Abstract
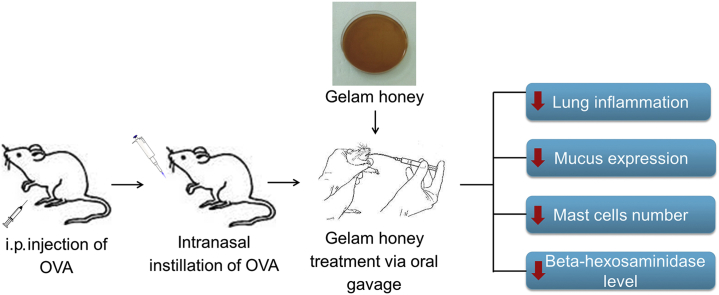
Allergic asthma is a chronic inflammatory disorder of the pulmonary airways. Gelam honey has been proven to possess anti-inflammatory property with great potential to treat an inflammatory condition. However, the effect of ingestion of Gelam honey on allergic asthma has never been studied. This study aimed to investigate the efficacy of Gelam honey on the histopathological changes in the lungs of a mice model of allergic asthma. Forty-two Balb/c mice were divided into seven groups: control, I, II, III, IV, V and VI group. All groups except the control were sensitized and challenged with ovalbumin. Mice in groups I, II, III, IV, and V were given honey at a dose of 10% (v/v), 40% (v/v) and 80% (v/v), dexamethasone 3 mg/kg, and phosphate buffered saline (vehicle) respectively, orally once a day for 5 days of the challenged period. Mice were sacrificed 24 h after the last OVA challenged and the lungs were evaluated for histopathological changes by light microscopy. All histopathological parameters such as epithelium thickness, the number of mast cell and mucus expression in Group III significantly improved when compared to Group VI except for subepithelial smooth muscle thickness (p < 0.05). In comparing Group III and IV, all the improvements in histopathological parameters were similar. Also, Gelam honey showed a significant (p < 0.05) reduction in inflammatory cell infiltration and beta-hexosaminidase level in bronchoalveolar lavage fluid. In conclusion, we demonstrated that administration of high concentration of Gelam honey alleviates the histopathological changes of mice model of allergic asthma.
Keywords: Allergic asthma, Anti-inflammatory, Honey, Ovalbumin, Mice
Abbreviations: BALF, Bronchoalveolar lavage fluid; DXN, Dexamethasone; i.p., Intraperitoneal injection; PBS, Phosphate buffered saline; PAS, Periodic acid Schiff; OVA, Ovalbumin
Graphical abstract
1. Introduction
Asthma is a chronic inflammatory disorder of the pulmonary airways characterized by bronchial hyper-responsiveness and airway obstruction caused by inflammation, mucus hypersecretion and airway wall remodeling.1 In susceptible individual, the inflammation causes the symptoms of wheezing, coughing, chest tightness and breathlessness.2 The respiratory airways of an asthmatic patient show abnormalities such as epithelial denudation, goblet cell metaplasia, subepithelial thickening, increased airway smooth muscle mass, bronchial gland enlargement, angiogenesis and alterations in extracellular matrix components involving large and small airways.3 The incidence of asthma diseases has increased dramatically in the last decade. Approximately 300 million people worldwide suffer from asthma and it can be fatal if left untreated. Currently, inhaled corticosteroids are the most effective available treatment for asthma and they are used as the first-line therapy for persistent asthma in adults and children in many countries. However, systemic absorption of inhaled corticosteroids may have deleterious effects over long term use.4 Thus, pursuit on effective and safe alternative medication for this disease management has become part of the major interest in scientific field nowadays.
Honey as a complementary medicine is increasingly being used as an adjunct and also as a substitute for effective and proven therapies in asthmatics among Malaysians.5 It has been shown that honey exhibits various biological properties including antibacterial, antioxidant, antifungal and as wound healing promoter. In addition to these biological effects, recent studies found honey to possess anti-inflammatory and immunomodulatory properties.6, 7, 8, 9 Since the main pathological feature of allergic asthma is caused by complex interactions between immunological mediators that are produced by the inflammatory process, this study aims to evaluate the efficacy of honey as an immunotherapeutic agent based on the pathogenesis of asthma. Many reports have highlighted the efficacy of immunomodulatory and anti-inflammatory agents to treat patients with asthma, thus it is likely to hypothesize that honey, which is cheap and readily available natural product may also be a potential therapeutic agent for asthma.10, 11, 12, 13 To our knowledge, no comprehensive studies thus far have addressed whether ingestion of honey is effective to treat allergic asthma. Therefore, this study is aimed to investigate the efficacy of Malaysian Gelam honey on the mediator and lung histopathology in a murine model of allergic asthma. The present approach may be useful to evaluate novel therapeutic modalities for asthma treatment and provide an evidence-based recommendation for the efficacy of honey as a complementary treatment for asthma.
2. Materials and methods
2.1. Honey sample
Local Apis mellifera honey from the floral source of Melaleuca spp. (Gelam) tree was used in this study. The honey was supplied by the Department of Agriculture Malaysia and irradiated with 25 kGy gamma irradiation using radioactive source Cobalt 60 for sterilization purposes at Bizworth Gammarad, Malaysian Nuclear Agency.
2.2. Reagents
Aluminum hydroxide was purchased from Fisher Scientific (Waltham, MA, USA). OVA (Grade V), Dexamethasone (DXN), Phosphate buffered saline (PBS), Toluidine Blue O and Periodic Acid Schiff stain were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.3. Animals
All animal studies were conducted in accordance with the criteria of the investigations and Universiti Teknologi MARA Committee of Animal Research and Ethics (UiTM CARE) guidelines concerning the use of experimental animals. A total of forty-two female Balb/c mice, 8–12 weeks old, were obtained from Laboratory Animal Facility and Management (LAFAM), Faculty of Pharmacy, UiTM Puncak Alam Campus. The animals underwent acclimatization period for one week and were given normal mouse diet along with filtered tap water ad libitum.
2.4. Experimental design
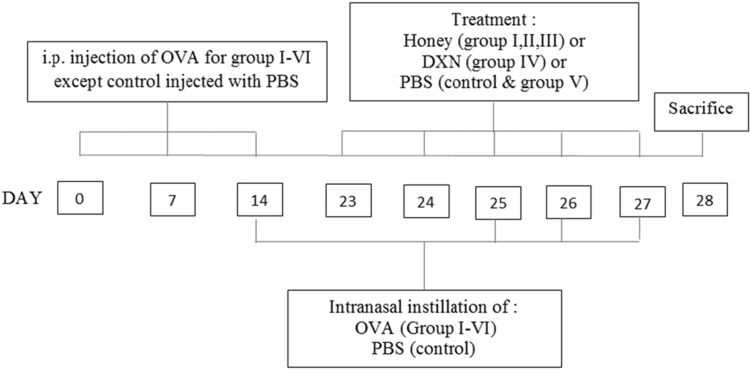
Mice were sensitized and challenged with OVA, as previously described with slight modification.14 Mice were randomly divided into seven groups (n = 6). The mice in study groups I, II, III, IV, V and VI were immunized on days 0, 7, and 14 by intraperitoneal (i.p.) injection of 50.0 μg chicken ovalbumin (OVA) with 1 mg aluminum hydroxide as an adjuvant in a total volume of 100 μl of PBS. The mice were then anesthetized and challenged by intranasal instillations of 100 μg OVA in 50 μl PBS on days 14, 25, 26 and 27. The mice in the control group were given PBS without OVA.
Treatment for groups I, II, III and IV started on days 23–27 where mice were fed via oral gavage (10 μl/g body weight) with 10%, 40%, 80% (v/v) honey and 3 mg/kg DXN diluted in PBS respectively, 1 h prior to OVA administration (Fig. 1). Groups V and control received PBS only while group VI received no treatment. Animals recovered quickly from the procedure with only mild discomfort. At the end of the experiment (day 28), the mice were sacrificed by dissecting the thoracic region under anesthesia using an intraperitoneal injection of pentobarbital (75 mg/kg).
Fig. 1.
Schematic diagram of the experimental protocol in mice. The mice were divided into seven groups (n = 6). All mice except control were immunized on days 0, 7 and 14 by intraperitoneal (i.p.) injection of 50.0 μg chicken ovalbumin (OVA) emulsified in 1.0 mg aluminum hydroxide adjuvant in a total volume of 100 μl of PBS. Mice in groups I, II, III, and IV were fed via oral gavage with 10%, 40% and 80% v/v honey (10 μl/g body weight) and dexamethasone (DXN) at 3 mg/kg respectively at days 23–27. Groups V and control received PBS only while group VI received no treatment. On days 14, 25, 26 and 27 mice were anesthetized and challenged by intranasal instillations of 100 μg OVA in 50 μl PBS. All mice were sacrificed on day 28.
2.5. Histopathological analysis of lung
2.5.1. Inflammatory cell infiltration
At 24 h after the last challenge, mice were sacrificed and the lungs were removed and fixed in 10% buffered formalin prior to embedding in paraffin. The lung tissues were sectioned into 5 μm thick and stained with hematoxylin and eosin (H&E) to assess inflammatory cell infiltration. Inflammation was evaluated using a semi-quantitative scoring system with a grading scale ranging from 0 to 3 as described previously by Jang et al.15 A value of 0 was assigned when no inflammation was detected; [1] was assigned when occasional cuffing with inflammatory cells was observed; [2] was assigned when most bronchiole or vessels were surrounded by a thin layer (one to five cells) of inflammatory cells; and a value of [3] was assigned when most bronchiole or vessel were surrounded by a thick layer (>5 cells) of inflammatory cells.
2.5.2. Quantitation of airway mucus expression
Mucus secretion was visualized using Periodic-acid Schiff staining. Paraffin embedded lung tissue sections were de-waxed and hydrated. Following rehydration, tissue sections were treated with Periodic-acid Solution (Aqueous solution, 1 g/l, Sigma–Aldrich) for 10 min. After washing repeatedly in water, slides were covered with Schiff's reagent solution (Sigma–Aldrich) for 15 min. Slides were then washed in water and immersed in Harris Hematoxylin, followed by a differentiation in acid alcohol (HCL concentration 4 ml; 95% EtOH—396 ml) followed by water again. The tissue sections were dehydrated through different concentrations of alcohol, and sections were mounted in mounting medium and the coverslips were applied. Mucus expression levels in the airway were quantified by counting PAS-positive and PAS-negative epithelial cells in each bronchiole. Results are expressed as the percentage of PAS-positive cells per bronchiole, which was calculated as the number of PAS-positive epithelial cells per bronchiole divided by the total number of epithelial cells in each bronchiole.15
2.5.3. Toluidine blue staining for mast cell
Number of mast cells was evaluated via toluidine blue staining in the connective tissue and smooth muscle layer underneath the epithelial of the bronchiole.16 Photomicrographs were taken by Leica MC170 HD camera which was adapted on Leica DM2500 model microscope. The histopathological analysis was carried out with Leica Application Suite version 4.3.0 software.
2.6. Total cell counting
Bronchoalveolar lavage fluid (BALF) was obtained by intratracheal instillation. The lungs were lavaged three times with 0.8 ml of sterile PBS. The BALF from each sample was centrifuged (4 °C, 420 g, 15 min), and supernatants were stored at −80 °C for beta hexosaminidase analysis. Cell pellets were resuspended in 200 μl sterile PBS for total cell counts using hemacytometer and 100 μl of cell suspension were applied to a slide by cytospinning followed by staining with Giemsa. At least 200 cells were counted per slide.14
2.7. Beta hexosaminidase assay
Beta hexosaminidase activity was determined as a measure of mast-cell degranulation. BALF (50 μl) was incubated with 50 μl 5 mM p-nitrophenyl N-acetyl-b-d-glucosaminide in 50 mM sodium citrate buffer (pH 4.5) at 37 °C for 2 h. Reactions were terminated with 200 μl 0.2 M glycine-NaOH, pH 10.6, and A405 nm determined spectrophotometrically, and values were reported as OD.17
2.8. Statistical analysis
GraphPad Prism version 6.00 for Windows was used for the statistical analysis. All values are expressed as means ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA followed by a multiple comparison Tukey test. p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of Gelam honey on OVA-induced histopathological changes in lung tissue
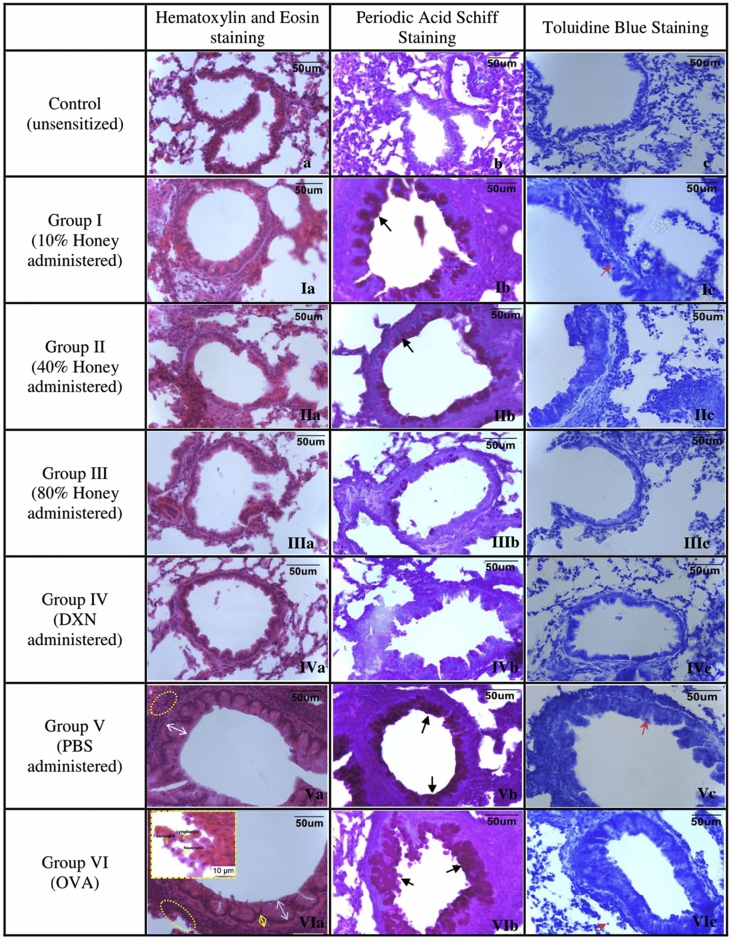
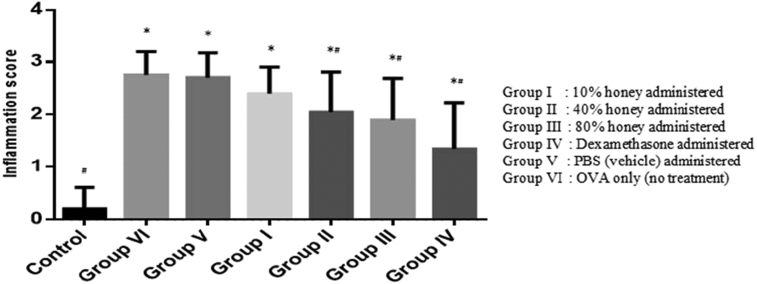
Histopathological analyses of mice lung tissues revealed that the OVA group developed inflammatory changes, including airway epithelium thickening and peribronchial infiltrates composed of numerous inflammatory cells such as eosinophil, neutrophils and lymphocytes (Fig. 2VIa). However, mice treated with honey shows marked reduction in the thickening of the airway epithelium and infiltration of inflammatory cells in the peribronchiolar region (Fig. 2IIa,IIIa,IVa). Dexamethasone also showed decreased in inflammatory cells infiltration and significantly reduced the airway epithelium thickness as compared to group VI (p < 0.05) (Fig. 2IVa). Fig. 3 shows that Gelam honey (40% and 80% v/v) and dexamethasone significantly decreased inflammatory cell infiltration in the lung as opposed to group VI (p < 0.05). A significant difference in the epithelium thickness and inflammatory cell infiltration between OVA and control groups demonstrating that airway inflammation has been successfully established.
Fig. 2.
Light microscopic findings of groups. Control; I, 10% honey; II, 40% honey; III, 80% honey; IV, DXN; V, PBS; VI, OVA group (magnification: ×400). In representative histological images, lung tissues were stained with H&E (a, 2nd column), PAS (b, 3rd column) and toluidine blue (c, 4th column). In the control group, light microscopic findings (a) revealed a regular respiratory epithelium and airways and normal PAS-stained (b) and toluidine blue staining (c) of lung tissues. In the asthma-induced group, H&E staining (VIa) revealed thickened epithelium (white arrow with two heads), thickened subepithelial smooth muscle (yellow arrow with two heads), and inflammatory cells infiltration (ellipsoid area). High numbers of mucin production (black arrow) were seen with PAS-staining (VIb), and mast cells (red arrow) with toluidine blue staining (VIc). In the honey treated groups, the pathological changes, cellular infiltration around airways, epithelial cell thickness and mucin production were less than in asthma-induced and PBS treated groups. Increase in honey concentration (10%–80%) reduced the mucin production.
Fig. 3.
Effect of Gelam honey in lung tissue of OVA-challenged mice. Quantitative analysis of inflammatory cell infiltration in lung sections was performed. Mean scores were obtained from 5 or 6 mice in each group. *p < 0.05 vs. control group; #p < 0.05 vs. OVA group.
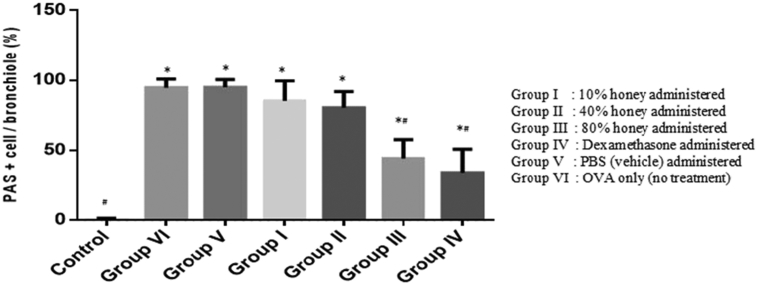
3.2. Quantitation of airway mucin expression
The percentage of airway epithelium that stained positively with periodic acid Schiff (PAS) in OVA treated group was significantly greater than in control mice (p < 0.05) (Fig. 2VIb and b). Additionally, the administration of 80% (v/v) of Gelam honey and dexamethasone significantly reduced the percentage of airway epithelium staining positively with PAS compared with untreated group (Fig. 2IIIb, IVb and VIb). However, no significant difference was seen in mucus expression of 80% (v/v) honey treated group and dexamethasone (Fig. 4). This showed that the ingestion of 80% (v/v) honey helps to abate the mucus production similar to dexamethasone, the gold standard treatment of asthma.
Fig. 4.
Effect of Gelam honey in lung tissue of OVA-challenged mice. Quantitative analysis of mucus production in lung sections was performed. Mean scores were obtained from 5 or 6 mice in each group. *p < 0.05 vs. control group; #p < 0.05 vs. OVA group.
3.3. Toluidine blue staining of mast cell
Table 1 shows that the number of mast cells per bronchiole in OVA group were significantly higher compared to control (p < 0.05). In contrast, administration of dexamethasone and 80% (v/v) honey treated groups significantly reduced the number of mast cells in the lungs of mice exposed to OVA (p < 0.05). Administration of lower dosage of Gelam honey (10% and 40% v/v) showed a minor reduction in the number of mast cells when compared to OVA group although not significantly different.
Table 1.
Comparison of epithelium and subepithelial smooth muscle thickness and mast cell number between groups (mean ± SD, n = 6).
| Control group | OVA + 10% honey (Group I) | OVA + 40% honey (Group II) | OVA + 80% honey (Group III) | OVA + DXN (Group IV) | OVA + PBS (Group V) | OVA (Group VI) | |
|---|---|---|---|---|---|---|---|
| Epithelium thickness (μm) | 15.155 ± 3.012a | 24.622 ± 5.269bc | 18.274 ± 4.023a | 16.709 ± 3.719a | 16.642 ± 2.695a | 28.474 ± 9.811bc | 27.562 ± 7.368bc |
| Subepithelial smooth muscle thickness (μm) | 2.851 ± 0.928 | 3.726 ± 1.189c | 3.164 ± 1.352 | 2.903 ± 0.750 | 3.142 ± 0.751 | 4.061 ± 1.913bc | 3.443 ± 1.665 |
| Mast cells/bronchiole | 0.550 ± 0.759a | 1.750 ± 1.209c | 1.450 ± 0.945 | 1.300 ± 0.923a | 1.150 ± 0.813a | 2.000 ± 1.257c | 2.250 ± 1.333bc |
p < 0.05 in groups vs. OVA.
p < 0.05 in groups vs. dexamethasone.
p < 0.05 in groups vs. control.
3.4. Total cell counting
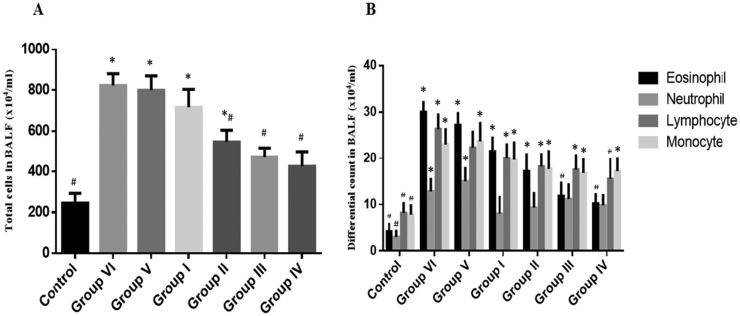
As shown in Fig. 5, exposure to OVA significantly increased the number of total inflammatory cells in BALF compared to the control group (p < 0.05). However, Gelam honey at 40% and 80% (v/v) significantly reduced the number of inflammatory cells in a dose-dependent manner (p < 0.05). DXN also significantly reduced the number of inflammatory cells (p < 0.05).
Fig. 5.
Effect of Gelam honey on inflammatory cell infiltration of OVA-challenged mice. The total cells and differential cells in BALF were counted as described in methods. 10% honey administered (Group I), 40% honey administered (Group II), 80% honey administered (Group III), Dexamethasone administered (Group IV), PBS (vehicle) administered (Group V) and OVA only (Group VI). The data represented as mean ± SD (n = 6). *p < 0.05 vs. control group; #p < 0.05 vs. OVA group.
3.5. Beta-hexosaminidase in bronchoalveolar lavage fluid (BALF)
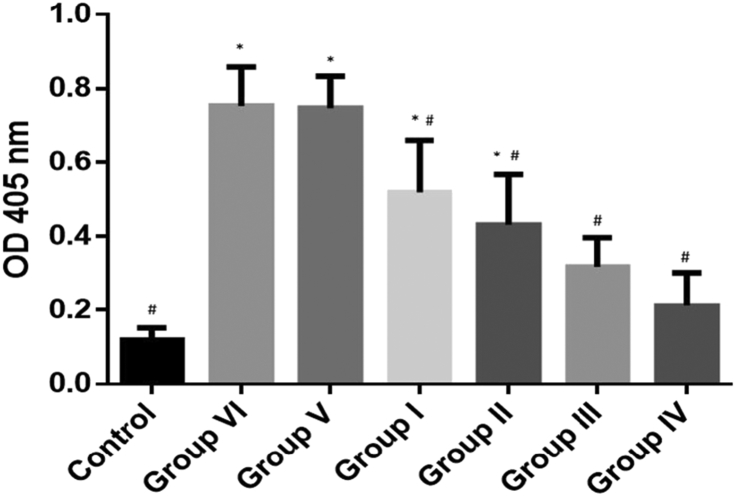
Fig. 6 shows that the level of beta hexosaminidase (an indicator of degranulation) in BALF of OVA group was significantly higher compared to control (p < 0.05). Having said that, the level of beta hexosaminidase in 10%, 40%, 80% honey treated and dexamethasone groups were attenuated (p < 0.05).
Fig. 6.
Beta hexosaminidase level in bronchoalveolar lavage fluid (BALF). 10% honey administered (Group I), 40% honey administered (Group II), 80% honey administered (Group III), Dexamethasone administered (Group IV), PBS (vehicle) administered (Group V) and OVA only (Group VI). The data represented as mean ± SD (n = 6). *p < 0.05 vs. control group; #p < 0.05 vs. OVA group. OD = optical density.
4. Discussion
In this study, we demonstrated that Gelam honey effectively inhibited airway inflammation in OVA-induced allergic asthma mice model. OVA-induced asthma has been recognized as a disease characterized by mucus overproduction, increased levels of inflammatory cells influx into the lungs, airway occlusion and thickness of the bronchial wall which are the hallmark of allergic asthma.14 The changes in inflammatory cell influx and histopathological of the lungs of OVA-induced mice show that airways inflammation have been generated successfully in accordance with the previous studies.14, 16, 18 Our study uses female Balb/c mice as it has been suggested by previous research that Balb/c female mice is an IgE-high responder to many allergens and develop a good T helper cell type 2 (Th2)-biased immunological response.19 Besides, they may be more susceptible to allergic airway inflammation than male mice due to the hormone progesterone present in female worsened the allergic airway inflammation while androgen in male has an opposite effect.20 Administrations of Gelam honey in our study have reduced the histopathological characteristics of airway inflammation. This is in line with the previous study done by Kamaruzaman and colleagues demonstrating that treatment in a rabbit model of OVA-induced asthma with aerosolized honey (25% and 50% v/v) significantly alleviated inflammatory cell influx, airway inflammation and mucus secreting goblet cell hyperplasia.13 They noted the reduction in the thickness of the airway epithelial and mucosal region of honey treated group.
Mucus overproductions are the prominent histopathological feature in allergic asthma and this can be observed in OVA-induced mice.21 In this study, treatment with 80% (v/v) Gelam honey markedly reduced the mucus expression in the lung of OVA-induced mice. This finding demonstrated the efficacy of Gelam honey in reducing the mucus overproduction on OVA-induced allergic asthma mice. In addition, a study done by Sulaiman et al (2011) showed that honey consumed by Malaysian pilgrims performing Hajj in Mecca helps to reduce the acute respiratory symptoms such as a sore throat and rhinitis.22 Numerous studies have established the potential of honey as an anti-inflammatory agent. Prakash and colleagues (2008) have investigated the effect of Manuka honey on inflammatory bowel disease and found that honey reduced the colonic inflammation in rats.23 Additionally, inflammation has always being related to reactive oxygen species (ROS) where overproduction of ROS can lead to the compromised cellular functioning and increased inflammation by damaging nucleic acids, lipids, proteins, and mitochondria.24 In the case of allergic asthma, inflammatory cells recruited to asthmatic airways are capable of generating reactive oxygen species (ROS) thus contributed to tissue injury and inflammatory reactions in the airways.25 Our study shows that the administration of 80% (v/v) Gelam honey helps to alleviate the inflammatory effect of OVA-induced asthma. The phenolic compounds found in Gelam honey such as ellagic acid, quercetin, chrysin and caffeic acid may contributed to the antioxidative and radical scavenging properties.26 Jung et al (2008) and Park et al (2009) reported the administration of caffeic acid phenethyl ester and quercetin in OVA-induced mice resulted in a marked improvement of luminal narrowing by reducing the mucus secretion in the airway and decreasing the infiltration of inflammatory cells within the peribronchiolar and perivascular regions.27, 28 Moreover, other compounds such as ellagic acid and chrysin also markedly inhibited lung eosinophilic inflammation and goblet cell hyperplasia in the airways of murine asthma model.29 Consistent with the results of histopathological analysis of the lung tissues, mice exposed to OVA exhibited an increasing number of inflammatory cells in BALF. Our findings showed that honey abated OVA-induced inflammatory cells infiltrating into the airways as shown by a significant drop in total cell, eosinophil, neutrophil, lymphocyte and monocyte counts in BALF (Fig. 5).
Mast cells have long been associated with allergic asthma as these cells are the only resident cells in the airway that can form an interaction with allergen by means of Immunoglobulin E (IgE) bound to the high affinity IgE receptor (FcɛRI).30 Mast cell degranulation was estimated by measuring the levels of mediators being released including beta hexosaminidase in BALF.17, 31 Fig. 6 shows increase of beta hexosaminidase level in OVA-induced group compared to control which indicating mast cell mediator release. However, there were a significant drop in beta hexosaminidase level of honey treated and dexamethasone groups. As shown in this study, the administration of 80% (v/v) of Gelam honey in our study significantly reduced the number of mast cells in OVA-induced group. Thus, the results indicate that the ingestion of high concentration of Gelam honey has the potential to reduce the number of mast cells hence, attenuate OVA-induced asthmatic condition.
5. Conclusion
In conclusion, we demonstrated that Malaysian Gelam honey at 80% (v/v) has the potential to mitigate OVA-induced allergic asthma of mice by alleviating asthma-related histopathological changes in the airway. The ability of Gelam honey to reduce mucus overproduction, epithelium thickness, the number of mast cells, inflammatory cells and beta-hexosaminidase level suggested that Gelam honey may prove to be a useful therapeutic approach for the treatment of allergic asthma. Therefore, more comprehensive studies are needed to better understand the mechanisms by which honey reduces asthma disease.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgement
This work was supported by Ministry of Higher Education Malaysia under the Exploratory Research Grant Scheme (ERGS). Grant code: 600-RMI/ERGS 5/3 (53/2012).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Nur Salme Suhana Shamshuddin, Email: salme.suhana@yahoo.com.my.
Rozaini Mohd Zohdi, Email: rozainizohdi@puncakalam.uitm.edu.my.
References
- 1.Agrawal D.K., Shao Z. Pathogenesis of allergic airway inflammation. Curr Allergy Asthma Rep. 2010;10(1):39–48. doi: 10.1007/s11882-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zakaria J., Sann L.M., Hashim Z. Asthma severity and environmental health risk factor among asthmatic primary school children in the selected areas. Am J Appl Sci. 2012;9(10):1553. [Google Scholar]
- 3.Mauad T., Bel E.H., Sterk P.J. Asthma therapy and airway remodeling. J Allergy Clin Immunol. 2007;120(5):997–1009. doi: 10.1016/j.jaci.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P.J., Adcock I.M. How do corticosteroids work in asthma? Ann Intern Med. 2003;139:359–370. doi: 10.7326/0003-4819-139-5_part_1-200309020-00012. [DOI] [PubMed] [Google Scholar]
- 5.Mokhtar N., Chan S. Use of complementary medicine amongst asthmatic patients in primary care. Med J Malays. 2006;61(1):125–127. [PubMed] [Google Scholar]
- 6.Mohd Zohdi R., Abu Bakar Zakaria Z., Yusof N., Mohamed Mustapha N., Abdullah M.N. Gelam (Melaleuca spp.) honey-based hydrogel as burn wound dressing. Evid Based Complement Altern Med eCAM. 2012:843025. doi: 10.1155/2012/843025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussein S.Z., Mohd Yusoff K., Makpol S., Mohd Yusof Y.A. Gelam honey inhibits the production of proinflammatory, mediators NO, PGE(2), TNF-alpha, and IL-6 in carrageenan-induced acute paw edema in Rats. Evid Based Complement Altern Med eCAM. 2012:109636. doi: 10.1155/2012/109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao L.K., Abdul Razak S.L., Ismail N. Malaysian gelam honey reduces oxidative damage and modulates antioxidant enzyme activities in young and middle aged rats. J Med Plants Res. 2011;5(23):5618–5625. [Google Scholar]
- 9.Sayadi S.A., Mohd Zohdi R., Ramasamy K., Hamid K.A. IEEE Symposium on Business, Engineering and Industrial Applications; Bandung, Indonesia: 2012. Antimicrobial Activity of Malaysian Honeys on Selected Bacterial in Gut Flora. [Google Scholar]
- 10.Yang E.J., Lee J.S., Song B.B., Yun C.Y., Kim D.H., Kim I.S. Anti-inflammatory effects of ethanolic extract from Lagerstroemia indica on airway inflammation in mice. J Ethnopharmacol. 2011;136(3):422–427. doi: 10.1016/j.jep.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 11.Chen M.L., Wu C.H., Hung L.S., Lin B.F. Ethanol extract of Perilla frutescens suppresses allergen-specific Th2 responses and alleviates airway inflammation and hyperreactivity in ovalbumin-sensitized murine model of asthma. Evid Based Complement Altern Med eCAM. 2015:324265. doi: 10.1155/2015/324265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang E.J., Lee J.S., Yun C.Y. Inhibitory effects of Duchesnea chrysantha extract on ovalbumin-induced lung inflammation in a mouse model of asthma. J Ethnopharmacol. 2008;118(1):102–107. doi: 10.1016/j.jep.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Kamaruzaman N.A., Sulaiman S.A., Kaur G., Yahaya B. Inhalation of honey reduces airway inflammation and histopathological changes in a rabbit model of ovalbumin-induced chronic asthma. BMC Complement Altern Med. 2014;14:176. doi: 10.1186/1472-6882-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Xiong H., Cheng Y. Effects of taraxasterol on ovalbumin-induced allergic asthma in mice. J Ethnopharmacol. 2013;148(3):787–793. doi: 10.1016/j.jep.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Jang H.Y., Kwon O.K., Oh S.R., Lee H.K., Ahn K.S., Chin Y.W. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2012;50(11):4042–4050. doi: 10.1016/j.fct.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Firinci F., Karaman M., Cilaker-Micili S., Bagrıyanık A., Uzuner N., Karaman O. The effect of atorvastatin on lung histopathology in a murine model of chronic asthma. Allergol Immunopathol. 2014;42(4):355–361. doi: 10.1016/j.aller.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Hiroshima Y., Hsu K., Tedla N. S100A8 induces IL-10 and protects against acute lung injury. J Immunol. 2014;192(6):2800–2811. doi: 10.4049/jimmunol.1302556. [DOI] [PubMed] [Google Scholar]
- 18.Pinelli V., Marchica C.L., Ludwig M.S. Allergen-induced asthma in C57Bl/6 mice: hyper-responsiveness, inflammation and remodelling. Respir Physiol Neurobiol. 2009;169(1):36–43. doi: 10.1016/j.resp.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Nials A.T., Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Models Mech. 2008;1(4–5):213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melgert B.N., Postma D.S., Kuipers I. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2005;35(11):1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 21.Henderson W.R.J., Chi E.Y., Maliszewski C.R. Soluble IL-4 receptor inhibits airway in a mouse model of asthma. J Immunol. 2000;164:1086–1095. doi: 10.4049/jimmunol.164.2.1086. [DOI] [PubMed] [Google Scholar]
- 22.Sulaiman S.A., Hasan H., Deris Z. The benefit of Tualang honey in reducing acute respiratory symptoms among Malaysian hajj pilgrims: a preliminary study. J ApiProduct ApiMedical Sci. 2011;3(1):38–44. [Google Scholar]
- 23.Prakash A., Medhi B., Avti P.K., Saikia U.N., Pandhi P., Khanduja K.L. Effect of different doses of Manuka honey in experimentally induced inflammatory bowel disease in rats. Phytother Res. 2008;22(11):1511–1519. doi: 10.1002/ptr.2523. [DOI] [PubMed] [Google Scholar]
- 24.Zuo L., Otenbaker N.P., Rose B.A., Salisbury K.S. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol Immunol. 2013;56(1–2):57–63. doi: 10.1016/j.molimm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Choi J.H., Hwang Y.P., Lee H.S., Jeong H.G. Inhibitory effect of Platycodi radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2009;47(6):1272–1279. doi: 10.1016/j.fct.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Aljadi A.M., Kamaruddin M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85(4):513–518. [Google Scholar]
- 27.Jung W.K., Lee D.Y., Choi Y.H. Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci. 2008;82(13–14):797–805. doi: 10.1016/j.lfs.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Park H.J., Lee C.M., Jung I.D. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int Immunopharmacol. 2009;9(3):261–267. doi: 10.1016/j.intimp.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Zhou E., Fu Y., Wei Z., Yang Z. Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 2014;5(9):2106–2112. doi: 10.1039/c4fo00384e. [DOI] [PubMed] [Google Scholar]
- 30.Stone K.D., Prussin C., Metcalfe D.D. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson M.S., Taylor M.D., Balic A., Finney C.A.M., Lamb J.R., Maizels R.M. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202(9):1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]