Abstract
Objective
The purpose of the study is to investigate potential of antioxidant property of ethanolic root extract of Asparagus racemosus Linn (EEAR).
Methods
In vitro evaluation antioxidant property of EEAR was done using various methods like DPPH scavenging activity, hydroxyl radical scavenging activity, and nitric oxide scavenging activity. HPTLC fingerprint analysis was performed for qualitative determination of possible number of components from the ethanolic extract. Acute toxicity study was performed in Wistar rat and an OECD guideline 423 was followed.
Results
The yield value was found 0.96% from EEAR. A concentration of 468.57 ± 3.002 μg/ml of probable antioxidant material from EEAR was required to scavenge 50% of DPPH. The IC50 value of EEAR were found to be 508.17 ± 7.37 μg and 416.57 ± 5.08 μg when determined by hydroxyl radical and nitric oxide scavenging assay respectively. The reducing powers of EEAR was 0.295 ± 0.0037 at 125 μg/ml and increased to 0.934 ± 0.0005 at 500 μg/ml. HPTLC fingerprint data supports several basic informations like isolation, purification, quality evaluation and standardization. No sign of toxicity was observed after treated with 2000 mg/kg of EEAR.
Conclusion
The obtained data highlight the potential role of EEAR as a source of natural antioxidants.
Keywords: Asparagus racemosus, Antioxidant activity, HPTLC, Acute toxicity, Free radical
Abbreviations: AHRF, Asthagiri Herbal Research Foundation; DPPH, 2,2-diphenyl-1-picrylhydrazyl; HPTLC, high performance thin layer chromatography; OECD, The Organisation for Economic Co-operation and Development
Graphical abstract
1. Introduction
Free radicals can defined as some free entities having one or more unpaired electrons which play a vital role in the development of various human diseases including aging.1 They can generate as a by-product from various endogenous (like normal cellular metabolism) or may be exogenous (like irradiation) processes. Free radicals can easily react with reactive oxygen species (ROS) and make themselves active radical. ROS are different activated forms of oxygen and of two distinct types, free activated oxygen radicals (like superoxide anion radical , hydroxyl radical OH−) and non-free activated oxygen radicals (like hydrogen peroxide H2O2, singlet oxygen 1O2). Human cells are exposed 10,000 oxidative hits every second by these activated ROS.2
Free radicals are well known for degrading food products, resulting in off taste and reduced shelf-life. Antioxidants are able to manage the degradation of food products by deactivating active free radicals thus act as preservative.3 To minimize the deterioration, dietary products contain a pervasive amount of flavonoids, a class of polyphenols having strong antioxidant activity.4
Like Trolox Equivalent Antioxidant Capacity (TEAC) assay, several methods are established to identify most potent antioxidants by comparing antioxidant capacity of enormous compounds.
In chemistry, oxidation is a chemical reaction in which an electron is transfer to an oxidizing agent from any substance. An antioxidant (counter part of oxidant) is chemical substance which reduces the rate of a particular oxidation reaction in a definite extent, hence preventing oxidative damage to the cells and biochemicals. They can prevent uncontrolled production of ROS, protein damage and DNA stand disruption and ensuring good health.5, 6 Development of diseases is highly co-related with oxidative damage by free radicals. In this regard, association of LDL oxidation in cardiovascular disease can be taken as example. This LDL oxidation by free radical acts as a precursor in various life threatening diseases like atherosclerosis, cardiovascular disease etc by triggering various chemical or enzymatic pathways.
According to scientists, antioxidant rich foods or dietary supplements can able to reduce the cell damage by free radicals. A regular diet of antioxidants from plants is very essential to maintain proper health as plants are rich source of organic antioxidant chemicals.7 Certain diseases like aging can be slow down or prevent with an adequate diet of antioxidant rich food.
Keeping the advantage and importance of antioxidant in daily life, many nutraceutical companies have launched various forms of antioxidant (like carotene, vitamin C, vitamin E, selenium, resveratrol etc) as dietary supplement.
The principle aim of this research work is to find out antioxidant potential of EEAR i.e. ethanolic root extract of Asparagus racemosus Linn.
1.1. Plant introduction8
A. racemosus is growing at low altitude in shade and available throughout Asia, Africa, and Australia. In India, A. racemosus is used as traditional medicine by tribal people in various parts of the country. It is belongs to Asparagaceae family. Basically A. racemosus is a woody climber of average 1–2 m in height. Small, uniform and pine needles leaves are the unique characteristics of this plant with whitish coloured flowers. Shatawari, satamuli, vrishya are few of the names in Indian languages which is used to describe this miracle plant. The term “shatawari” means “curer of a hundred diseases”.
1.2. Traditional uses of A. racemosus Linn.
A. racemosus having anti-ulcer,9 antitussive activity,10 antineoplastic activity,11 antistress effects,12 immunomodulatory activities,9 hypoglycemic, hypolipidemic,13 antidiarrhoeal, antiseptic, bronchitis and hyperacidity,14 immunoadjuvant and antilithiatic affect,9 wound healing property.15
1.3. Phytochemical evaluation study
From literature survey it was proved that A. racemosus Linn. possesses shatvarin I to VI (Steroidal saponins),16 asparanin A,17 racemosol,18 racemofuran,19 flavonoids (kaempferol, quercetin, and rutin),20 sitosterol,21 diosgenin and quercetin 3-glucourbnides etc.22, 23
2. Materials and methods
2.1. Collection of plant
The plant materials were collected from Kulasekharam of Kanyakumari district and authenticated by Prof. P. Jeyaraman Chief Botanist PARC, Chennai, India.
2.2. Preparation of plant extracts24
Air-dried roots (1.5 kg) were extracted (hot maceration) with absolute ethanol (3 L) at a temperature not exceeding 55 °C for 4 days and filtered. This filtrate was concentrated by rotary evaporator. This concentrated material was used for further study.
2.3. Source of chemicals
All the chemicals were of analytical reagent grade and purchased from Loba chemie, ACROS Organics, Merck lab, S.D. Fine chemicals, Fluke.
2.4. In vitro antioxidant evaluation
2.4.1. DPPH method
Free radical scavenging assay of ethanolic extract of A. racemosus (EEAR) was measured by DPPH method.25
| DPPH percentage inhibition (%) = Abs (control) − Abs (sample)/Abs (control) × 100 |
2.4.2. Hydroxyl radical scavenging assay
The free radical scavenging capacity of EEAR was measured by hydroxyl radical scavenging method.26
| Hydroxyl scavenging activity (%) = Abs (control) − Abs (sample)/Abs (control) × 100. |
2.4.3. Nitric oxide scavenging assay
Nitric oxide scavenging method was used to determine the antioxidant activity of EEAR.27
| Nitric oxide scavenging assay (%) = Abs (control) − Abs (sample)/Abs (control) × 100. |
2.4.4. Total reducing ability
The reducing power of the root extract was determined by different concentrations of the ethanolic extract, phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1%), trichloroacetic acid (10%), ferric chloride (1%).28
2.5. HPTLC analysis
HPTLC of EEAR samples were identified by AHRF method. The sample applicator used for the procedure was CAMAG Linomat IV. The applied sample was scanned by CAMAG TLC Scanner II.
Volume of sample loaded: 10 μl
Mobile phase: Chloroform:Acetic acid:MethanolWater (5:3.5:1.5:1)
λmax: 254 nm
Lamp: Deuterium
2.6. Determination of toxicity
2.6.1. Experimental animals
Adult male Wistar rats weighing 150–180 g were used for the study. The animals were fed a standard laboratory diet and tap water ad libitum. The care and use of the animals were strictly in accordance to the guidance of the Institutional Ethical Committee (constituted under the guidelines Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Reg. No. – 1237/PO/C/2008/CPCSEA). After one week of habituation, animals were subjected to the experiments. Each animal was tested only once. All efforts were made to minimize animal suffering.
2.6.2. Acute oral toxicity study (OECD423)29, 30, 31
For carrying out oral toxicity study OECD guidelines 423 was followed. It is a stepwise procedure with three animals per step. Depending on the mortality and/or morbidity of the animals a few steps may be necessary to judge the toxicity of the test substance. This procedure has advantage over other methods because of minimal usage of animals while allowing for acceptable data. The method uses defined doses (2000 mg/kg body weight) and the results allow a substance to be ranked and classified according to the globally harmonized system. The concentrated extract was dissolved in distilled water for oral administration. The starting dose of the plants extract was 2000 mg/kg bodyweight (p.o). The dose was administered to the mice which were fasted overnight with water ad libitum and observed for signs of toxicity symptoms like change in skin colour, salivation, diarrhoea, sleep, tremors, convulsions and also respiratory, autonomic and central nervous system effects.
2.7. Statistical analysis
Results were shown as mean ± S.D. for each group (where, number of each in-vitro antioxidant experiment, n = 3; number of experimental animal, n = 6). SPSS 9.0 for Windows (Chicago, IL, USA) software was used for statistical analysis. For multiple comparisons, one-way analysis of variance (ANOVA) was performed. In cases where ANOVA showed significant differences, Tukey test was performed. P < 0.01 was considered to be statistically significant.
3. Results
3.1. Percentage yield study
The percentage yield of the EEAR was found to be 0.96%
3.2. DPPH radical scavenging assay
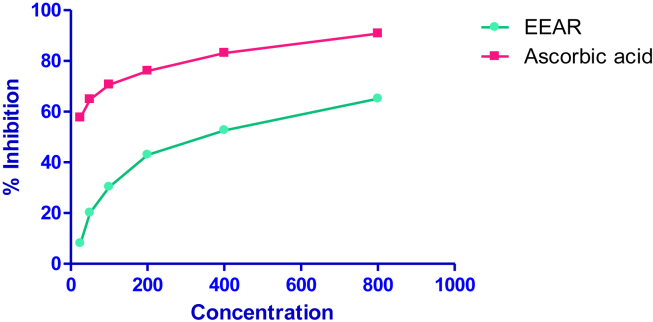
Fig. 1 shows the antiradical activity of the root extract of EEAR. The scavenging activity was increased with the increasing concentrations (25–800 μg). IC50 value (the amount of antioxidant material required to scavenge 50% of free radical in the assay system) of EEAR was found to be 468.57 ± 3.002 μg/ml. On the other hand ascorbic acid possesses IC50 value 45.27 ± 0.28 μg/ml.
Fig. 1.
Free radical scavenging activity of EEAR by DPPH method.
3.3. Hydroxyl radical scavenging assay
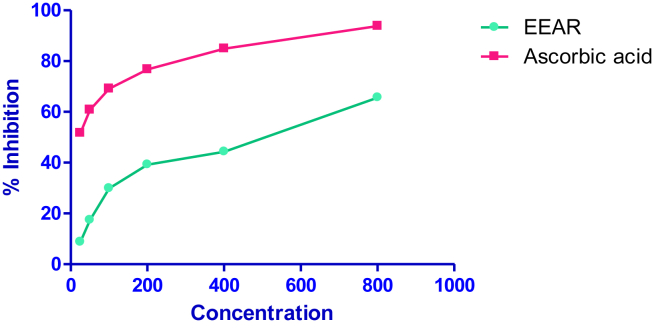
Fig. 2 shows the antioxidant capacity of EEAR by scavenging the hydroxyl radical. The antioxidant activity was increased from 8.76 ± 0.59% at 25 μg/ml to 65.63 ± 0.61 at 800 μg/ml concentration. The IC50 value of EEAR was found to be 508.17 ± 7.37 μg/ml. By the same time ascorbic acid having 12.3 ± 0.73 μg/ml.
Fig. 2.
Free radical scavenging activity of EEAR by hydroxyl radical scavenging method.
3.4. Nitric oxide scavenging assay
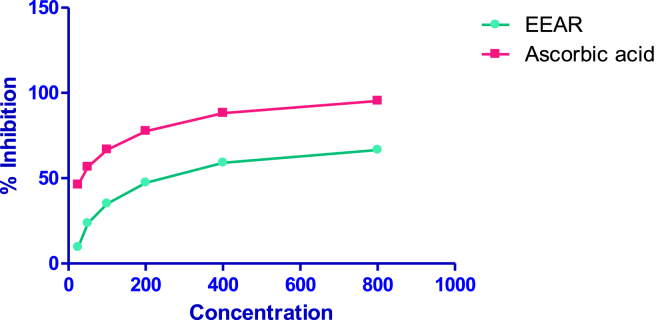
Fig. 3 shows the appreciable antioxidant activity by scavenging the nitric oxide. The value of the antioxidant assay increased from 9.54 ± 0.41% at 25 μg/ml to 66.57 ± 0.43% at 800 μg/ml. The IC50 value was found to be 416.57 ± 5.08 μg/ml. In this experiment, ascorbic acid showed IC50 value of 10.82 ± 1.02 μg/ml.
Fig. 3.
Nitric oxide scavenging activity of EEAR.
3.5. Total reducing ability
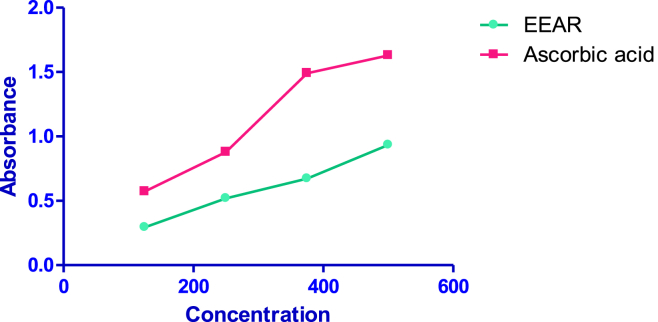
The result shows that the EEAR possesses antioxidant properties which could react with free radicals to stabilize and terminate radical chain reactions. The reducing powers of EEAR were 0.295 ± 0.0037 at 125 μg/ml and increased to 0.934 ± 0.0005 at 500 μg/ml (Fig. 4). The standard drug ascorbic acid shows the reducing power 1.63 at 500 μg/ml.
Fig. 4.
Determination of reducing power of EEAR.
3.6. HPTLC analysis
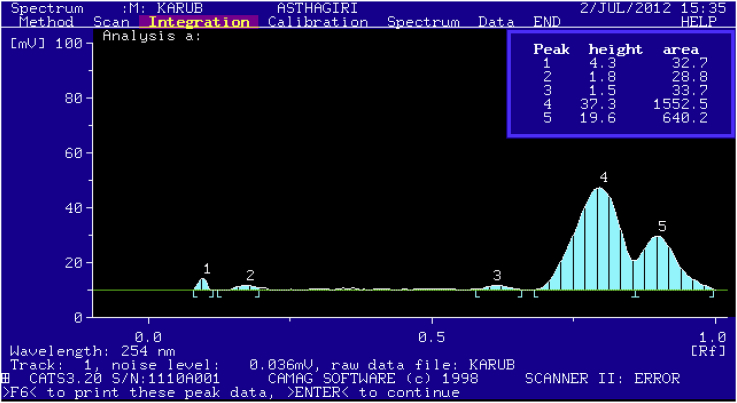
The chromatographic profiles of the EEAR were performed on silica gel 60F254. The plate using Chloroform:Acetic acid:Methanol:Water (5:3.5:1.5:1) as mobile phase was given in Fig. 5. Thin layer chromatography profiles revealed 2 distinct spots under UV (254 nm). This was again confirmed in HPTLC chromatogram (Fig. 6) where The Rf values and the peak area percentage were observed and given in Table 1.
Fig. 5.
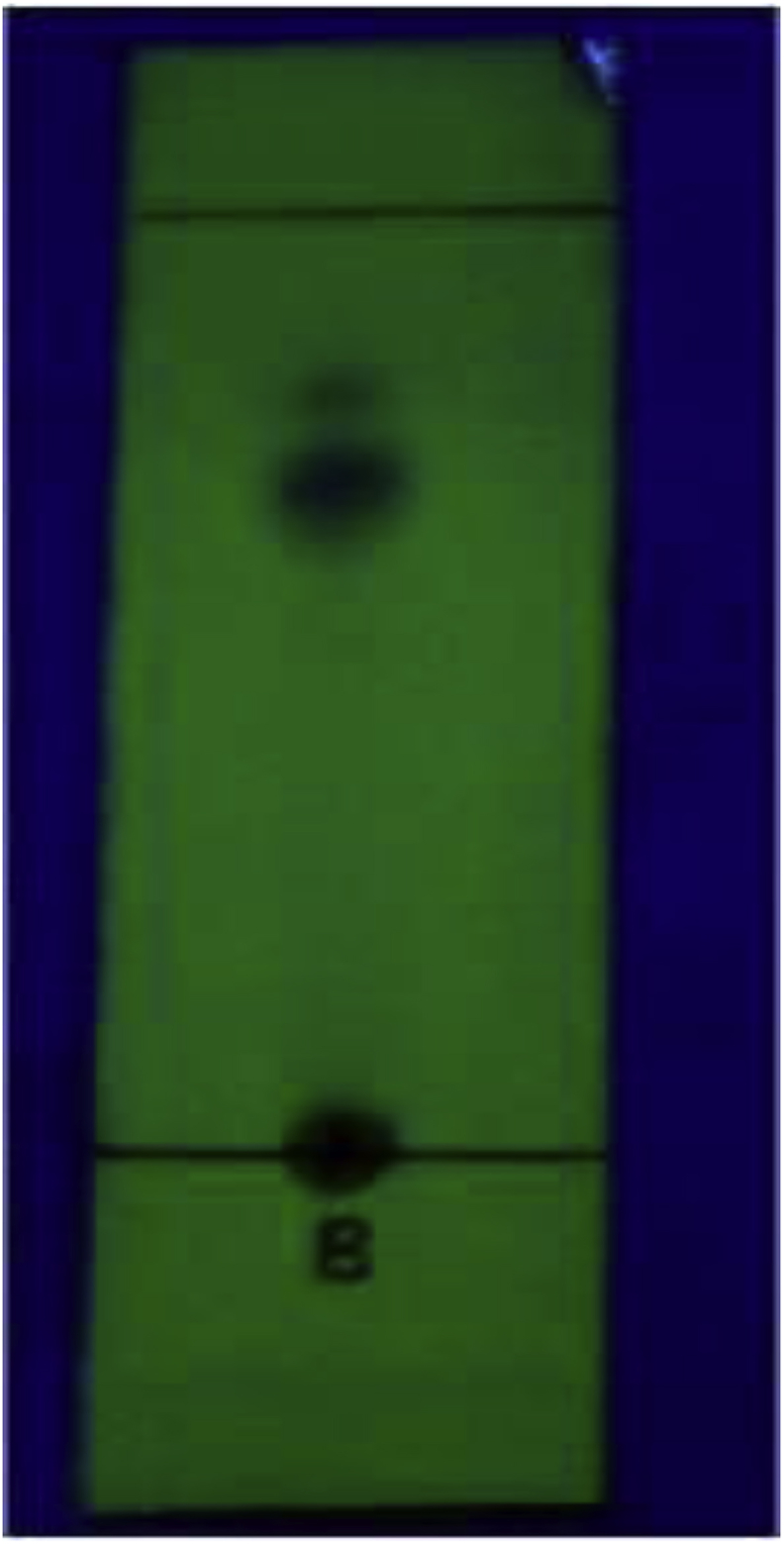
TLC chromatogram of EEAR.
Fig. 6.
HPTLC chromatogram of EEAR.
Table 1.
HPTLC fingerprint of ethanolic extract of EEAR.
| Peak | Rf | λmax | Height | Area in mV |
|---|---|---|---|---|
| 4 | 0.7 | 254 | 37.3 | 1552.5 |
| 5 | 0.9 | 254 | 19.6 | 640.2 |
4. Discussion
Three different radical scavenging assay has been performed to find antioxidant potential of EEAR.
DPPH becomes diamagnetic molecule after gets reduced into its hydrazine form by electron donation by the antioxidants. λmax of DPPH, a stable free radical is at 517 nm. Decrease in absorption at 517 nm was the characteristic marker of DPPH reduction. Here the result was determined as a ratio between percentage of absorbance decrease of DPPH radical in presence of extract to the absorbance of DPPH radical alone at 517 nm. Based on this research work it may be presumed that DPPH gets reduced to its corresponding hydrazine form by EEAR. This reduction leads to colour change of DPPH from purple to yellow depending on number of electron taken up.32, 33
In biochemical system, superoxide radical and H2O2 react together and form different forms of ROS viz. hydroxyl radical and singlet oxygen. They are responsible for various life threading diseases like carcinogenesis, cytotoxicity and aging by altering or damaging DNA. From the experimental data it may be speculated that EEAR can prevent the damaging properties of ROS at cellular level as it may able to quench hydroxyl radical and scavenge ROS. So, hydroxyl radical scavenging activity of this extract is directly conferring its antioxidant potential.
Nitric oxide is a lipophilic molecule. At physiological pH it reacts with oxygen and produce nitrite ion. Nitric oxide is very essential for controlling of vasodilation; signal transmission, inflammatory response etc.34 Scavengers of nitric oxide compete with oxygen and inhibit the production of nitric oxide.35 This research shows that EEAR may be act as antioxidant by inhibiting the production of nitric oxide.
Antioxidant activity of polyphenols can be examined by reducing power. The reducing power is governed by presence of reductones, which exhibit antioxidant property by donating hydrogen atom and breaking free radical chain reaction. Higher the electron transfer ability, more reducing power activity and more antioxidant activity.
In the present study, ferric ion is gets reduced into ferrous ion by antioxidants present in the ethanolic extract which leads to a colour change, depending upon their reducing power capacity.36
Proper identification and quality control of specific plant species can be done by HPTLC fingerprint analysis. This chromatographic data can provide various basic informations like chemical compounds for identification, isolation and purification of a particular plant species.
In HPTLC first 3 peaks was observed due to solvent. Presence of active constituents in EEAR was confirmed by peak 4 and 5. Although further study is required to identify those compounds. This HPTLC fingerprint analysis profile of EEAR may be used for quality evaluation and standardization.
For acute toxicity studies mortality was not observed in the groups treated with EEAR at the dose of 2000 mg/kg (Table 2, Table 3).
Table 2.
Acute toxicity dose 2000 mg/kg (P.O). Initial observation (Day-I) (n = 3).
| Parameters observed | I h | II h | III h | IV h |
|---|---|---|---|---|
| Piloerection | − | − | − | − |
| Oedema | − | − | − | − |
| Urine stains | − | − | − | − |
| Alopecia | − | − | − | − |
| Loss of writing reflex | − | − | − | − |
| Circling | − | − | − | − |
| Nasal sniffing | − | + | + | + |
| Lacrimation | − | − | − | − |
| Seizures | − | − | + | + |
| Righting reflex | + | − | + | + |
| Grip strength | + | − | − | − |
| Eye closure at touch | + | + | + | + |
| Rearing | + | − | − | − |
| Straub tail | − | − | − | − |
+: Presence.
−: Absence.
Table 3.
Acute toxicity-daily observation (n = 3).
| Parameters observed | Day-1 | Day-2 | Day-3 | Day-4 |
|---|---|---|---|---|
| Piloerection | − | − | − | − |
| Oedema | − | − | − | − |
| Urine stains | − | − | − | − |
| Alopecia | − | − | − | − |
| Loss of writing reflex | − | − | − | − |
| Circling | − | − | − | − |
| Nasal sniffing | − | + | + | + |
| Lacrimation | − | − | − | − |
| Seizures | − | − | + | + |
| Righting reflex | + | − | + | + |
| Grip strength | + | − | − | − |
| Eye closure at touch | + | + | + | + |
| Rearing | + | − | − | − |
| Straub tail | − | − | − | − |
+: Presence.
−: Absence.
5. Conclusion
EEAR showed relevant antioxidant property. This study provides experimental support for the traditional medicinal plants.
To ensure therapeutic efficacy and quality control of the drug along with its identification, this HPTLC data will serve as reference standard for scientists engaged. So along with antioxidant property of this plant, HPTLC fingerprint data of root extract of EEAR can be used as diagnostic tool for the correct identification of the plant and also useful to estimate genetic variability in their population.
Based on the toxicity study, EEAR was found to be non-toxic at a dose of 2000 mg/kg.
Still molecular studies of the above mentioned plant may open up new hope in drug discovery and research.
Conflict of interest statement
The authors declare no conflicts of interest.
Acknowledgment
Authors are grateful to Asthagiri Herbal Research Foundation and C. L. Baid Metha College of Pharmacy for providing us the laboratory facilities. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Sanchez M.C., Larrauri J.A., Saura F.C. Free radical scavenging capacity of selected red, rose and white wines. J Sci Food Agric. 1999;79:1301–1304. [Google Scholar]
- 2.Onkar P., Bangar J., Karodi R. Evaluation of antioxidant activity of traditional formulation Giloysatva and hydroalcoholic extract of the Curculigo orchioides gaertn. J Appl Pharm Sci. 2012;02:209–213. [Google Scholar]
- 3.Concepcion N.M., Pilar M.M., Martin A. Free radical scavenger and anti-hepatotoxic activity of Rosmarinus tomentosus. Planta Med. 1993;59:312–314. doi: 10.1055/s-2006-959688. [DOI] [PubMed] [Google Scholar]
- 4.Robak J., Gryglewski R.J. Flavonoids are scavengers of superoxide anions. Biochem Pharmcol. 1998;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 5.Govindarajan R., Vijaykumar M., Rawat A.K., Mehrotra S. Free radical scavenging potential of Picrorhiza kurrooa Royle ex Benth. Indian J Exp Biol. 2003;41:875–879. [PubMed] [Google Scholar]
- 6.Jayaprakasha G.K., Singh R.P., Sakariah K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in-vitro. Food Chem. 2001;73:285–290. [Google Scholar]
- 7.Babu B.H., Shylesh B.S., Padikkala J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia. 2001;72:272–277. doi: 10.1016/s0367-326x(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 8.Alok S., Jain S.K., Verma A., Kumar M., Mahor A., Sabharwal M. Plant profile, phytochemistry and pharmacology of Asparagus racemosus (Shatavari): a review. Asian Pac J Trop Dis. 2013;3:242–251. [Google Scholar]
- 9.Sairam K., Priyambada S., Aryya N.C., Goel R.K. Gastroduodenal ulcer protective activity of Asparagus racemosus: an experimental, biochemical and histological study. J Ethnopharmacol. 2003;86:1–10. doi: 10.1016/s0378-8741(02)00342-2. [DOI] [PubMed] [Google Scholar]
- 10.Mandal S.C., Kumar C.K.A., Mohana L.S. Antitussive effect of Asparagus racemosus root against sulfur dioxide-induced cough in mice. Fitoterapia. 2000;71:686–689. doi: 10.1016/s0367-326x(00)00151-9. [DOI] [PubMed] [Google Scholar]
- 11.Rao A.R. Inhibitory action of Asparagus racemosus on DMBA-induced mammary carcinogoenesis in rats. Int J Cancer. 1981;28:607–610. doi: 10.1002/ijc.2910280512. [DOI] [PubMed] [Google Scholar]
- 12.Kamat J.P., Boloor K.K., Devasagayam T.P.A., Venkatachalam S.R. Antioxidant properties of Asparagus racemosus against damage induced by γ-radiation in rat liver mitochondria. J Ethnopharmacol. 2000;71:425–435. doi: 10.1016/s0378-8741(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran V., Mandal D., Payyavala U. Hypoglycemic, antioxidant and hypolipidemic activity of Asparagus racemosus on streptozotocin-induced diabetic in rats. Adv Appl Sci Res. 2011;2:179–185. [Google Scholar]
- 14.Chawla A., Chawla P., Mangalesh, Roy R.C. Asparagus racemosus (Willd): biological activities & its active principles. Indo Glob J Pharm Sci. 2011;1:113–120. [Google Scholar]
- 15.Prabhath K.G., Satish K.M.C., Rajput R. Wound healing profile of Asparagus racemosus (Liliaceae) wild. CPR. 2011;1:111–114. [Google Scholar]
- 16.Gaitonde B.B., Jetmalani M.H. Antioxytocic action of saponin isolated from Asparagus racemosus Willd (Shatavari) on uterine muscle. Arch Int Pharmacodyn Ther. 1969;179:121–129. [PubMed] [Google Scholar]
- 17.Hayes P.Y., Jahidin A.H., Lehmann R., Penman K., Kitching W., De Voss J.J. Steroidal saponins from the roots of Asparagus racemosus. Phytochemistry. 2008;69:796–804. doi: 10.1016/j.phytochem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Sekine T., Fukasawa N., Murakoshi I., Ruangrungsi N.A. 9, 10-Dihydrophenanthrene from Asparagus racemosus. Phytochemistry. 1997;44:763–764. [Google Scholar]
- 19.Wiboonpun N., Phuwapraisirisan P., Tip-pyang S. Identification of antioxidant compound from Asparagus racemosus. Phytother Res. 2004;18:771–773. doi: 10.1002/ptr.1526. [DOI] [PubMed] [Google Scholar]
- 20.Bopana N., Saxena S. Asparagus racemosus—ethnopharmacological evaluation and conservation needs. J Ethnopharmacol. 2007;110:1–15. doi: 10.1016/j.jep.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Bose S., Show S., Hazra M., Sarkar T. Comparative study of Antioxidant activity of herbal drugs and their formulations using Asparagus racemosus and Centella asiatica. Am J PharmTech Res. 2012;2:391–398. [Google Scholar]
- 22.Subramanian S.S., Nair A.G.R. Occurrence of Diosgenin in Asparagus racemosus leaves. Curr Sci. 1969;38(17):414. [Google Scholar]
- 23.Tambvekar N.R. Ayurvedic drugs in common eye conditions. J Natl Integr Med Assoc. 1985;27:13–18. [Google Scholar]
- 24.Dey P., Mukherjee M., Bhakta T., Ghosh T.K. Preliminary phytochemical studies of leaf extracts of Molineria recurvata. J Chem Pharm Res. 2012;4:3727–3730. [Google Scholar]
- 25.Wong S.P., Leong L.P., Koh J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. [Google Scholar]
- 26.Ramakrishna H., Murthy S.S., Divya R., MamathaRani D.R., Panduranga G.M. Hydroxy radical and DPPH scavenging activity of crude protein extract of Leucas linifolia: a folk medicinal plant. Asian J Plant Sci Res. 2012;2:30–35. [Google Scholar]
- 27.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 28.Yildirim A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson M., Jestoi M., Nathanail A.V. Application of OECD guideline 423 in assessing the acute oral toxicity of moniliformin. Food Chem Toxicol. 2013;53:27–32. doi: 10.1016/j.fct.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Sankhari J.M., Jadeja R.N., Thounaojam M.C., Devkar R.V., Ramachandran A.V. Safety evaluation of Eugenia jambolana seed extract. Asian Pac J Trop Med. 2010;3:982–987. [Google Scholar]
- 31.Nana H.M., Ngane R.A., Kuiate J.R. Acute and sub-acute toxicity of the methanolic extract of Pteleopsis philodendron stem bark. J Ethnopharmacol. 2011;137(1):70–76. doi: 10.1016/j.jep.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 32.Shirwaikar A., Rajendran K., Kumar C.D. In vitro antioxidant studies of Annona squamosal Linn. Leaves. Indian J Exp Biol. 2004;42:803–807. [PubMed] [Google Scholar]
- 33.Prasad N.K., Divakar S., Shivamurthy G.R., Aradhya S.M. Isolation of a free radical-scavenging antioxidant from water spinach (Ipomoea aquatic Forsk) J Sci Food Agric. 2005;85:1461–1468. [Google Scholar]
- 34.Rees D.D., Palmer R.M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcocci L., Maguire J.J., Droy-Lefaix M.T., Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 36.Sravani T., Padmaa M.P. Antioxidant activity of Hedychium spicatum Buch.- Ham. Rhizomes. Indian J Nat Prod Resour. 2012;3:354–358. [Google Scholar]