Abstract
Background
Invasive pneumococcal disease (IPD) is a major cause of illness and death among children worldwide. 10-valent pneumococcal conjugate vaccine (PCV10) was introduced as part of the Mozambican routine immunization program in April 2013. We characterized the IPD burden in a rural area of Mozambique before PCV introduction and estimated the potential impact of this intervention.
Methods
We conducted population-based surveillance for IPD, defined as S. pneumoniae isolated from blood or cerebrospinal fluid, among children <5 years old admitted to Manhiça District Hospital, a referral hospital in a rural area with high prevalence of human immunodiciency virus infection. S. pneumoniae was identified using standard microbiologic methods and serotyped using sequential multiplex PCR or Quellung. IPD incidence was calculated among cases from a defined catchment area.
Results
From January 2001 through December 2012, we isolated 768 cases of IPD, 498 (65%) of which were bacteraemic pneumonia episodes. A total of 391 (51%) were from the catchment area, yielding IPD incidence rates of 479, 390 and 107 episodes per 100,000 children-years at risk among children <12, 12–23 and 24-<60 months old, respectively. The overall IPD incidence fluctuated and showed a downward trend over time. In these same age groups, in-hospital death occurred in 48 (17%), 26 (12%), and 21 (13%) of all IPD cases, respectively. Overall 90% (543/603) of IPD isolates were available for serotyping; of those, 65% were covered by PCV10 and 83% by PCV13. Among 77 hospital deaths associated with serotyped IPD, 49% and 69% were caused by isolates included in the PCV10 and PCV13, respectively.
Conclusions
We describe very high rates of IPD among children in rural Mozambique that were declining before PCV introduction. Children <1 year old have the greatest incidence and case fatality; although the rates remain high among older groups as well. Most IPD episodes and many deaths among children <5 years old will likely be prevented through PCV10 introduction in Mozambique.
Introduction
Streptococcus pneumoniae is a leading cause of bacterial pneumonia, bacteremia/sepsis, and acute meningitis in children worldwide [1]. The World Health Organization (WHO) estimated that approximately 541,000 children less than 5 years old died in 2008 of pneumococcal disease [2], and most pediatric pneumococcal deaths occur in developing countries [1]. In 2007, based on demonstrated efficacy and safety of a 7-valent and a 9-valent pneumococcal conjugate vaccine (PCV) [3–6], WHO recommended that PCV be included in routine immunization programs, especially in countries with high mortality rates among children under the age of 5 years and high prevalence of HIV infection [7]. In 2009 and 2010, 10-valent (PCV10) and 13-valent (PCV13) PCVs were licensed, offering improved coverage for prevalent pneumococcal serotypes, particularly in Africa where serotypes 1 and 5 are frequent [8]. PCVs have been increasingly introduced in sub-Saharan Africa in recent years [9]. However, due to difficulties in building the necessary laboratory and epidemiologic capacities, few African settings have high-quality population-based surveillance for invasive pneumococcal disease.
Since 2001, the Centro de Investigação em Saúde de Manhiça (CISM) has been conducting surveillance for invasive bacterial disease among children in rural Mozambique [10–12]. Data from this surveillance system were instrumental in prompting the Mozambican Ministry of Health to apply for GAVI Alliance support to introduce Hib conjugate vaccine in August, 2009 and PCV10 in April, 2013. PCV10 was introduced using a 3-dose schedule (given at 2, 3, and 4 months of age) with no catch-up campaign. We examined pre-PCV surveillance data in order to describe the burden of invasive pneumococcal disease (IPD) before vaccine introduction and to estimate the anticipated impact of PCV introduction on pneumococcal illness and death among children in Mozambique.
Material and methods
Study area and population
Surveillance for invasive pneumococcal disease (IPD) is routinely conducted at Manhiça District Hospital (MDH), a referral district hospital in Southern Mozambique. Details of the study site have been described elsewhere [13]. Manhiça district has a total population of approximately 165,000 persons, 18% of which are children <5 years old [14]. The area is rural, malaria-endemic [15] and with a high burden of human immunodeficiency virus (HIV); HIV prevalence among pregnant woman was estimated to be 24% in 2003 to 2005 [16] and overall community prevalence peaked at 39% in 2011 [17]. Within the district, CISM has maintained a continuous demographic surveillance system (DSS) since 1996, recording life events (pregnancy, births, deaths and migration in or out of the study area) and performing updates biannually from 1996 to 2012. The DSS area initially included approximately 36,000 people; by 2005 it had been expanded to include 92,000 people; which correspond to 58% of the total district population in Manhiça [13].
The protocol for population-based invasive pneumococcal surveillance described in this study was reviewed and approved by the Mozambican National Bioethics Committee, the Institutional Review Board of the Clinic Hospital of Barcelona (Barcelona, Spain), and was determined to be non-human subjects research by the U.S. Centers for Disease Control and Prevention.
Morbidity surveillance and sample collection
Since January 1997, CISM has operated pediatric morbidity surveillance at MDH and within a network of 5 peripheral health centers within the DSS area [10, 12]. In these health facilities, trained clinical personnel perform physical examinations and complete standardized forms to record demographic information, presenting signs and symptoms, and clinical outcomes. During the study period, HIV testing results were not routinely available; because of concern about stigma. HIV testing was often performed anonymously and therefore the results are not linkable to the IPD surveillance database. At MDH, the only site with admission facilities and linked to demographic surveillance, 1–3 mL of blood for culture are obtained by venipuncture upon admission and before administration of antimicrobials for all children <2 years requiring hospitalization and for those 2-<15 years with axillary temperature ≥39°C or other signs of severe disease. Lumbar puncture to collect cerebrospinal fluid (CSF) is systematically performed on all children with suspected meningitis and admitted infants <29 days old.
Bacteriological procedures
Pediatric blood culture bottles (Pedibact®, Becton-Dickinson, Franklin Lakes, NJ) were inoculated with 1–3 ml of whole blood and incubated in an automated system (BACTEC® 9050 Becton-Dickinson, Franklin Lakes, NJ) for 4 days. Post-incubation specimens from blood culture bottles with a Gram stain compatible with pneumococcus were sub-cultured onto blood agar and incubated overnight at 37°C in 5% CO2. CSF samples were first stained by Gram and subsequently cultured onto blood agar, chocolate blood agar and thioglycolate broth media for 72h, at 37°C in an atmosphere of 5% CO2. Thioglycolate broths were subcultured on chocolate blood agar media and incubated for 72h. Pneumococci were identified by α-hemolysis and typical colony morphology and confirmed by optochin sensitivity.
Serotyping of pneumococcal isolates was consistently performed starting in 2003. Pneumococcal strains were initially serotyped at the CDC Streptococcus laboratory by Quellung reaction with serotype-specific antiserum [18]. Since 2006, serotyping has been performed at CISM using sequential multiplex PCR [19], with quality control and resolution of serogroups that cannot be differentiated by PCR done at CDC in Atlanta by Quellung method.
All pneumococcal isolates from 2008 to 2010 were tested for antibiotic susceptibility using broth microdilution method. Isolates were classified as susceptible, intermediate or resistant according to the definitions of the National Committee for Clinical and Laboratory Standards Institute (CLSI) for bacteremia [20] for 2011.
Definitions
A case of IPD was defined as the isolation of S. pneumoniae from a normally sterile site (such as blood or CSF) in a child <5 years of age presenting to MDH. Cases with S. pneumoniae detected in CSF (CSF only or in both CSF and blood) were classified as pneumococcal meningitis; all other cases with S. pneumoniae in the blood were classified as bacteraemia. Among bacteraemia cases, those with cough and/or breathing difficulties plus tachypnea were classified as bacteraemic pneumonia. Case-fatality for IPD episodes excludes patients with unknown outcome; 90 children out of 670 (because were referred to referral hospital or absconded from the hospital), and thus represents in-hospital mortality. PCV10 serotypes include 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F; because of potential cross-protection against serotype 6A [21], we also examined PCV10 serotypes plus 6A. PCV13 includes the same pneumococcal serotypes included in the PCV10 plus 3, 6A and 19A.Weight-for-age z-score was calculated using WHO child growth chart standards [22].
Data management and analysis
Data from standardized forms were double entered using visual Fox Pro version 2.6 (Microsoft Corporation, Redmond, WA) at CISM. Proportions were compared using the X2 test. We calculated the incidence rates of IPD, pneumococcal bacteremia and pneumococcal meningitis among children <1 year, 1–2 years, 2-<5 years from the DSS area. Recurrent detection of S. pneumoniae within 14 days of the first positive culture was considered the same episode of IPD. To estimate incidence, person-time of follow-up for children in the demographic surveillance area was calculated per 100,000 children-years at risk (CYR) using dates of birth and death, excluding periods of outmigration. Negative binomial regression models were developed to estimate incidence rates overall and by age-group. Models were estimated with random intercept to take into account repeated measures, since children can belong to several age categories or to several calendar years during the surveillance period.
Statistical analyses were performed using STATA software (version 12.0, STATA Corporation, College Station, TX).
Results
From 1st January 2001 to 31st December 2012, there were 41,106 admissions to MDH among children <5 years of age, of which 48% were from the DSS catchment area. Per hospital protocol, blood culture was indicated for nearly all admitted children (n = 41,032, 99.8%); and it was performed for 34,947 (85%) of admissions with an indication. CSF culture was done for 3,347 patients, representing 8% of all admissions and 56% (3,347/6,006) of those with an indication for lumbar puncture per hospital protocol. The proportion of eligible patients undergoing lumbar puncture was lowest during the first two years of surveillance (569/1,264, 45%); in more recent years the proportion ranged from 82% to 85%.
A total of 768 IPD cases were identified, including 498 (65%) bacteraemic pneumonia episodes, 218 (28%) bacteraemia (non-pneumonia) cases and 52 (7%) pneumococcal meningitis cases (Table 1). Among pneumococcal meningitis cases, 35 (67%) had S. pneumoniae isolated from blood in addition to being detected in the CSF. Overall, 55% (425/768) of IPD cases were in males and 76% (584/768) occurred among children under the age of <24 months. Among 64 cases (8%) with documented HIV status, 34 (53%) occurred in HIV-infected children. Among 667 cases with known nutritional status, 159 (24%) had severe malnutrition. Hospital death occurred in 48 (17%), 26 (12%), and 21 (13%) of all cases aged <12 months, 12–23 months and 24-<60 months old respectively (p = 0.078). Overall case fatality was 14% (95/670), and significantly higher among pneumococcal meningitis cases (38%; 15/40) compared with non-meningitis syndromes (13%, 80/630; p = 0.001), among cases with known outcomes. Almost half (51%; 48/95) of IPD death occurred among infants.
Table 1. Characteristics of IPD cases (n = 768) detected from January 2001 to December 2012 at Manhiça District Hospital, in Mozambique.
| Characteristics | Bacteraemic pneumonia | Bacteraemia (non-Pneumonia) | Meningitis | Overall |
|---|---|---|---|---|
| n = 498 | n = 218 | n = 52 | N = 768 | |
| n (%) | n (%) | n (%) | n (%) | |
| Gender: male | 269 (54) | 127 (58) | 29 (56) | 425 (55) |
| Age (months) | ||||
| Median (interquartile range) | 14 (8 to 24) | 11 (7 to 20) | 8.5 (4 to 18) | 13 (7 to 23) |
| 0–11 | 195 (39) | 111 (51) | 30 (58) | 336 (44) |
| 12–23 | 174 (35) | 62 (28) | 12 (23) | 248 (32) |
| 24-<60 | 129 (26) | 45 (21) | 10 (19) | 184 (24) |
| Malnutrition* | 135 (30) | 48 (27) | 22 (45) | 22 (45) |
| None | 135 (30) | 48 (27) | 22 (45) | 22 (45) |
| Mild | 86 (20) | 51 (28) | 13 (27) | 150 (23) |
| Moderate | 104 (24) | 38 (21) | 11 (22) | 153 (23) |
| Severe | 112 (26) | 44 (24) | 3 (6) | 159 (24) |
| Seasonality | ||||
| Rainy season (October–March) | 234 (47) | 96 (44) | 26 (50) | 356 (46) |
| Hospitalized duration (days) | ||||
| Median (interquartile range) | 6 (3 to 9) | 5 (3 to 8) | 3 (2 to 5) | 5 (3 to 9) |
| Case fatality by age group** | ||||
| 0–11 months | 24/169 (14) | 12/97(12) | 12/24(50) | 48/290 (17) |
| 12–23 months | 18/151 (12) | 6/52 (12) | 2/9 (22) | 26/212 (12) |
| 24-<60 months | 17/119 (14) | 3/42 (7) | 1/7 (14) | 21/168 (13) |
| Overall | 59/439 (14) | 21/191 (11) | 15/40 (38) | 95/670 (14) |
*Among for 667 IPD cases with complete weight or age data for weight for age Z-score (waz) calculation (waz >-2 to <-1 Mild; waz>-3 to <-2 Moderate; waz<-3 severe).
** Among 670 IPD cases with known outcome (missing date in 47 among infants (<12 months), 36 among children age 12-<24m and 16 among children age 24-<60).
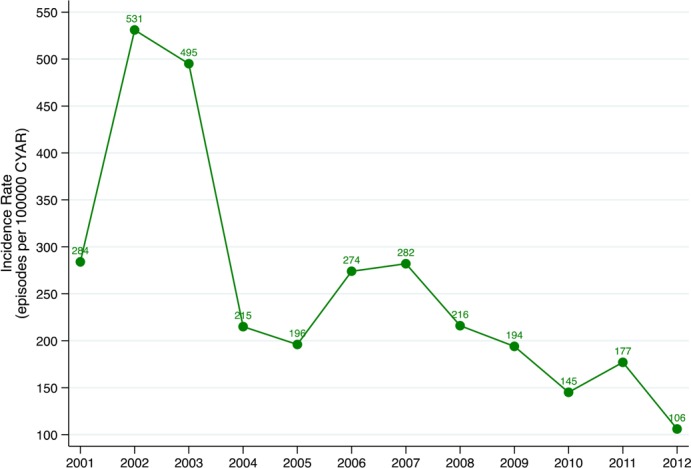
Incidence rate: Among 391 IPD cases from the DSS area, 4 were in children who had a positive pneumococcal culture within the prior 15 days; thus a total of 387 unique IPD episodes were detected. The overall incidence was 245 episodes per 100,000 CYR (Table 2). Rates were highest among infants 0–11 months (479 episodes per 100,000 CYR) and among children aged 12–23 months (390 episodes per 100,000 CYR). Incidence rates for all children <5 years varied over time, with a peak incidence of 531 episodes per 100,000 CYR in 2002–2003 (Fig 1). Overall, during the study period there was a trend toward declining incidence rates, with the lowest rate observed in 2012 (106 episodes per 100,000 CYR).
Table 2. Incidence rate of IPD among children <5 years old in rural Mozambique per different age groups, in Manhiça District Hospital.
| Age groups (months) | Episodes | Time at risk | Incidence | IRR# | 95% |
|---|---|---|---|---|---|
| (CYR) | (Episodes per 100,000 CYR*) | Conf. Interval | |||
| 0–11 | 163 | 34,018 | 479 | 1 | |
| 12–23 | 126 | 32,345 | 390 | 0.82 | (0.65–1.04) |
| 24–59 | 98 | 91,848 | 107 | 0.23 | (0.17–0.29) |
| TOTAL | 387 | 158,211 | 245 | - | - |
* CAR = children-years at risk.
# IRR = Incidence Rate Ratio.
Fig 1. Annual incidence rates of IPD cases among children <5 years of age admitted at Manhiça District Hospital, in Mozambique between 2001–2012.
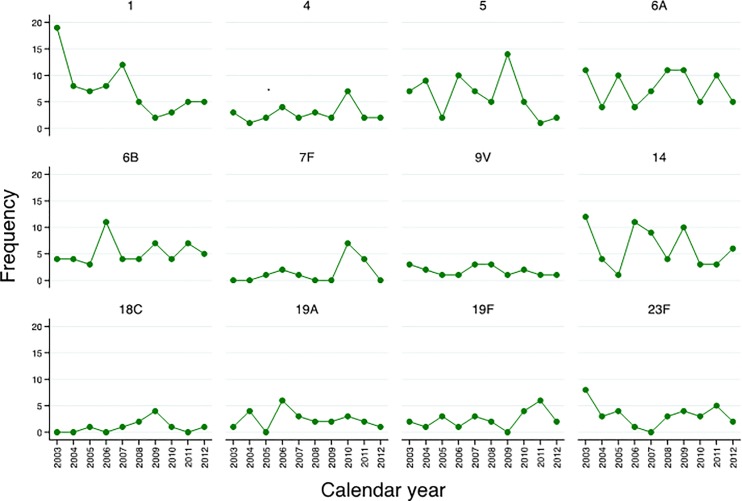
Serotypes: From 603 IPD episodes occurring in 2003–2012, 543 (90%) isolates were available for serotyping. Among those, 5 (1%) were non-typable. Among the remaining 538, 37 different serotypes were detected. The most prevalent serotypes were 6A (13.9%), 1 (13.2%), 14 (11.2%), 5 (10.7%), 6B (9.0%), and 23F (6.1%), (Fig 2). Three (<1%) isolates were classified as 6AB by PCR but no isolate was available to definitively determine serotype by Quellung. The relative contribution of different serotypes varied over time, with the greatest number of serotypes 1, 14 and 23F cases observed in 2003 and in 2009 the greatest number it was recorded for serotypes 5, 6A and 14 (Fig 3).
Fig 2. Serotypes distribution of pneumococcal isolates (n = 546) in blood and CSF of children <5 years of age admitted at Manhiça District Hospital, in Mozambique: 2003–2012.
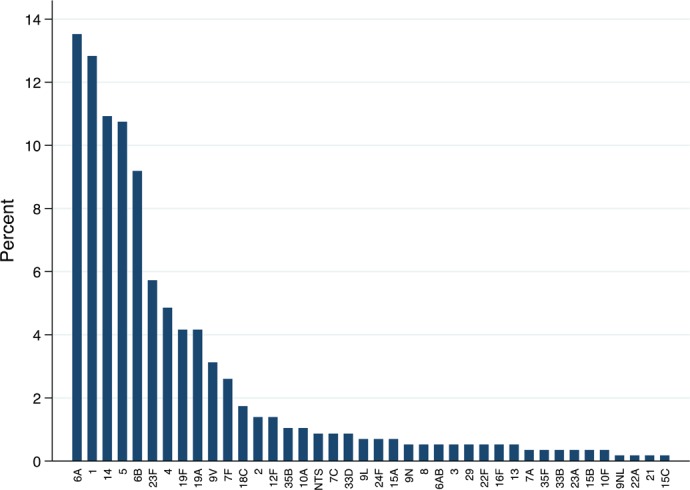
Fig 3. Number of IPD cases caused by most common vaccine types among children <5 years of age admitted at Manhica District Hospital, 2003–2012.
The proportion of cases due to serotypes included in PCV10 and PCV13 were 65% and 83%, respectively, with the difference attributable primarily to serotypes 6A and 19A. If we assume cross protection against 6A from PCV10, then the proportion of cases covered by PCV10 increases to 78%. Serotype coverage did not vary significantly by clinical syndrome, age group or by year (Table 3). Serotype data were available for 77 IPD in-hospital deaths; 38 (49%) were due to serotypes included in PCV10 and 52 (69%) due to serotypes included in PCV13.
Table 3. Proportion of pneumococcal disease syndromes potentially preventable by PCV formulation, among children < 5 years of age in Mozambique.
| PCV10 | PCV13 | |||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| Overall | 383/588 | 65 | 488/588 | 83 |
| Clinical syndromes | ||||
| Bacteraemic pneumonia | 247/375 | 66 | 310/375 | 83 |
| Bacteraemia (non-pneumonia) | 105/165 | 64 | 141/165 | 85 |
| Pneumococcal Meningitis | 31/48 | 65 | 37/48 | 77 |
| Age group | ||||
| 0–11 months | 166/263 | 63 | 210/263 | 80 |
| 12–23 months | 118/187 | 63 | 159/187 | 85 |
| 24–59 months | 99/138 | 72 | 119/138 | 86 |
Antimicrobial susceptibility: Table 4 summarizes pneumococcal antimicrobial resistance of 195 IPD isolates between 2008 and 2010. Resistance to cotrimoxazole was most common (54.4%), followed by tetracycline (23.6%) and chloramphenicol (14.4%). Resistance to beta lactam antibiotics was low, with <1% resistant to penicillin or ampicillin. Among 106 isolates resistant to cotrimoxizale, 68 (64%) were vaccine serotypes.
Table 4. Antibmicrobial susceptibility for 195 invasive pneumococcal isolates with antimicrobila susecptability performed from children <5 years old, hospitalized at Manhiça District Hospital, Mozambique.
| Antibiotics | Intermediate* | Resistant* | Range MICs |
|---|---|---|---|
| n (%) | n (%) | ||
| Erythromycin | 0 | 7 (3.6) | ≤0.03 to >32 |
| Penicillin # | 0 | 1 (0.5) | ≤0.03 to 4 |
| Amoxicillin | 0 | 1 (0.5) | ≤0.03 to 4 |
| Cefotaxime | 0 | 0 | ≤0.06 to 1 |
| Clindamycin | 0 | 3 (1.5) | ≤0.03 to >2 |
| Cotrimoxazole | 57 (29.2) | 106 (54.4) | ≤0.12 to >4 |
| Chloramphenicol | - | 28 (14.4) | ≤2 to >8 |
| Tetracycline | 0 | 46 (23.6) | ≤2 to >8 |
# Using CLSI 2011 breakpoints 4ug/ml for intermediate and ≥8ug/ml for resistant for non-meningitis isolates.
*The percentage indicates the pneumococcal isolates resistant or intermediate among the 195 invasive pneumococcal isolates tested for antibiotic susceptibility
Discussion
We demonstrate an extremely high, yet dynamic, burden of paediatric pneumococcal disease in rural Mozambique before PCV introduction. The overall incidence observed during the twelve year period examined was 245 cases of IPD per 100,000 CYR among children <5 years old, which is higher than what has been reported for this age group from other African sites. Consistent with studies from other locations [23–27], the greatest burden was observed among infants <12 months (479 per 100,000 CYR), followed by 12–23 month olds (390 per 100,000 CYR). Even these very high incidences, however, are likely an underestimate, since more than 50% of paediatric deaths in the study area occur at home with no previous hospital attendance [28]. With a case fatality proportion of 14% overall and 29% among meningitis cases, our findings highlight the importance of S. pneumoniae as a cause of death among young children and the need to prevent pneumococcal deaths in order to achieve Millennium Development Goal 4.
PCV10 was introduced in the routine infant immunization program in Mozambique in April, 2013. Extrapolating the observed incidence rates in 2012 to 4,332,000 Mozambican children <5 years of age in 2012 [14], we estimate 4,592 hospitalizations for IPD and 643 IPD in-hospital deaths among children <5 years olds nationwide occur each year. Assuming 65% of these IPD cases and 49% of the deaths could be prevented by PCV10, approximately 2,985 admissions and 315 deaths could be averted annually. Available data on PCV10 impact [29] and effectiveness [30] from other countries suggest that it is highly protective against vaccine-type IPD among young children; however, these studies have been conducted in places with relatively low HIV prevalence. Data on PCV effectiveness against IPD among HIV-infected children are limited and inconclusive [5, 31]. Continued surveillance for IPD in Mozambique, an area with a very high burden of both HIV and pneumococcal disease, will provide important data on the benefits of PCV10 introduction in populations with a high-HIV prevalence.
Based on the serotype results, PCV13 would offer protection against a higher proportion of IPD cases in Mozambique (83% versus 65% coverage for PCV10 and 78% coverage for PCV10+6A). Since the two serotypes that primarily account for the difference were 6A and 19A, the true difference between the coverage of the two formulations may depend upon cross-protection from vaccine serotypes (6B and 19F). PCV7, which included serotype 6B, was shown in a case-control study to be 76% (95% CI 39, 90) effective against IPD caused by serotype 6A [32] and rates of 6A IPD fell in variety of settings where PCV7 was introduced [33, 34]. Immunogenicity studies suggest that PCV10 should also provide cross-protection against serotype 6A [35, 36]. A case-control study in Brazil did not find significant PCV10 effectiveness against serotype 6A IPD, although power was limited [30]. That study did show an effectiveness of 82.2% (95% CI 10.7–96.4) against serotype 19A. However pneumococcal carriage data from Kenya show no impact of PCV10 introduction on carriage with serotype 6A or 19A [37]. The uncertainty about PCV10 cross protection underscores the need to monitor trends in non-vaccine serotypes following PCV10 introduction in Mozambique.
Our data also highlight the challenges in evaluating PCV10 impact due to the dynamic nature of pneumococcal disease. The incidence initially increased from 2001 to 2002, followed by a general downward trend over the course of the study period, albeit with year-to-year variation and relative increases observed in 2006 and 2011. The annual incidence ranged considerably from year to year, with a 5-fold difference between the lowest (106 per 100,000 CYR in 2012) and highest (531 per 100,000 CYR in 2002) annual rates measured. Certain pneumococcal serotypes (such as serotypes 1 and 5) can fluctuate over time, dramatically altering IPD trends. From 2003 on serotype 1 disease declined steadily, which may represent the resolution of a serotype 1 outbreak. The increase in overall IPD seen from 2001 to 2002 (before serotyping was routinely performed) is difficult to explain, but could also represent fluctuations in serotype 1. The general decline in IPD incidence over the course of the study period may have been influenced by a variety of factors including HIV prevalence and clinical management, socioeconomic and nutritional status. Improved access to antiretroviral treatment for HIV has been associated with a decline in IPD burden among HIV-infected children in South Africa [38] and HIV-infected adults in Malawi [39, 40]. Attempts to assess PCV10 impact by studying IPD trends over time must take into account pre-vaccine trends, and may be complemented by other methods (such as case-control vaccine effectiveness studies) or trends in other outcomes (i.e. pneumonia, pneumococcal carriage).
Antibiotic resistance data derived from this study are reassuring. Despite a high prevalence of penicillin resistance among pneumococcal isolates from other settings [41–43] we found less than 1% of strains to be resistant to beta-lactam antibiotics. These data support the empirical use of penicillin for management of pediatric pneumonia and suspected bacteremia/sepsis.
Several factors should be considered when interpreting these data. Only a single blood culture specimen was collected for each patient and lumbar punctures were performed for less than half of the children with suspect meningitis. Children with severe illness may die at home due to distance to health facilities and other barriers to care. HIV testing results were not available for the most IPD cases during this pre-PCV period and estimates of HIV prevalence in pediatric population are not available. The lack of data on HIV status make it impossible to calculate HIV-specific IPD incidence rates, and severely limits our understanding of driving forces behind the pre-PCV decline in IPD. Therefore these incidence estimates represent a minimum burden of disease. Nonetheless we report a remarkably high burden of invasive pneumococcal illness and death in this rural African setting with a high prevalence of HIV and limited access for antiretroviral treatment among children HIV-infected. Our findings suggest that recently introduced PCV10 will have a major impact on hospitalizations, death and health care costs in Mozambique. Efforts to measure that impact must take into account the complex nature of IPD and pre-vaccine trends in disease.
Supporting information
(ZIP)
Acknowledgments
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This project was supported with Bacterial Disease Surveillance funds from; the Program for Appropriate Technology in Health (PATH) through to the pneumonia and Pneumococcus surveillance study (GAT.770-790-01350-LPS), Bill and Melinda Gates Foundation through Center for Vaccine Development, University of Maryland, School of Medicine (Grant: S00957) and from the U.S. Agency for International Development (Fixed Obligation Grant: AID-656-f-12-00001, TFA-656-12-000003) through to the Surveillance for Paediatric Pneumonia and Invasive Pneumococcal Disease in Mozambique. The study was also supported by CISM core funding provided by the Spanish Agency for International Cooperation (Ministry of Foreign Affairs, Spain). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Jorge Uqueio for coordinating clinical aspects of the study, and our colleagues from the Manhiça District Hospital and from CISM for collecting and processing samples and completing questionnaires. We also acknowledge the study participants and their parents, along with the district health authorities for their collaboration in the research activities ongoing in the Manhiça district hospital.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was supported with Bacterial Disease Surveillance funds from; the Program for Appropriate Technology in Health (PATH) through to the pneumonia and Pneumococcus surveillance study (GAT.770-790-01350-LPS), Bill and Melinda Gates Foundation through Center for Vaccine Development, University of Maryland, School of Medicine (Grant: S00957) and from the U.S. Agency for International Development (Fixed Obligation Grant: AID-656-f-12-00001, TFA-656-12-000003) through to the Surveillance for Paediatric Pneumonia and Invasive Pneumococcal Disease in Mozambique. The study was also supported by CISM core funding provided by the Spanish Agency for International Cooperation (Ministry of Foreign Affairs, Spain). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008 2013 [cited 2015 25 June]. Available from: http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/.
- 3.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5. doi: 10.1097/01.inf.0000027926.99356.4c [DOI] [PubMed] [Google Scholar]
- 4.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–46. doi: 10.1016/S0140-6736(05)71876-6 [DOI] [PubMed] [Google Scholar]
- 5.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–8. doi: 10.1056/NEJMoa035060 [DOI] [PubMed] [Google Scholar]
- 6.O'Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, Brown L, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362(9381):355–61. doi: 10.1016/S0140-6736(03)14022-6 [DOI] [PubMed] [Google Scholar]
- 7.Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2007;82(12):93–104. [PubMed] [Google Scholar]
- 8.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Progress in introduction of pneumococcal conjugate vaccine—worldwide, 2000–2012. Morbidity and mortality weekly report. 2013;62(16):308–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Sigaúque B, Roca A, Mandomando I, Morais L, Quinto´ L, Sacarlal J, et al. Community-Acquired Bacteremia Among Children Admitted to a Rural Hospital in Mozambique. The Pediatric Infectious Disease Journal. 2009;28(2):108–13 doi: 10.1097/INF.0b013e318187a87d [DOI] [PubMed] [Google Scholar]
- 11.Mandomando I, Sigauque B, Morais L, Espasa M, Valles X, Sacarlal J, et al. Antimicrobial drug resistance trends of bacteremia isolates in a rural hospital in southern Mozambique. The American journal of tropical medicine and hygiene. 2010;83(1):152–7. doi: 10.4269/ajtmh.2010.09-0578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roca A, Sigauque B, Quinto L, Mandomando I, Valles X, Espasa M, et al. Invasive pneumococcal disease in children<5 years of age in rural Mozambique. Trop Med Int Health. 2006;11(9):1422–31. doi: 10.1111/j.1365-3156.2006.01697.x [DOI] [PubMed] [Google Scholar]
- 13.Sacoor C, Nhacolo A, Nhalungo D, Aponte JJ, Bassat Q, Augusto O, et al. Profile: Manhica Health Research Centre (Manhica HDSS). Int J Epidemiol. 2013;42(5):1309–18. doi: 10.1093/ije/dyt148 [DOI] [PubMed] [Google Scholar]
- 14.Institute Nacional de Estatistica (INE) M. Indicadores Socio-Demograficos do Censo Populacional 2007. http://www.ine.gov.mz/estatisticas/estatisticas-demograficas-e-indicadores-sociais (accessed 20 October 2015) 2009.
- 15.Saute F, Aponte J, Almeda J, Ascaso C, Abellana R, Vaz N, et al. Malaria in southern Mozambique: malariometric indicators and malaria case definition in Manhica district. Trans R Soc Trop Med Hyg. 2003;97(6):661–6. [DOI] [PubMed] [Google Scholar]
- 16.Menendez C, Bardaji A, Sigauque B, Romagosa C, Sanz S, Serra-Casas E, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One. 2008;3(4):e1934 doi: 10.1371/journal.pone.0001934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez R, Munguambe K, Aponte J, Bavo C, Nhalungo D, Macete E, et al. High HIV prevalence in a southern semi-rural area of Mozambique: a community-based survey. HIV medicine. 2012;13(10):581–8. doi: 10.1111/j.1468-1293.2012.01018.x [DOI] [PubMed] [Google Scholar]
- 18.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33(10):2759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. Journal of clinical microbiology. 2006;44(1):124–31. doi: 10.1128/JCM.44.1.124-131.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement M100-S20. Wayne, PA, USA: CLSI; 2010. 2011. [Google Scholar]
- 21.Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène J-P, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. The Pediatric infectious disease journal. 2009;28(4 Suppl):S66–S76. doi: 10.1097/INF.0b013e318199f8ef [DOI] [PubMed] [Google Scholar]
- 22.WHO. WHO child growth standards and the identification of severe acute malnutrition in infants and children 2011. [PubMed]
- 23.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275 [DOI] [PubMed] [Google Scholar]
- 24.Usen S, Adegbola R, Mulholland K, Jaffar S, Hilton S, Oparaugo A, et al. Epidemiology of invasive pneumococcal disease in the Western Region, The Gambia. Pediatr Infect Dis J. 1998;17(1):23–8. [DOI] [PubMed] [Google Scholar]
- 25.Campbell JD, Kotloff KL, Sow SO, Tapia M, Keita MM, Diallo S, et al. Invasive pneumococcal infections among hospitalized children in Bamako, Mali. The Pediatric infectious disease journal. 2004;23(7):642–9. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez R, Munguambe K, Aponte J, Bavo C, Nhalungo D, Macete E, et al. High HIV prevalence in a southern semi-rural area of Mozambique: a community-based survey. HIV Med.13(10):581–8. doi: 10.1111/j.1468-1293.2012.01018.x [DOI] [PubMed] [Google Scholar]
- 27.von Gottberg A, Cohen C, de Gouveia L, Meiring S, Quan V, Whitelaw A, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003–2008. Vaccine. 2013;31(38):4200–8. doi: 10.1016/j.vaccine.2013.04.077 [DOI] [PubMed] [Google Scholar]
- 28.Sacarlal J, Nhacolo AQ, Sigauque B, Nhalungo DA, Abacassamo F, Sacoor CN, et al. A 10 year study of the cause of death in children under 15 years in Manhica, Mozambique. BMC Public Health. 2009;9:67 doi: 10.1186/1471-2458-9-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemri wellcome trust. The Pneumococcal Conjugate Vaccine Impact Study (PCVIS) [21 September 2015]. Available from: http://www.kemri-wellcome.org/index.php/en/studies_inner/75.
- 30.Domingues CM, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. The Lancet Respiratory medicine. 2014;2(6):464–71. doi: 10.1016/S2213-2600(14)70060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371(20):1889–99. doi: 10.1056/NEJMoa1401914 [DOI] [PubMed] [Google Scholar]
- 32.Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–502. doi: 10.1016/S0140-6736(06)69637-2 [DOI] [PubMed] [Google Scholar]
- 33.CDC CfDCaP. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148 2008 2008 Feb 15. Report No. [PubMed] [Google Scholar]
- 34.Cohen C, von Mollendorf C, de Gouveia L, Naidoo N, Meiring S, Quan V, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in HIV-infected and -uninfected children in south africa: a matched case-control study. Clin Infect Dis. 2014;59(6):808–18. doi: 10.1093/cid/ciu431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesikari T WJ, Chevallier B, Karvonen A, Czajka H, Arsène J-P, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. The Pediatric infectious disease journal. 2009;28 (4 Suppl):S66–S76. doi: 10.1097/INF.0b013e318199f8ef [DOI] [PubMed] [Google Scholar]
- 36.Prymula R SL. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines. 2009;8 (11):1479–500. doi: 10.1586/erv.09.113 [DOI] [PubMed] [Google Scholar]
- 37.Hammitt LL, Akech DO, Morpeth SC, Karani A, Kihuha N, Nyongesa S, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health. 2014;2(7):e397–405. doi: 10.1016/S2214-109X(14)70224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Moore DP, Klugman KP, et al. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS. 2011;25(4):453–62. doi: 10.1097/QAD.0b013e328341b7f1 [DOI] [PubMed] [Google Scholar]
- 39.Everett D, Mukaka M, Denis B, Gordon S, Carrol E, van Oosterhout J, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PloS one. 2011;6(3):e17765–e. doi: 10.1371/journal.pone.0017765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everett D MM, Denis B, Gordon S, Carrol E, van Oosterhout J, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PloS one. 2011;6 (3):e17765–e. doi: 10.1371/journal.pone.0017765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song JH; Jung SI KK, et al. High Prevalence of Antimicrobial Resistance among Clinical Streptococcus pneumoniae Isolates in Asia (an ANSORP Study). Antimicrob. Agents Chemother. 2004; 48 (6): 2101–2107. Antimicrob Agents Chemother. 2004;48 (6)(6):2101–7. doi: 10.1128/AAC.48.6.2101-2107.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borg MA, Tiemersma E, Scicluna E, Sande-Bruinsma Nvd, Kraker M, Monen J, et al. Prevalence of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae isolates reported by laboratories in the southern and eastern Mediterranean region. Clin Microbiol Infect. 2009;15(3):232–7. doi: 10.1111/j.1469-0691.2008.02651.x [DOI] [PubMed] [Google Scholar]
- 43.Crowther-Gibson P, Cohen C, Klugman KP, de Gouveia L, von Gottberg A, Group for Enteric R, et al. Risk factors for multidrug-resistant invasive pneumococcal disease in South Africa, a setting with high HIV prevalence, in the prevaccine era from 2003 to 2008. Antimicrob Agents Chemother. 2012;56(10):5088–95. doi: 10.1128/AAC.06463-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.