the samples. After 6 days of drying, the glass transition temperature of samples stabilized, and remained stable after up to 9 days of drying (data not shown).
Determination of the glass transition temperature
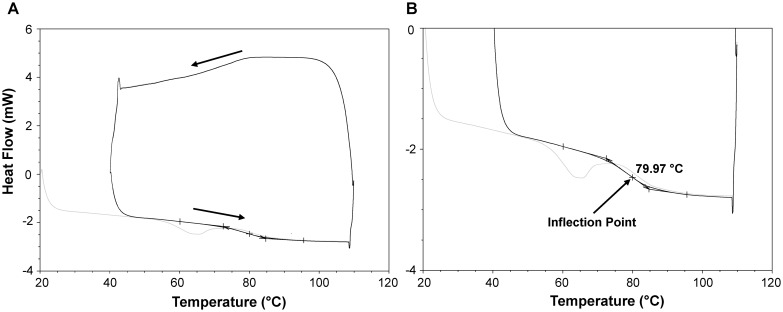
After removing equilibrated DSC pans from the mason jars, the pans were immediately weighed and hermetically sealed with aluminum hermetic lids (part number 901684.901, TA Instruments), using a Tzero Sample Press (TA Instruments). The glass transition temperatures of the samples were then determined using a Q2000 DSC with an RCS90 cooling system (TA Instruments), following the ASTM standard test method [33]. Briefly, samples underwent two cycles in which they were heated at 10°C/min to a temperature 20–30°C higher than the expected glass transition temperature, and then cooled at 20°C/min to a temperature approximately 50°C lower than the expected glass transition temperature. The glass transition temperature was determined from the inflection point observed in the second heating cycle, using TA Universal Analysis software. Fig 1 is representative of a typical experiment. The DSC temperature and heat flow were calibrated using an indium standard. The accuracy of the temperature calibration was periodically checked by measuring the melting temperature of purified water (Ricca Chemical Company), resulting in a measured melting point within ±1 K of the expected value.
Fig 1. Representative DSC data (A) and zoomed in version showing the glass transition region (B).
Light grey line is the initial ramp up in temperature which is done to erase thermal history in the sample. The test proceeds counter-clockwise, as indicated by the arrows. The glass transition temperature was determined from the inflection point in the second heating cycle, as illustrated in panel B (arrow).
Models for predicting the glass transition temperature
Various models have been used to predict glass transition temperatures of mixtures, as reviewed recently by Katkov and Levine [34] and Corti et al [35]. The most commonly used are the Gordon-Taylor (GT) model and the Couchman-Karasz (CK) model. The GT model allows prediction of the glass transition temperature of a mixture as a function of the glass transition temperatures of the pure components and a parameter k that characterizes the strength of the interaction between the two components [36]:
| (1) |
where w is the mass fraction and the subscripts 1 and 2 refer to water and solute, respectively. While the GT model is typically used for binary solutions, we use it here to characterize more complicated solutions by treating all non-water components as a single “lumped” component, as has been described previously [37]. The GT model was fit to experimental data using a least squares approach.
The CK model allows prediction of Tg,mix for a mixture given the glass transition temperature (Tg,i) and the corresponding change in heat capacity (ΔCp.i) for the i-th pure component [38]:
| (2) |
where n is the number of components in the mixture.
Determination of moisture content
All samples used for the determination of moisture content were equilibrated in chambers with defined relative humidity environments shown in Table 2. For trehalose-water samples, additional saturated-salt relative humidity chambers were also used, namely LiCl (11% relative humidity), and MgCl2 (33% relative humidity). Those relative humidity chambers were not used for the other sample compositions, due to space constraints. After equilibration of samples in the mason jars, the aluminum pans were weighed and placed into an oven at 95°C to remove any residual water. Samples were removed from the oven after 7 days of drying, and weighed again. This dry mass was compared to the initial sample mass to determine the mass fraction of water in the original sample, as well as the residual moisture content (g water/g dry mass). The resulting moisture content data were fit using the Brunauer, Emmett and Teller (BET) model [39]:
| (3) |
where m is the residual moisture content, aw is the water activity, and mm and c are constants. To obtain best-fit values of mm and c, Eq 3 was linearized [40] and fit to the data using linear regression.
Results and discussion
Moisture sorption isotherms
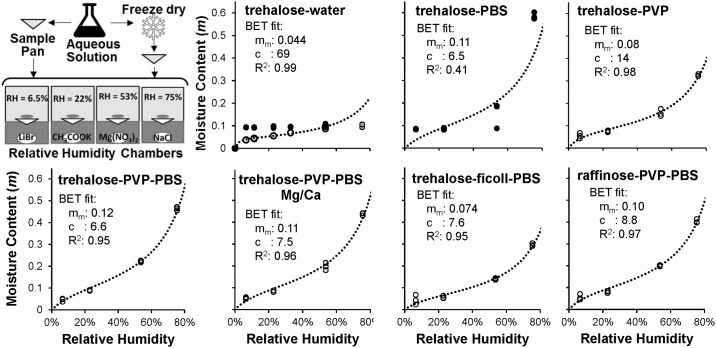
Dry basis moisture content values for various mixtures are shown in Fig 2 as a function of relative humidity. When binary trehalose-water mixtures were dried from an aqueous solution, the moisture content observed after equilibration was about 0.104 at a relative humidity of 54% and dropped slightly to 0.095 at a relative humidity of 33%. However, further decreases in relative humidity did not decrease the moisture content, suggesting that the samples may not have achieved equilibrium for relative humidities below 33%. In contrast, when aqueous trehalose solutions were freeze-dried before equilibration in controlled relative humidity environments, much lower moisture contents were observed in the samples. For example, as shown in Fig 2, the moisture content for a relative humidity of 6.5% was 0.037 for the freeze-dried sample, much lower than the value of 0.093 that was obtained when the sample was prepared by directly drying an aqueous solution. The BET fit to the data for freeze-dried trehalose is excellent over the relative humidity range 6.5%-54%, but substantially overestimates the moisture content for a relative humidity of 75%. The uncharacteristically low moisture content of 0.10 observed at 75% relative humidity may be a result of crystallization of trehalose dihydrate, as has been suggested previously [41]. Overall, our moisture content results for freeze-dried trehalose-water mixtures are very similar to those in previous reports [41, 42].
Fig 2. Moisture sorption isotherms.
Moisture content in samples prepared either as an aqueous solution (black symbols) or as a freeze-dried matrix (white and grey symbols) was measured after equilibration in chambers with controlled relative humidity. The BET model (Eq 3) was fit (dotted lines) to observations from freeze-dried samples, except for the trehalose-PBS case, in which aqueous solution data were used for fitting; in the trehalose-water experiment, outlier values at 75% relative humidity (grey symbols) were omitted from the fit. See Table 1 for detailed composition of each mixture.
Trehalose-PBS mixtures repeatedly failed to produce a dry sample after freeze-drying. This may have been caused, at least in part, by sample inhomogeneity due to collapse of the matrix during freeze-drying. Thus, for trehalose-PBS mixtures, samples were equilibrated from aqueous solution and not from the freeze-dried matrix. In this case, the samples were allowed to equilibrate for 3 months. As shown in Fig 2, trehalose-PBS mixtures yielded moisture content values that remained below 0.18 for relative humidities up to 54%, but increased sharply to 0.60 when the relative humidity increased to 75%. The data are somewhat scattered for the trehalose-PBS mixtures, and the BET fit to the data is poor, especially at the highest relative humidity value. Nonetheless, the results are approximately consistent with published results for mixtures of trehalose, PBS and water [31].
Similar problems with cake collapse during freeze drying were not observed for the other mixtures. The moisture sorption isotherms for all mixtures containing PVP were similar, yielding moisture contents ranging from about 0.04 to 0.47 over the range of relative humidities tested (see Fig 2). This is consistent with previously published results for binary PVP-trehalose mixtures [43]. Samples with Ficoll retained less moisture than did PVP mixtures, yielding moisture contents ranging from 0.02 to 0.30.
Glass transition temperatures
Trehalose-water mixtures
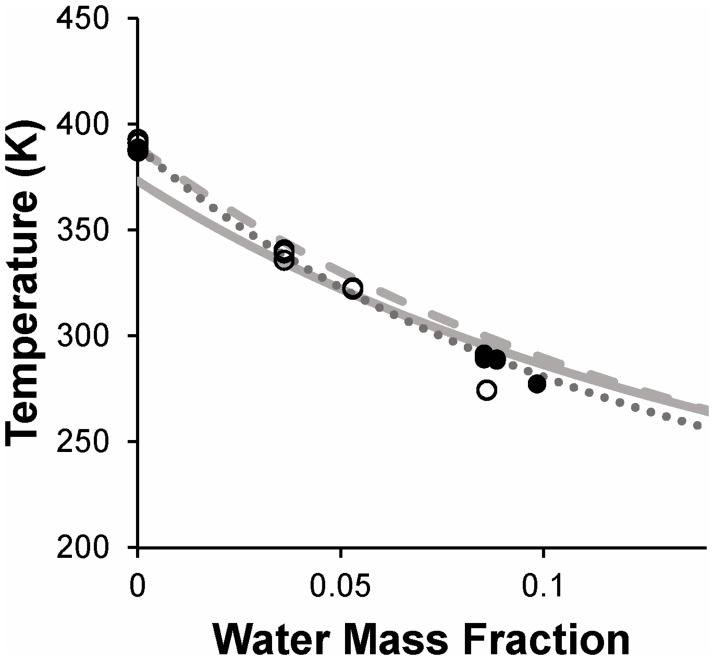
Fig 3 shows glass transition temperatures obtained for binary mixtures of trehalose and water. Pure trehalose yielded an average glass transition temperature (Tg) of 389 K; the eight replicates had Tg values that ranged from 387 K to 393 K. Literature values for the glass transition temperature of pure trehalose vary widely (reviewed in [29]), but several recent reports place Tg between 386 K and 394 K [43–47], consistent with our result. As expected, the glass transition temperature of trehalose-water mixtures decreased substantially as the water mass fraction increased, reaching about 280 K at 10% water content; this represents a decrease in Tg by more than 100 K. Chen and colleagues [29] compiled Tg data for trehalose-water mixtures from over 20 sources and fit the data using a GT model. As shown in Fig 3, our Tg data are reasonably consistent with the GT model of Chen et al. [29]. By fitting the GT model (Eq 1) to our own experimental results, we estimated a value k = 6.8 for the interaction parameter. Our estimate of the GT interaction parameter is higher than the value reported by Chen et al. (k = 5.2) [29] but slightly lower than the k-value reported by Crowe et al. (k = 7.5) [14]. Fig 3 also shows CK model (Eq 2) predictions based on published thermophysical properties of water and trehalose (Table 3). Overall, the results shown in Fig 3 demonstrate that our Tg results for trehalose-water mixtures are consistent with those reported in previous studies.
Fig 3. Glass transition temperature of binary trehalose-water mixtures.
Samples were prepared either by equilibrating aqueous solution (black symbols) or freeze-dried matrix (white symbols) in various relative humidity environments. The solid grey line shows a fit of the GT model to literature data, as reported by Chen et al. [29]. The dotted line shows the best-fit GT model (Eq 1) to the data from the current study. The dashed line represents predictions of the CK model (Eq 2) using the parameters shown in Table 3.
Table 3. Parameters for use in the CK model.
Trehalose-PVP-water mixtures
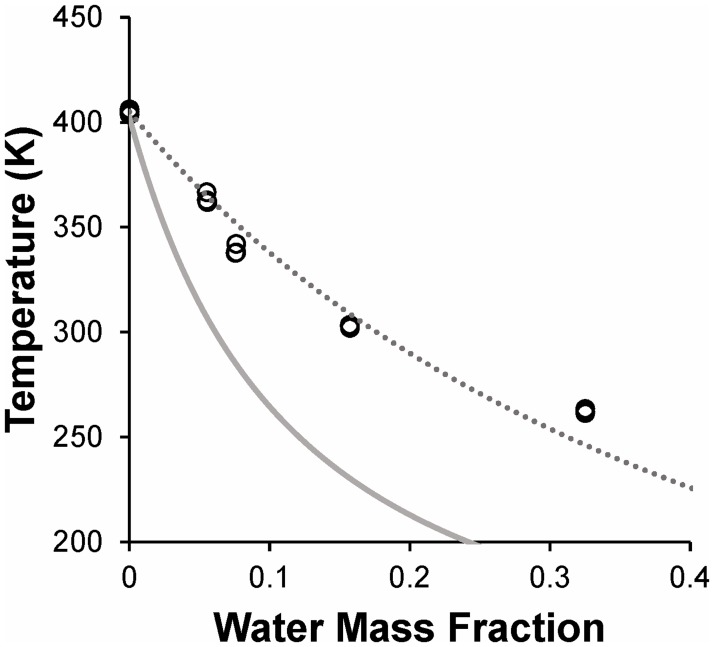
Fig 4 depicts the glass transition temperature as a function of moisture content for the ternary system trehalose-PVP-water. Compared to binary trehalose-water mixtures (Fig 3), the addition of PVP was found to increase the glass transition temperature. The glass transition temperature of dry trehalose-PVP (Tg = 405 K) was about 16 K higher than the Tg for dry trehalose, and Tg values were up to 30 K higher in the presence of PVP, over the full range of moisture contents tested. To our knowledge, there are no published studies of the glass transition properties of trehalose-PVP mixtures for PVP of the same molecular weight (40 kDa) as in the current study. Zhang and Zografi used PVP of a higher molecular weight, and reported a glass transition temperature of about 420 K for dry PVP-trehalose at the same mass ratio of trehalose to PVP as that used in our experiments [43]. This slightly higher Tg value is consistent with the expected increase in Tg with PVP molecular weight [48].
Fig 4. Glass transition temperature of trehalose-PVP-water mixtures.
Samples were prepared by equilibrating freeze-dried matrix in various relative humidity environments. The dotted line shows the best fit of the GT model (Eq 1) to the data. The solid line shows predictions of the CK model (Eq 2) using the parameters shown in Table 3.
As shown in Fig 4, the GT model (Eq 1) yields an adequate fit to the data. In contrast, predictions of the CK model (Eq 2) were found to deviate greatly from the experimental observations for this ternary system, increasingly underestimating Tg as moisture content increased. The CK model assumes that intermolecular interactions between the components of the mixture are negligible. Because the CK model fails to predict Tg for trehalose-PVP-water mixtures, it is likely that the components do not behave in a manner consistent with the model assumptions. Previous studies of PVP-water mixtures have also shown large discrepancies between experimental data and the predictions of the CK model [48]. As a result, we did not attempt to use the CK model for analysis of the other sugar-polymer mixtures investigated in our study. The failure of the CK model is unfortunate, because the CK model would have allowed prediction of the glass transition point for mixtures of arbitrary composition, using only measurements made on the individual components, whereas the GT model requires experimental measurement of Tg for each mixture of interest.
Trehalose-PBS mixtures
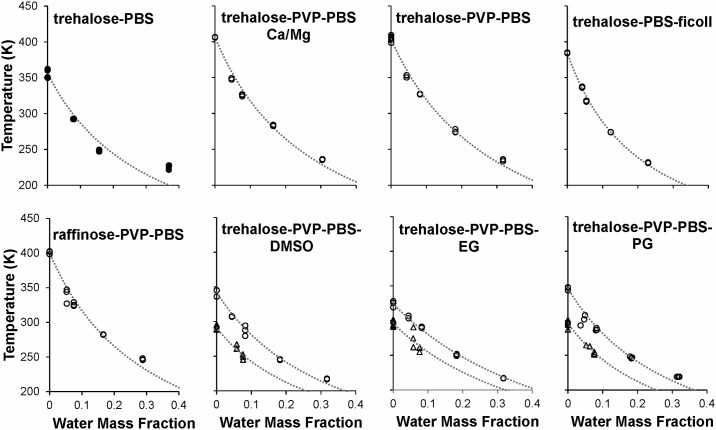
The glass transition temperatures for mixtures of trehalose and PBS are shown in Fig 5 as a function of water content. Compared with binary trehalose-water mixtures, the trehalose-PBS mixtures had a substantially lower glass transition temperature at low water content, which suggests that PBS salts impair the glass forming ability of trehalose. This result is consistent with other studies on the effects of PBS and similar physiological buffers on the glass transition temperature of trehalose mixtures [31, 37, 44, 46].
Fig 5. Glass transition temperatures of various mixtures containing PBS.
Samples were prepared either by equilibrating aqueous solution (filled symbols) or freeze-dried matrix (open symbols) in various relative humidity environments. The dotted lines show the best-fit GT model to each data set. Mixtures containing penetrating CPAs at concentrations of 0.25 M and 0.5 M are shown as circles and triangles, respectively. See Table 1 for detailed composition of each mixture.
Sugar-PBS mixtures containing PVP or Ficoll
Compared with a baseline desiccation medium containing trehalose and PBS, mixtures that also include a polymer component had a higher glass transition temperature (Fig 5). In particular, the addition of PVP to trehalose-PBS increased Tg by about 40 K over the entire range of water mass fractions tested. Addition of Ficoll increased Tg to a lesser extent, resulting in Tg values about 30 K higher than those observed in the baseline trehalose-PBS desiccation medium. The presence of calcium and magnesium in the PBS did not have an appreciable effect on the glass transition temperature of trehalose-PVP-PBS mixtures. Replacement of trehalose with raffinose resulted Tg values that were similar to those obtained for trehalose-PVP-PBS mixtures. The dominant influence of PVP on Tg is most likely due to its high mass fraction relative to the other components, which may explain the apparent insensitivity of the glass transition temperature to variations in the buffer and sugar composition.
Trehalose-PBS-PVP mixtures containing penetrating CPAs
Fig 5 also depicts the effects of penetrating CPAs on the glass transition temperature of desiccation solutions. Compared to a baseline mixture containing trehalose, PVP and PBS without CPA, mixtures that also include a penetrating cryoprotectant additive (DMSO, EG, or PG) had a lower glass transition temperature. This decrease in Tg for the mixture is consistent with the lower Tg of each individual CPA compared with the glass transition points of pure trehalose and pure PVP. Interestingly, each of the three CPAs investigated had a similar effect; for dry mixtures, Tg decreased by nearly 55 K upon addition of 0.25 M CPA, and by nearly 110 K upon addition of 0.5 M CPA. In general, the drop in Tg as water content increased was less pronounced for mixtures containing a penetrating CPA than for mixtures without such additives.
Comparison of mixtures
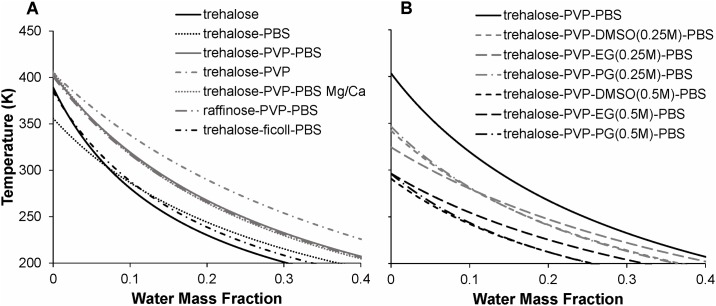
To facilitate comparison of the multi-component mixtures, the best-fit GT model for each mixture is plotted in Fig 6. The left panel of Fig 6 depicts the effects of sugars (trehalose and raffinose), polymers (PVP and Ficoll) and PBS salts on the glass transition temperature, whereas the right panel depicts the effects of penetrating CPAs (DMSO, EG, and PG) at two concentrations. The corresponding best-fit GT model parameters are summarized in Table 4. Several trends in the glass transition data are apparent in Fig 6:
Fig 6. Comparison of best-fit GT models.
(A) Effect sugars, polymers and PBS salts on the glass transition temperature. (B) Effect of CPAs on the glass transition temperature.
Table 4. Best-fit GT model parameters (see Eq 1).
| Composition of desiccation solution | Tg2 (K) | k |
|---|---|---|
| 0.1 M trehalose in water | 389 | 6.8 |
| 0.1 M trehalose in PBS | 356 | 4.1 |
| 0.1M trehalose, 10% PVP | 405 | 3.0 |
| 0.1 M trehalose, 10% Ficoll in PBS | 385 | 5.7 |
| 0.1 M trehalose, 10% PVP in PBS | 403 | 4.1 |
| 0.1 M trehalose, 10% PVP in PBS Mg/Ca | 407 | 4.4 |
| 0.1 M raffinose, 10% PVP in PBS | 401 | 4.2 |
| 0.1 M trehalose, 10% PVP, 0.25 M DMSO in PBS | 342 | 3.8 |
| 0.1 M trehalose, 10% PVP, 0.25 M EG in PBS | 324 | 2.8 |
| 0.1 M trehalose, 10% PVP, 0.25 M PG in PBS | 347 | 4.0 |
| 0.1 M trehalose, 10% PVP, 0.5 M DMSO in PBS | 291 | 4.1 |
| 0.1 M trehalose, 10% PVP, 0.5 M EG in PBS | 296 | 3.2 |
| 0.1 M trehalose, 10% PVP, 0.5 M PG in PBS | 296 | 4.4 |
Compared with trehalose-water, trehalose-PBS has a lower Tg, particularly at low water content (differences become less pronounced as water content increases);
Addition of the polymers PVP or Ficoll to trehalose-PBS increases Tg;
PVP raises Tg to a greater extent than does Ficoll;
Addition of any of the penetrating cryoprotectants DMSO, EG or PG to trehalose-PBS-PVP substantially decreases Tg;
Addition of calcium and magnesium to PBS does not have a discernible effect on the Tg of trehalose-PVP-PBS mixtures;
Sugar-PVP-PBS mixtures yield similar Tg values whether the sugar component is trehalose or raffinose.
Conclusion
To our knowledge, this study provides the first measurements of the glass transition temperature in cell desiccation media containing a mixture of sugars, polymers and penetrating CPAs in physiological buffer. In general, the polymers PVP and Ficoll had a stabilizing effect; the addition of these polymers to a baseline desiccation media containing trehalose and PBS increased Tg substantially at any given moisture content. On the other hand, the addition of DMSO, EG or PG to the desiccation mixture decreased Tg in a concentration-dependent manner.
Our results provide a means for estimating the extent of drying necessary to achieve a glass transition temperature sufficiently high for stability during storage. For stable storage in the dried state, it has been suggested that Tg should be about 50 K above the storage temperature [49]. Thus, our results indicate that a desiccation solution containing 0.1 M trehalose and 10% PVP in PBS would need to be dried to a water mass fraction of about 5% for stable storage at room temperature. For storage in a refrigerator, less extensive drying to ~9% water content would be sufficient for stability. Addition of membrane-permeable CPAs to this desiccation mixture offers the potential for protection of intracellular organelles, but increases the extent of drying necessary for stable storage. Our results suggest that room-temperature storage of a desiccation solution containing 0.25 M CPA is not feasible, even after complete drying of the sample. However, it appears to be possible for such a solution to achieve stable storage in a standard refrigerator or freezer after drying to water mass fractions of ~3% and ~6%, respectively. These examples illustrate the potential of our results to facilitate design of promising multi-component mixtures for cell desiccation.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
This work was supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (Grant No. R21 EB018538).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (Grant No. R21 EB018538) to Ali Eroglu. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dobrinsky JR. Advancements in cryopreservation of domestic animal embryos. Theriogenology. 2002;57(1):285–302. [DOI] [PubMed] [Google Scholar]
- 2.Fickel J, Wagener A, Ludwig A. Semen cryopreservation and the conservation of endangered species. Eur J Wildlife Res. 2007;53(2):81–9. [Google Scholar]
- 3.Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update. 2016;22(4):440–9. doi: 10.1093/humupd/dmw007 [DOI] [PubMed] [Google Scholar]
- 4.Hunt CJ. Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus Med Hemother. 2011;38(2):107–23. doi: 10.1159/000326623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torok Z, Satpathy G, Banerjee M, Bali R, Little E, Novaes R, et al. Preservation of trehalose-loaded red blood cells by lyophilization. Cell Preserv Technol. 2005;3(2):96–111. [Google Scholar]
- 6.Chakraborty N, Chang A, Elmoazzen H, Menze MA, Hand SC, Toner M. A Spin-Drying Technique for Lyopreservation of Mammalian Cells. Ann Biomed Eng. 2011;39(5):1582–91. doi: 10.1007/s10439-011-0253-1 [DOI] [PubMed] [Google Scholar]
- 7.Patrick J, Comizzoli P, Elliott G. Dry Preservation of Spermatozoa: Considerations for Different Species. Biopreserv Biobank. 2017;15(2):158–68. doi: 10.1089/bio.2016.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di G, Wang J, Liu M, Wu CT, Han Y, Duan H. Development and evaluation of a trehalose-contained solution formula to preserve hUC-MSCs at 4 degrees C. J Cell Physiol. 2012;227(3):879–84. doi: 10.1002/jcp.23066 [DOI] [PubMed] [Google Scholar]
- 9.Ma X, Jamil K, Macrae TH, Clegg JS, Russell JM, Villeneuve TS, et al. A small stress protein acts synergistically with trehalose to confer desiccation tolerance on mammalian cells. Cryobiology. 2005;51(1):15–28. doi: 10.1016/j.cryobiol.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 10.Li S, Chakraborty N, Borcar A, Menze MA, Toner M, Hand SC. Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. Proc Natl Acad Sci U S A. 2012;109(51):20859–64. doi: 10.1073/pnas.1214893109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitaula R, Elmoazzen H, Toner M, Bhowmick S. Desiccation tolerance in bovine sperm: a study of the effect of intracellular sugars and the supplemental roles of an antioxidant and a chelator. Cryobiology. 2009;58(3):322–30. doi: 10.1016/j.cryobiol.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 12.Wolkers WF, Walker NJ, Tablin F, Crowe JH. Human platelets loaded with trehalose survive freeze-drying. Cryobiology. 2001;42(2):79–87. doi: 10.1006/cryo.2001.2306 [DOI] [PubMed] [Google Scholar]
- 13.Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–99. doi: 10.1146/annurev.ph.54.030192.003051 [DOI] [PubMed] [Google Scholar]
- 14.Crowe LM, Reid DS, Crowe JH. Is trehalose special for preserving dry biomaterials? Biophys J. 1996;71(4):2087–93. doi: 10.1016/S0006-3495(96)79407-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T, Acker JP, Eroglu A, Cheley S, Bayley H, Fowler A, et al. Beneficial effect of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology. 2001;43(2):168–81. doi: 10.1006/cryo.2001.2360 [DOI] [PubMed] [Google Scholar]
- 16.Eroglu A, Russo MJ, Bieganski R, Fowler A, Cheley S, Bayley H, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol. 2000;18(2):163–7. doi: 10.1038/72608 [DOI] [PubMed] [Google Scholar]
- 17.Eroglu A, Toner M, Toth TL. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil Steril. 2002;77(1):152–8. [DOI] [PubMed] [Google Scholar]
- 18.Eroglu A, Bailey SE, Toner M, Toth TL. Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol Reprod. 2009;80(1):70–8. doi: 10.1095/biolreprod.108.070383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch AL, Chen R, Slater NK. pH-responsive polymers for trehalose loading and desiccation protection of human red blood cells. Biomaterials. 2011;32(19):4443–9. doi: 10.1016/j.biomaterials.2011.02.062 [DOI] [PubMed] [Google Scholar]
- 20.Satpathy GR, Torok Z, Bali R, Dwyre DM, Little E, Walker NJ, et al. Loading red blood cells with trehalose: a step towards biostabilization. Cryobiology. 2004;49(2):123–36. doi: 10.1016/j.cryobiol.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 21.Shirakashi R, Kostner CM, Muller KJ, Kurschner M, Zimmermann U, Sukhorukov VL. Intracellular delivery of trehalose into mammalian cells by electropermeabilization. J Membr Biol. 2002;189(1):45–54. doi: 10.1007/s00232-002-1003-y [DOI] [PubMed] [Google Scholar]
- 22.Abazari A, Meimetis LG, Budin G, Bale SS, Weissleder R, Toner M. Engineered Trehalose Permeable to Mammalian Cells. PLoS ONE. 2015;10(6):e0130323 doi: 10.1371/journal.pone.0130323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eroglu A, Lawitts JA, Toner M, Toth TL. Quantitative microinjection of trehalose into mouse oocytes and zygotes, and its effect on development. Cryobiology. 2003;46(2):121–34. [DOI] [PubMed] [Google Scholar]
- 24.Lovelock JE. The protective action of neutral solutes against haemolysis by freezing and thawing. Biochem J. 1954;56(2):265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott GD, Wang S, Fuller BJ. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74–91. doi: 10.1016/j.cryobiol.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 26.Eroglu A. Cryopreservation of mammalian oocytes by using sugars: Intra- and extracellular raffinose with small amounts of dimethylsulfoxide yields high cryosurvival, fertilization, and development rates. Cryobiology. 2010;60(3 Suppl):S54–9. doi: 10.1016/j.cryobiol.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Y, Quan GB, Liu XZ, Ma EP, Liu A, Jin P, et al. Improved preservation of human red blood cells by lyophilization. Cryobiology. 2005;51(2):152–64. doi: 10.1016/j.cryobiol.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Natan D, Nagler A, Arav A. Freeze-drying of mononuclear cells derived from umbilical cord blood followed by colony formation. PLoS ONE. 2009;4(4):e5240 doi: 10.1371/journal.pone.0005240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen T, Fowler A, Toner M. Literature review: supplemented phase diagram of the trehalose-water binary mixture. Cryobiology. 2000;40(3):277–82. doi: 10.1006/cryo.2000.2244 [DOI] [PubMed] [Google Scholar]
- 30.Rahman MS, Al-Marhubi IM, Al-Mahrouqi A. Measurement of glass transition temperature by mechanical (DMTA), thermal (DSC and MDSC), water diffusion and density methods: A comparison study. Chem Phys Lett. 2007;440(4–6):372–7. [Google Scholar]
- 31.Sitaula R, Bhowmick S. Moisture sorption characteristics and thermophysical properties of trehalose-PBS mixtures. Cryobiology. 2006;52(3):369–85. doi: 10.1016/j.cryobiol.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 32.Greenspan L. Humidity Fixed-Points of Binary Saturated Aqueous-Solutions. J Res Nbs a Phys Ch. 1977;81(1):89–96. [Google Scholar]
- 33.ASTM Standard E1356-08. Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry. ASTM International, West Conshohocken, PA2014.
- 34.Katkov II, Levine F. Prediction of the glass transition temperature of water solutions: comparison of different models. Cryobiology. 2004;49(1):62–82. doi: 10.1016/j.cryobiol.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 35.Corti HR, Angell CA, Auffret T, Levine H, Buera MP, Reid DS, et al. Empirical and theoretical models of equilibrium and non-equilibrium transition temperatures of supplemented phase diagrams in aqueous systems (IUPAC Technical Report). Pure Appl Chem. 2010;82(5):1065–97. [Google Scholar]
- 36.Gordon M, Taylor JS. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Noncrystalline copolymers. Journal of Applied Chemistry. 1952;2(9):493–500. [Google Scholar]
- 37.Reis J, Sitaula R, Bhowmick S. Water activity and glass transition temperatures of disaccharide based buffers for desiccation preservation of biologics. J Biomedical Science and Engineering. 2009;2:594–605. [Google Scholar]
- 38.Couchman PR, Karasz FE. Classical Thermodynamic Discussion of Effect of Composition on Glass-Transition Temperatures. Macromolecules. 1978;11(1):117–9. [Google Scholar]
- 39.Brunauer S, Emmett PH, Teller E. Adsorption of Gases in Multimolecular Layers. J Am Chem Soc. 1938;60(2):309–19. [Google Scholar]
- 40.Timmermann EO, Chirife J, Iglesias HA. Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? J Food Eng. 2001;48(1):19–31. [Google Scholar]
- 41.Iglesias HA, Chirife J, Buera MP. Adsorption isotherm of amorphous trehalose. J Sci Food Agric. 1997;75(2):183–6. [Google Scholar]
- 42.Lammert AM, Schmidt SJ, Day GA. Water activity and solubility of trehalose. Food Chem. 1998;61(1–2):139–44. [Google Scholar]
- 43.Zhang J, Zografi G. Water vapor absorption into amorphous sucrose-poly(vinyl pyrrolidone) and trehalose-poly(vinyl pyrrolidone) mixtures. J Pharm Sci. 2001;90(9):1375–85. [DOI] [PubMed] [Google Scholar]
- 44.Weng LD, Elliott GD. Distinctly Different Glass Transition Behaviors of Trehalose Mixed with Na2HPO4 or NaH2PO4: Evidence for its Molecular Origin. Pharm Res. 2015;32(7):2217–28. doi: 10.1007/s11095-014-1610-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weng LD, Elliott GD. Dynamic and thermodynamic characteristics associated with the glass transition of amorphous trehalose-water mixtures. Phys Chem Chem Phys. 2014;16(23):11555–65. doi: 10.1039/c3cp55418j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohtake S, Schebor C, Palecek SP, de Pablo JJ. Effect of pH, counter ion, and phosphate concentration on the glass transition temperature of freeze-dried sugar-phosphate mixtures. Pharm Res. 2004;21(9):1615–21. [DOI] [PubMed] [Google Scholar]
- 47.Surana R, Pyne A, Suryanarayanan R. Effect of preparation method on physical properties of amorphous trehalose. Pharm Res. 2004;21(7):1167–76. [DOI] [PubMed] [Google Scholar]
- 48.Buera MD, Levi G, Karel M. Glass-Transition in Poly(Vinylpyrrolidone)—Effect of Molecular-Weight and Diluents. Biotechnol Prog. 1992;8(2):144–8. [Google Scholar]
- 49.Hancock BC, Shamblin SL, Zografi G. Molecular Mobility of Amorphous Pharmaceutical Solids Below Their Glass-Transition Temperatures. Pharm Res. 1995;12(6):799–806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.