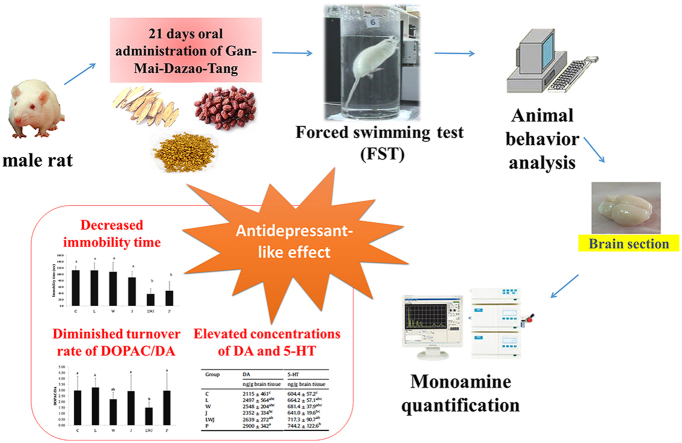
Abstract
Depression is a highly prevalent and recurrent mental disorder that impacts all aspects of human life. Undesirable effects of the antidepressant drugs led to the development of complementary and alternative therapies. Gan-Mai-Da-Zao-Tang (甘麥大棗湯, gān mài dà zǎo tang) is a traditional herbal formula commonly used for the treatment of depression, but lack of scientific proof on its mechanism. It consisted of Glycyrrhiza uralensis Fisch. (licorice), Triticum aestivum L. (wheat) and Zizphus jujuba Mill. (jujube). The objective of this study is to investigate the antidepressant effects of Gan-Mai-Dazao-Tang and its ingredients in rats exposed to forced swimming test (FST). The 72 of male Nerl: Wistar rats (8 weeks old) were randomized into control (10 mL/kg bw H2O), licorice (0.4 g/kg bw), wheat (1.6 g/kg bw), jujube (0.5 g/kg bw), Gan-Mai-Da-Zao-Tang (2.5 g/kg bw of licorice: wheat: jujube in ratio of 5:20:6) and Prozac (18 mg/kg bw) groups. Samples were administered by oral gavage for 21 days. FST was performed on 21st day, with 15 min for pretest followed by 5 min for real test. Then, the animals were sacrificed and brain tissues were collected for monoamines analyses. The Gan-Mai-Da-Zao-Tang (LWJ) showed significantly down-regulation of immobility time, 3,4-dihydroxyphenylacetic acid (DOPAC) and DOPAC/dopamine (DA) turnover rates, and also enhanced the concentration of serotonin (5-HT) and DA in brain tissues, as compared with the control. The LWJ stated the potent antidepressant-like effect by modulating these monoamines concentration, while the licorice, wheat and jujube did not reported significant results as compared with control group. The positive control (Prozac) was noted with significantly reduction in body weight and appetite. In conclusion, the antidepressant-like effects of LWJ might be mediated by the regulation of monoamine neurotransmitters. Thus, it could beneficial in depression treatment as a complementary approach.
Keywords: Gan-Mai-Da-Zao-Tang, Antidepressant, Forced swim test, Monoamines, Monoamine metabolite
Graphical abstract
1. Introduction
Major depressive disorder (MDD) is characterized by pervasive low mood, marked loss of interest, fatigue, sleep difficulties, irritability, poor concentration, suicidal ideation, feeling worthless, excessive guilt and loss of appetite for at least one month which causes clinically significant distress or functional impairment.1 The World Health Organization (WHO) reported that depression is the fourth highest contributor to the global burden of disease and is predicted to be in the first place by 2030.2 MDD affects not only people's performance at work, school and in daily life, but also their life satisfaction and perceived well-being.
Preclinical and clinical lines of evidence suggest that disturbed monoaminergic neurotransmission is one of most important mechanisms underlying the pathophysiology of depression.3 The monoamine hypothesis has demonstrated that serotonin (5-hydroxytryptamine or 5-HT), noradrenaline (NE) and dopamine (DA) are the important neurotransmitters involved in the etiology of depression.4 Indeed, most of the current antidepressants act on one or more mechanisms compatible with the monoamine hypothesis, such as inhibition of the reuptake of 5-HT and/or NE. A large amount of evidence from various studies has indicated that the levels of monoamine neurotransmitters (5-HT, NE and DA) in the brain increased compared with that of controls after antidepressant treatments.5
Today several kinds of antidepressant drugs have been widely available in the pharmaceutical market, including tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), noradrenergic and specific serotonergic antidepressants (NaSSAs).6 However, complaints such us nausea, sexual dysfunction, headache, insomnia, daytime somnolence, agitation and weight loss have often been reported by patients during the course of treatment, which can lead to treatment termination.7 The efficacy of the pharmacotherapy above-mentioned antidepressant drugs does not exceed 40–50% and is unsatisfactory.8 Furthermore, psychological treatments for depression are also commonly used, especially in middle to high-income countries. Despite its proven effectiveness, the limitations of psychotherapy include limited access to skilled providers, high cost, time-intensive nature and requirement of patients' participation and motivation. Faced with the limitations of the currently available treatments, the use of complementary and alternative medicine for depression is common. A national representative survey in the United States showed that 53.6% of people with self-reported depression used some forms of complementary and alternative therapies to treat depression.9 Many studies have been conducted to test the effectiveness of complementary and alternative therapies for mood disorders,10 suggesting that the demand for treatments other than pharmacotherapy and psychotherapy is present.
Traditional herbal medicine is one of the potential complementary and alternative therapies for depression and is widely used in Chinese culture. The Bupleurum Liver-Coursing Powder (柴胡疏肝散, chái hú shū gān sǎn), Free Wanderer Powder (逍遙散, xiáo yáo sǎn), and Gan-Mai-Da-Zao-Tang (甘麥大棗湯, gān mài dà zǎo tāng) are the commonly used and recommended herbal formulas for depression.9 Gan-Mai-Da-Zao-Tang (LWJ) contained Glycyrrhiza uralensis Fisch. (licorice), Triticum aestivum L. (wheat) and Zizphus jujube Mill. (jujube) as its major ingredients. It was first documented in Essential Prescriptions of the Golden Coffer (金匱要略, jīn guì yào lüè) for treating visceral irritation, in which female patients presented with low mood, a tendency to cry, possession-like behavior and frequent yawning.11 Additionally, previous study had been reported that after consumption of LWJ, the ICR mice significantly reduced the immobility times, which investigated by forced swim test and tail suspension test.12 Furthermore, rats with endogenous depression showed improvement in their body weight, hedonic effect, ability to expose and spontaneously motion, by continuously consumption of 2.5 g/kg of LWJ.13 Both of these previous studies did not mentioned its mechanism in suppressing depression. Therefore, given the common use of LWJ for depression, it is worthwhile to conduct an experiment to investigate its potential role on depression. The aim of this study was to determine the antidepressant-like effects of LWJ and its ingredients via forced swimming test (FST) model, and to explore its possible molecular mechanisms. This study hypothesized that FST depressive-like behavior of animals is caused by the dysregulation of monoamine neurotransmitters and treatment with LWJ might ameliorate these behavioral effects.
2. Material and methods
2.1. Materials
G. uralensis Fisch. (licorice), T. aestivum L. (wheat) and Z. jujube Mill. (jujube) were purchased from Fu-Der Herbal Material Company (Taichung, Taiwan). All the herbs were identified by a herbal specialist and confirmed their appearance and characters in accordance with Taiwan Chinese Pharmacopoeia. The LWJ was prepared by mixing the licorice (L), wheat (W) and jujube (J) in the ratio of 5:20:6.13 The LWJ and its ingredients were extracted in distilled water (ratio 1:10). After boiling at 100 °C for 3 h, the extraction was collected and freeze-dried to powder form and then stored at −20 °C until use. The total yields for L, W, J, and LWJ were 22.80, 5.50, 21.90, and 8.61%, respectively.
2.2. Chemicals
Prozac (P) was provided by Eli Lilly Company, Taiwan. Ascorbic acid (Ultra, purity 99%), while pargyline and isoproterenol were purchased from Sigma (St. Louis, MO, USA). Ethylenediaminetetraacetic acid (EDTA) was obtained from Riedel-de Haen (Seelze, Germany), while the 1-octanesulfonic acid, sodium salt (SOS) was provided by J.T. Backer Inc. (Phillipsburg, NJ, USA). Sodium dihydrogen phosphate (NaH2PO4) was obtained from Merck KGaA (Darmstadt, Germany).
2.3. Experimental animals
The 72 male Nerl; Wistar rats (6 week old, 197.60 ± 8.40 g) were purchased from National Taiwan University Hospital (Taipei, Taiwan). The rats were housed individually in the Animal House Facility, Institute of Food Science and Technology, National Taiwan University at 22 ± 2 °C, 50–60% humidity, and under a 12 h/12 h light/dark cycle. Food (rodent chow diet, PMI Feed, St. Louis, MO, USA) and distilled water were provided ad libitum; consumption was recorded and the rats were weighed every week. All experiments were performed according to the guidelines of the Taiwan Animal Protection Law and were approved by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University.
2.4. Animal treatments
After 2 weeks of acclimation to laboratory conditions, animals were randomly assigned into 6 groups (12 rats per group): control (C), L, W, J, LWJ, and positive control (P) group. The 10 mL/kg bw of deionized water, 0.4 g/kg bw of licorice extract, 1.6 g/kg bw of wheat extract, 0.5 g/kg bw of jujube extract, 2.5 g/kg be of LWJ, and 18 mg/kg bw of Prozac,14 were administrated by oral gavage in each group, respectively, for 21 days.
2.5. Forced swimming test
Rats were subjected to the forced swim test on the 21st day after sample administration. A cylinder glass (20 cm in diameter) was prepared and filled up with water until 30 cm depth, in order to avoid the hind paws of rat touch the bottom part of cylinder. Animals were exposed to a pretest for 15 min. After the pretest, the rats were removed from cylinder, then was dried and placed into their original cage. After 24 h, they were received a real test session for 5 min in the same environment. The experiment was recorded by video to investigate their behaviors. The time that the rat remained immobile was quantified during a test period of 5 min. The total immobility time was defined as the total amount of time when the mice remained immobile or made only small limb movements necessary for floating.15 Immobility was regarded as depression-like behavior (behavioral despair).
2.6. Quantification of monoamines
The rats were sacrificed immediately by decapitation after the FST. The frontal cortex, striatum, hippocampus, and amydala were dissected on icy cold plate, which followed Glowinski method.16 The concentrations of serotonin (5-hydroxytryptophan, 5-HT), 5-hydroxyindoleacetic acid (5-HIAA), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were analyzed by high performance liquid chromatography (HPLC, BSA, West Lafayette, IN, USA), which coupled with electrochemical detector (ECD, BSA, West Lafayette, IN, USA).
The brain tissues were stored at −80 °C until use. The tissue was homogenized in 5 mL of extract solution, which according to the protocol of Cheng et al17 with slight modification. Due to the reason that the amygdala tissues of rats were too small, they were gathered by group and homogenized together. While the other tissues (frontal cortex, striatum and hippocampus) were homogenized individually. The extract solution was 0.1 N HCl, which contained 0.1 μM ascorbic acid, 1.5 mg/100 mL pargyline, and 50 pg/μl isoproterenol. Homogenates were centrifuged at 10000 ×g for 20 min at 4 °C. After filtration (0.22 μm), the supernatant was injected onto the HPLC column (BAS MF-6213, 3 μm, 3.2 × 100 mm). The mobile phase was made up of 20.5 g of NaH2PO4, 185 mg of EDTA, 130 mg of sodium 1-octanesulfonate (SOS), 75 mL of methanol, and 1 mL/L of triethylamine (TEA). The pH was adjusted to pH 3.0 by 85% phosphoric acid. The flow rate was 20 μL/min. The electrochemical detector (Range 10 nA, Filter 0.1 Hz, AppE cell 0.75 V) was coupled to the HPLC with autosampler (CMA 200 Refrigerated Microsampler, Stockholm, Sweden).
2.7. Statistical analysis
All results were expressed as mean ± standard deviation. The data of all groups were analyzed by a one-way analysis of variance (ANOVA) followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences. The p < 0.05 was considered as statistically significant.
3. Results
3.1. Effects of LWJ on body weight
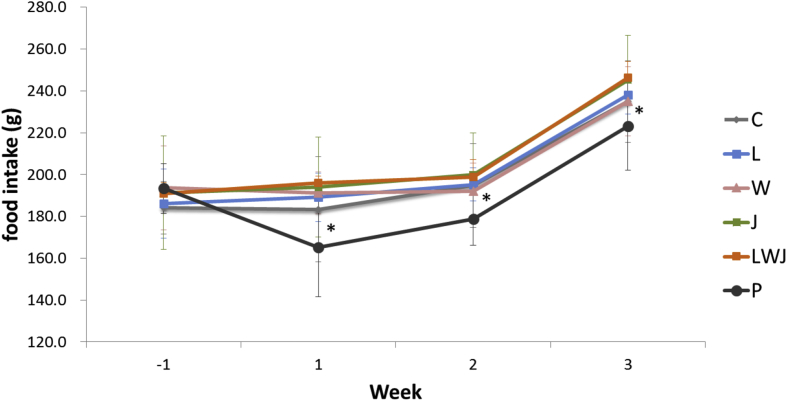
The body weight was measured weekly from 2 weeks before sample administration until the end of the experiment. As shown in Table 1, there were no significant differences in body weights between the groups on the weeks before treatment (-1 and -2 weeks). The body weight of rats exposed to drug (P) was significantly lower than that in the control group (p < 0.05), from 2nd week of treatment until end of the experiment. While the other treatments (L, W, J and LWJ) were not significantly affected on their body weights.
Table 1.
Body weight measurement of rats in experimental period.
| Group | Body weight (g) |
|||||
|---|---|---|---|---|---|---|
| -2 wk | -1 wk | 1 wk | 2 wk | 3 wk | 4 wk | |
| C | 202.7 ± 7.0 | 233.0 ± 7.7 | 283.4 ± 3.8 | 321.8 ± 7.4a | 358.1 ± 9.8a | 383.4 ± 13.7ab |
| L | 198.9 ± 8.5 | 235.2 ± 8.9 | 284.1 ± 9.8 | 323.5 ± 12.0a | 357.9 ± 12.2a | 386.9 ± 9.5a |
| W | 198.9 ± 7.0 | 234.2 ± 6.8 | 289.0 ± 5.8 | 328.4 ± 4.1a | 362.1 ± 8.9a | 387.4 ± 11.5a |
| J | 199.8 ± 6.4 | 235.6 ± 5.4 | 289.0 ± 13.4 | 331.6 ± 19.3a | 367.3 ± 19.7a | 395.7 ± 26.6a |
| LWJ | 200.2 ± 6.3 | 239.5 ± 8.5 | 291.1 ± 8.9 | 329.6 ± 10.2a | 360.4 ± 15.6a | 382.3 ± 16.3a |
| P | 199.9 ± 6.4 | 237.6 ± 5.3 | 288.7 ± 10.8 | 299.4 ± 11.8b | 335.6 ± 13.6b | 356.1 ± 15.7b |
Each data was represented as mean ± SD (n = 6). a, b are significantly different (p < 0.05). -2 and -1 week means the week before treatment started. Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
Next, the Fig. 1 was recorded the influence of appetite before and after the treatments. The positive control (P) significantly decreased the appetite of rats at the end of experiment, when compared with the control. Other treatments did not indicate significantly influenced on rat's appetite.
Fig. 1.
Effect of Gan-Mai-Da-Zao-Tang and its ingredients on animal's appetite. Data were represented as mean ± SD (n = 6). -1 week means that the week before treatment started. Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
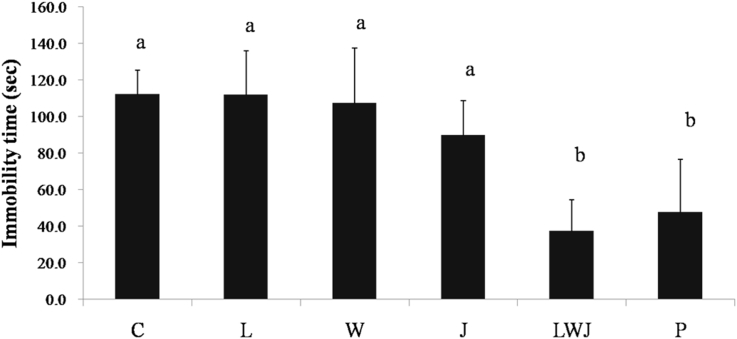
3.2. Effects of LWJ on animal behaviors
The immobility times of C, L, W, J, LWJ, and P group were noted as 112.0, 111.7, 107.2, 89.7, 37.2, and 47.5 s, respectively. As shown in Fig. 2, both of the LWJ and P groups were significantly reduced the duration of immobility in FST, as compared with the control.
Fig. 2.
Effect of Gan-Mai-Da-Zao-Tang and its ingredients on immobility time via forced swimming test. Data were expressed as mean ± SD (n = 6). a, b are significantly different (p < 0.05). Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
3.3. Effects of LWJ on concentrations of monoamines
The concentrations of 5-HT in frontal cortex were significantly enhanced in both LWJ and P group, when compared to control (Table 2). However, the concentration of 5-HT and 5-HIAA in hippocampus did not showed significant different between treatment and control group (Table 3).
Table 2.
Effect of Gan-Mai-Da-Zao-Tang and its ingredients on the concentrations of 5-HT and 5-HIAA in frontal cortex.
| Group | 5-HT |
5-HIAA |
|---|---|---|
| ng/g brain tissue | ||
| C | 604.4 ± 57.2c | 225.4 ± 89.3a |
| L | 664.2 ± 57.1abc | 156.2 ± 72.9a |
| W | 681.4 ± 37.9abc | 107.0 ± 86.3a |
| J | 641.0 ± 19.6bc | 156.0 ± 134.6a |
| LWJ | 717.3 ± 90.7ab | 111.4 ± 37.3a |
| P | 744.2 ± 122.6b | 121.0 ± 110.5a |
Each data were expressed as mean ± SD (n = 6). a, b are significantly different (p < 0.05). Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
Table 3.
Effect of Gan-Mai-Da-Zao-Tang and its ingredients on the concentrations of 5-HT and 5-HIAA in hippocampus.
| Group | 5-HT |
5-HIAA |
|---|---|---|
| ng/g brain tissue | ||
| C | 250.6 ± 60.4a | 467.3 ± 87.5a |
| L | 230.2 ± 96.0a | 464.0 ± 54.1a |
| W | 238.6 ± 74.3a | 420.0 ± 40.7a |
| J | 250.1 ± 73.2a | 460.1 ± 60.8a |
| LWJ | 237.3 ± 129.2a | 438.4 ± 33.8a |
| P | 281.7 ± 38.0a | 442.2 ± 45.7a |
Each data were expressed as mean ± SD (n = 6). a, b are significantly different (p < 0.05). Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
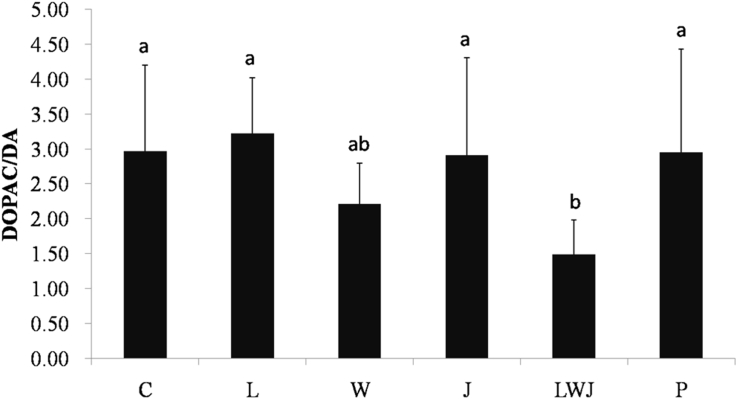
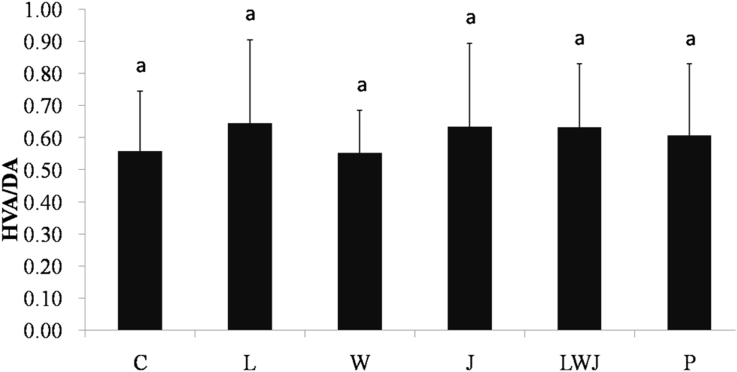
Additionally, the concentrations of DA, DOPAC and HVA in striatum were showed in Table 4. The DA was significantly improved in both LWJ and P group, while the DOPAC and DOPAC/DA ratio (Fig. 3) were only significantly decreased in LWJ group, as compared with the control. Next, the HVA/DA ratio did not showed significantly difference between groups (Fig. 4).
Table 4.
Effect of Gan-Mai-Da-Zao-Tang and its ingredients on the concentrations of DA, DOPAC and HVA in straitum.
| Group | DA |
DOPAC |
HVA |
|---|---|---|---|
| ng/g brain tissue | |||
| C | 2115 ± 461c | 5792 ± 1645a | 1368 ± 181a |
| L | 2497 ± 564abc | 5861 ± 1198a | 1541 ± 282a |
| W | 2548 ± 204abc | 4939 ± 1360ab | 1527 ± 309a |
| J | 2352 ± 334bc | 4632 ± 1091ab | 1705 ± 243a |
| LWJ | 2639 ± 272ab | 3916 ± 932b | 1619 ± 312a |
| P | 2900 ± 342a | 4917 ± 1083ab | 1763 ± 474a |
Each data were expressed as mean ± SD (n = 6). a, b are significantly different (p < 0.05). Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
Fig. 3.
Effect of Gan-Mai-Da-Zao-Tang and its ingredients on DOPAC/DA ratio in striatum. Data were represented as mean ± SD (n = 6). a, b are significantly different (p < 0.05). Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
Fig. 4.
Effect of Gan-Mai-Da-Zao-Tang and its ingredients on HVA/DA ratio in striatum. Data were represented as mean ± SD (n = 6). a, b are significantly different (p < 0.05). Statistical analyses were performed using one-way ANOVA, followed by Duncan's new multiple range test (MRT) in order to detect inter-group differences.
4. Discussion
Depression diagnosed by symptomatic criteria is a complex mood disorder, the heterogeneity of which suggests that multiple and different biological mechanisms may underlie its etiology. Despite the wide use of LWJ in Chinese medicine for treating mood disorders, there is a lack of scientific reports evaluating its pharmacological effects. In the present study, the effects of LWJ in the animal model of FST-induced depression were studied. Prozac was selected as positive control. It (a SSRI, also known as fluoxetine) is used as an antidepressant by presenting a high selectivity for the 5-HT transporter, thus modulating the concentration of serotonin in the synapses.18
The antidepressant effects of LWJ were suggested due to the synergistic effects of its individual components. Firstly, the licorice has long been valued as a demulcent, to relieve respiratory ailments, stomach burn including heart burn, gastritis, inflammatory disorders, skin diseases and liver problems.19 Secondly, the phenolic contents and antioxidant capacities in wheat have been studied to investigate its health benefits.20 In addition, the phenolic extract from the insoluble-bound fraction of whole wheat could inhibit the proliferation of human colon cancer cells in vitro.21 Thirdly, the jujube, which contains tannin, flavonoids, alkaloids and terpenoids, is widely used in Iranian folk medicine as antitussive, laxative and hypotensive agent.22 Local traditional healers used powders of stem bark and leaves of jujube to cure wounds and peptic ulcers. The pharmacological activities previously reported are antibacterial, anti-inflammatory, antimicrobial, anti-steroidogenic and antioxidant.23
On the other hand, there were no adverse events related to LWJ decoction was reported in this study. Previous study reported that the adverse events were more common in participants taking antidepressants than those taking LWJ decoction (Rate Ratio: 0.52, 95% CI: 0.32–0.82, P = 0.005, I2 = 37%).24 It was also found that LWJ decoction plus antidepressants was associated with less adverse events compared to antidepressants alone (Rate Ratio: 0.23, 95% CI: 0.08–0.68, P = 0.08, I2 = 0%).24
Besides, the importance of animal models has been proved during recent advances in experimental neuroscience, including modeling of human mood disorders such as depression. The assessment of depressive-like behaviors in FST is based on the observation of the immobility time when rodents are placed in an inescapable, water-containing cylinder. Mice would become immobile after an initial period of struggling, which is similar to human depression and is feasible to reversal by antidepressant drugs.2 The duration of immobility reflects state of hopelessness which is the cardinal symptom of depression in human.25 Both of the LWJ and P groups down-regulated the immobility duration in the FST after 21 days of treatment. However, this study noticed the negative effects of P treatment, which was characterized by growth retardation, decreased food and water intakes, tend to be more sensitive, irritate and repel harshly to the feeding tools (Data do not shown). These negative effects were consistent with the report of Oliveira.18 Administration of LWJ significantly ameliorated these abnormal behaviors and biochemical changes.
In addition, it is well known that the dysregulation of central nervous system is associated with the neurotransmitters noradrenaline, 5-HT and DA which plays an important role in the pathogenesis of depression. Low concentrations and high turnover rates of these neurotransmitters were found in the cerebrum of subjects with depression.26 Currently, the most efficacious treatment of major depression is considered to involve an increase in 5-HT and/or noradrenaline neurotransmission.27 In this study, FST decreased the levels of 5-HT in the frontal cortex, and also DA in the striatum. These effects were normalized by LWJ treatment. Thus, study suggested that the antidepressant-like effect of LWJ might be mediated by the serotonergic system and dopaminergic system.
The relatively low turnover rate of DA in the striatum of rats in the LWJ group compared with those in the control group is interesting. One possible explanation for lower turnover rate in the LWJ group might be the compensatory effect of monoamine homeostasis in the brain after the monoamine oxidase A (MAO-A) inhibition and upregulation of tyrosine hydroxylase (TH) levels,28 both could increase the amounts of DA in the cerebrum. Another possible reason might be that the elevated activity of catechol-O-methyl transferase increases DA metabolism after the inhibition of MAO-A.29 Since there were no negative effects were observed during the experiments, this study suggested that using traditional herbal medicine, LWJ might be a safe alternative to complementarily treat depression.
Furthermore, the precursors of neurotransmitters (tryptophan, tyrosine or phenylalanine), could up-regulate the concentration of serotonin and dopamine in the brain for nerves transmission.30 The licorice, wheat and jujube were found that they were rich in monoamine precursors. According to the previous study,31 licorice contained 225 mg of phenylalanine, 290 mg of tyrosine, 20 mg of tryptophan, and 36.3 g of carbohydrate. Wheat was recorded to have 670 mg of phenylalanine, 360 mg of tyrosine, 205 mg of tryptophan, and 76.8 g of carbohydrate. While the jujube stated about 225 mg of phenylalanine, 105 mg of tyrosine, 36 mg of tryptophan, and 61.6 g of carbohydrate. Therefore, in the current study, the enhancement of serotonin and dopamine by LWJ was suggested relevant to the high concentration of tyrosine and tryptophan. Furthermore, licorice, wheat and jujube were also reported to contain high amounts of carbohydrates. This may facilitate the tyrosine and tryptophan to pass through the blood brain barrier, and then enter into brain tissues. High contents of carbohydrates could stimulate the insulin secretion, then simultaneously raise plasma tryptophan, and reduce the concentrations of other macromolecular neutral amino acids (leucine, isoleucine, valine) in the blood. Thus, the up-regulation of the tyrosine and tryptophan would have higher competitive advantages than the other macromolecular neutral amino acids, to improve their concentrations in the blood. Hence, high percentages of wheat in the LWJ were hypothesized to provide higher concentrations of neurotransmitter precursors than the licorice and jujube, due to its higher carbohydrates contents, and then led to antidepressant-like effects. However, in this study, the wheat did not indicate any significant difference in the concentration of monoamines. Therefore, this study suggested that the antidepressant-like effects of LWJ may be a synergetic contribution by its ingredients.
Moreover, previous study was proved that the glycyrrhizin in licorice significantly decreased the decomposition of monoamine compounds by inhibiting serotonin and monoamine oxidase. The reduction of decomposition of monoamines caused the enhancement of serotonin and dopamine in mice brain tissues, then led to the antidepression.31 Additionally, the administration of 150 mg/kg bw of water extract of licorice could down-regulate the mice immobility times via FST and tail suspension test, which then showed potential antidepressant-like effects.32 However, the consumption of 0.4 g/kg bw of licorice for 21 days in this study did not improved the immobility times, and also concentrations of both serotonin and dopamine. It is most probably due to the different extraction method leads to different contents of active ingredients, which simultaneously affect their antidepressant-like effect directly.
The limitation of this study was that it was conducted in a short duration of only 21 days. Moreover, the FST is not a full spectrum analog of human depression. Even though there are exceptions, the FST has a considerable level of predictive validity, since it is reasonably sensitive to compounds that are effective in humans as anti-depressants and insensitive to those that are not effective.33 Since the behavioral outcome of the FST is one-dimensional it can only indicate the antidepressant efficacy of compound or experimental manipulations, but it cannot differentiate mechanistic differences between them. Also any manipulations that may affect the overall activity levels may potentially alter immobility in the FST leading to spurious conclusions. Therefore it is important to verify the results of FST with separate behavioral tests that measure overall activity such as the open-field test.34 It is beneficial to keep in mind that the FST does not represent the human condition, and to extent which underlying neurobiological mechanisms of the behaviors manifested by model animals in the FST and human depression overlap is not entirely clear.35 However, these types of limitations should not devalue the usefulness of FST as a drug discovery and validation tool. On the other hand, further experiments are necessary to clarify the exact molecular mechanisms underlying the behavioral effects of LWJ and the interplay between nitric oxide and serotonin. Further clinical analysis also should be done to assess the effect of LWJ on different types of depression.
5. Conclusion
In summary, the present study indicated that LWJ has antidepressant-like effects by the regulation of monoamines. It downregulated the DOPAC and DOPAC/DA ratio, and upregulated the 5-HT and DA concentartions, which led to the enhancement of monoamines in the brains. These findings revealed that Gan-Mai-Da-Zao-Tang has potential for depression treatment as complementary antidepressants or adjuvants.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.American Psychiatric Association . 5th ed. 2013. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC. [Google Scholar]
- 2.Liang Y., Yang X., Zhang X. Antidepressant-like effect of the saponins part of ethanol extract from SHF. J Ethnopharmacol. 2016;191:307–314. doi: 10.1016/j.jep.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Belmaker R., Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 4.Nutt D. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69(suppl E1):S4–S7. [PubMed] [Google Scholar]
- 5.Wang Y., Wang J., Hu X. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J Ethnopharmacol. 2016;179:9–15. doi: 10.1016/j.jep.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Sultana J., Italiano D., Spina E. Changes in the prescribing pattern of antidepressant drugs in elderly patients: an Italian, nationwide, population-based study. Eur J Clin Pharmacol. 2014;70(4):469–478. doi: 10.1007/s00228-013-1636-z. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . 2011. Mental Health Atlas. Geneva. [Google Scholar]
- 8.Lasoń W., Budziszewska B., Basta-Kaim A., Kubera M., Maes M. New trends in the neurobiology and pharmacology of affective disorders. Pharmacol Rep. 2013;65:1441–1450. doi: 10.1016/s1734-1140(13)71504-4. [DOI] [PubMed] [Google Scholar]
- 9.Yeung W., Chung K., Nga K., Yu Y., Ziea E., Ng B. A meta-analysis of the efficacy and safety of traditional Chinese medicine formula Ganmai Dazao decoction for depression. J Ethnopharmacol. 2014;153:309–317. doi: 10.1016/j.jep.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi N., AI-Bedah A. Mood disorders and complementary and alternative medicine: a literature review. J Neuropsychiatric Dis Treat. 2013;9:639–658. doi: 10.2147/NDT.S43419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Zhang Z. People's Medical Publishing House; Beijing: 2000. Jin-Gui-Yao-Lue. [Google Scholar]
- 12.Ma R., Yao H., Ku B., Qian R., Zang X., Guo J. Difference of the antidepressant effects between jieyu wan and its components in mouse models of depression. Chin J Clin Rehabil. 2005:16. [Google Scholar]
- 13.Ko T. Studies on the therapeutic effect of gan-mai-dazao-tang in experimental endogenous depression model. 1996.
- 14.Chen P., Hsieh C., Su K. The antidepressant effect of Gastrodia elata Bl. on the forced-swimming test in rats. Am J Chin Med. 2008;36(1):95–106. doi: 10.1142/S0192415X08005618. [DOI] [PubMed] [Google Scholar]
- 15.Lcg Moreno, Rolim H., Freitas R., Santos-Magalhães N. Antidepressant-like activity of liposomal formulation containing nimodipine treatment in the tail suspension test, forced swim test and MAOB activity in mice. Brain Res. 2016;1646:235–240. doi: 10.1016/j.brainres.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Glowinski J., Iversen L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheng F., Kuo J., Shih Y., Lai J., Ni D., Chia L. Simultaneous measurement of serotonin, catecholamines and their metabolites in mouse brain homogenates by high-performance liquid chromatography with a microbore column and dual electrochemical detection. J Chromatogr B Biomed Sci Appl. 1993;615(2):225–236. doi: 10.1016/0378-4347(93)80336-3. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira M. Fluoxetine and the mitochondria: a review of the toxicological aspects. Toxicol Lett. 2016;258:185–191. doi: 10.1016/j.toxlet.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C., Yu Y., Liang Y., Chen X. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int J Biol Macromol. 2015;79:681–686. doi: 10.1016/j.ijbiomac.2015.05.060. [DOI] [PubMed] [Google Scholar]
- 20.Okarter N., Liu C., Sorrells M., Liu R. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010;119:249–257. [Google Scholar]
- 21.Okarter N. Phenolic extracts from insoluble-bound fraction of whole wheat inhibit the proliferation of colon cancer cells. Life Sci Med Res. 2011;38:1–10. [Google Scholar]
- 22.Hamedi S., Arian A., Farzaei M. Gastroprotective effect of aqueous stem bark extract of Ziziphus jujuba L. against HCl/ethanol-induced gastric mucosal injury in rats. J Tradit Chin Med. 2015;35(6):666–670. doi: 10.1016/s0254-6272(15)30157-6. [DOI] [PubMed] [Google Scholar]
- 23.Asgarpanah J., Haghighat E. Phytochemistry and pharmacologic properties of Ziziphus spina christi (L.) Willd. Afr J Pharm Pharmacol. 2012;6(31):2332–2339. [Google Scholar]
- 24.Yeung W., Chung K., Ng K., Yu Y., Ziea E., Ng B. A meta-analysis of the efficacy and safety of traditional chinese medicine formula ganmai dazao decoction for depression. J Ethnopharmacol. 2014;153(2):309–317. doi: 10.1016/j.jep.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Castagné V., Moser, Roux S., Porsolt R. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011:11–18. doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- 26.Huang Z., Mao Q., Zhong X., Li X., Qiu F., Ip S. Mechanistic study on the antidepressant-like effect of danggui-shaoyao-san, a chinese herbal formula. Evid Based Complement Alternat Med. 2012:7. doi: 10.1155/2012/173565. 173565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye L., Hu Z., Du G. Antidepressant-like effects of the extract from Cimicifuga foetida L. J Ethnopharmacol. 2012;144(3):683–691. doi: 10.1016/j.jep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Kumar H., Kim I., More S., Kim B., Bahk Y., Choi D. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson's disease model. Evid Based Complement Alternat Med. 2013:13. doi: 10.1155/2013/514095. 173565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhutani M., Bishnoi M., Kulkarni S. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Meyers S. Use of neurotransmitter precursors for treatment of depression. Altern Med Rev. 2000;5(1):64–71. [PubMed] [Google Scholar]
- 31.Liu T., Yang W. Mechanism of gan-mai-dazao-tang on Mania in women via monoamine transmitters. J Basic Chin Med. 1995;1(2):55–56. [Google Scholar]
- 32.Dhingra D., Sharma A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006;30(3):449–454. doi: 10.1016/j.pnpbp.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Can A., Dao D., Arad M., Terrillion C., Piantadosi S., Gould T. The mouse forced swim test. J Vis Exp. 2012;59:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hascoét M., Bourin M. Mood and anxiety related phenotypes in mice. Neuromethods. 2009;42:85–118. [Google Scholar]
- 35.Bourin M., Fiocco A., Clenet F. How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol Clin Exp. 2001;16:9–21. doi: 10.1002/hup.178. [DOI] [PubMed] [Google Scholar]