Abstract
To help define the molecular basis of cellular signalling cascades, and their biological functions, there is considerable value in utilizing a high-quality chemical ‘probe’ that has a well-defined interaction with a specific cellular protein. Such reagents include inhibitors of protein kinases and small molecule kinases, as well as mimics or antagonists of intracellular signals. The purpose of this review is to consider recent progress and promising future directions for the development of novel molecules that can interrogate and manipulate the cellular actions of inositol pyrophosphates (PP-IPs) – a specialized, ‘energetic’ group of cell-signalling molecules in which multiple phosphate and diphosphate groups are crammed around a cyclohexane polyol scaffold.
Keywords: inositol phosphate, cell-signalling, drug, pharmacology, structure
Introduction – the multifunctionality of PP-InsPs
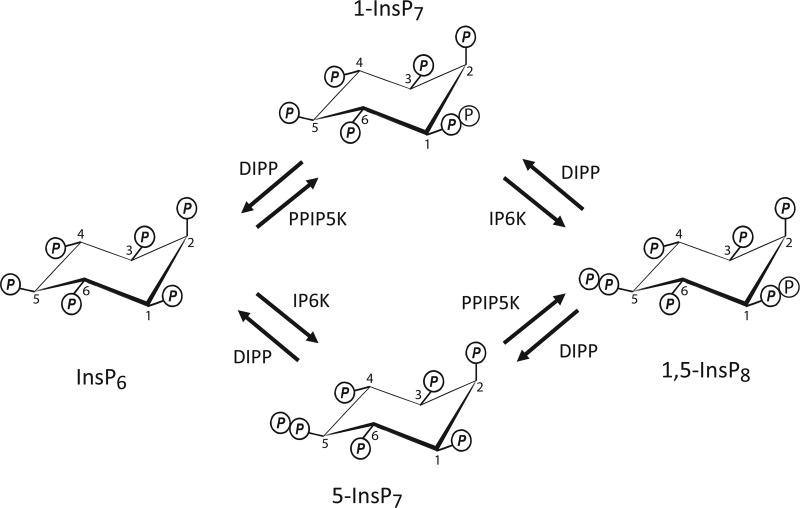
The inositol pyrophosphates (PP-IPs; Figure 1) comprise a unique class of cell-signalling molecules characterized by their functionally-significant and highly ‘energetic’ diphosphate groups [1,2]. As many as seven (‘InsP7’) or eight (‘InsP8’) phosphates are crammed around the six-carbon inositol ring; these molecules possess the most crowded 3D phosphate arrays seen in Nature [1]. The pathways of PP-InsP synthesis involve 5-kinases [inositol hexakisphosphate kinases (IP6Ks)] and 1-kinases [diphosphoinositol pentakisphophate kinases (PPIP5Ks)]. Thus, two InsP7 isomers are generated, distinguished by the presence of a diphosphate group at either the 5- or 1-position on the inositol ring; InsP8 has both of these diphosphates (Figure 1).
Figure 1. Synthesis and metabolism of the PP-InsPs.
The figure describes the metabolic reactions that account for the turnover of the PP-InsPs in both yeasts and mammalian cells. IP6K (Asp1 in Schizosaccharomyces pombe, Vip1 in Saccharomyces cerevisiae; PPIP5K (Ksc1 in yeasts); DIPP (Ddp1 in yeasts).
Genetic studies indicate that PP-InsPs can regulate many different biological processes. The manipulation of IP6K expression has shown PP-InsPs to participate in maintenance of telomere length [3,4], vesicle trafficking [5,6], apoptosis [7,8], autophagy [9], repair of DNA repair by homologous recombination [10,11], transcription of glycolytic genes [12], haemostasis [13], phagocytic and bactericidal activities of neutrophils [14], craniofacial morphogenesis [15], epigenetic modifications to chromatin [16] and exocytic insulin secretion [17]. Genetic targeting of PPIP5K/Vip expression has contributed to demonstrations that PP-InsPs regulate phosphate homoeostasis [18] and cellular morphogenesis [19] in yeasts, and stimulate IFN-β expression in mammalian cells during viral invasion [20].
Current evidence supports two different molecular mechanisms of action of PP-InsPs: association with specific ‘receptors’ [18,21–24] and the ability to non-enzymatically phosphorylate certain proteins [25,26] (since β-phosphates of PP-InsPs are transferred to a phosphoserine residue, the net result is actually protein pyrophosphorylation [25]). 5-InsP7, 1-InsP7 and InsP8 each appear to be equally effective as phosphate donors [25]. This absence of isomer specificity, and the ever-increasing number of proteins identified as targets, could help account for PP-InsP multifunctionality. However, it has been noted that there is no direct evidence this covalent modification occurs in vivo [1,2]. There are as yet no antibody-based or proteomic methods that can address this issue [27]. An indirect, ‘back-phosphorylation’ assay for protein pyrophosphorylation has been developed [28], although such methodology may instead act as a bioassay for the degree of endogenous CK2-mediated protein phosphorylation [29].
There are other actions of PP-InsPs that are isomer-specific. For example, Saccharomyces cerevisiae express a cyclin kinase regulator-protein that is activated by 1-InsP7, but not by 5-InsP7 [18]. There is also selectivity in the inhibition by PP-InsPs of the binding of PtdIns(3,4,5)P3 to pleckstrin-homology domains: 5-InsP7 generally appears to be more potent than both 1-InsP7 and InsP8 [24]. The large number of PtdIns(3,4,5)P3-binding proteins that 5-InsP7 may regulate [23,24] could contribute to PP-InsP multifunctionality.
PP-InsP mimicry
Phosphates and diphosphates are typically targets of active cellular phosphatases, so PP-InsPs are particularly labile in cells and cell extracts. Therefore, there is interest in preparing biologically-stable PP-InsP analogues for functional studies, such as screening cell and tissue lysates for candidate receptors. This research topic has only developed recently – a comprehensive 2010 review of inositide analogues was unable to list any stable PP-InsP mimics [30]. In no small part, that situation reflected synthetic challenges imposed by the high charge density of PP-InsPs and the involvement of unstable phospho-anhydrides and phospho-esters. Characterization and purification of PP-InsP analogues has also been hindered by their lack of UV-absorbance and low level of optical rotation. All of these problems are at last being circumvented [31–34].
There has been a particular focus on replacing the metabolically labile diphosphates in PP-InsPs with α-phosphonoacetic acid esters (PA) or methylenebisphosphonates (PCP) [31–34]. One application for such molecules is for experiments performed in vitro that aim to distinguish between proposed alternative mechanisms of interaction of PP-InsPs with proteins: non-covalent binding compared with non-enzymatic transfer of the ‘energetic’ β-phosphates [25,26]. The latter phenomenon cannot be recapitulated by the metabolically stable analogues. Thus, phosphate transfer can be excluded as the mechanism by which 5-InsP7 enhances CK2 activity (by attenuating inhibition of CK2 by NOPP140), since 5-PCP-InsP7 reproduces this effect [35]. Nevertheless, in circumstances in which a biological effect of a natural PP-InsP is not imitated by a PA- or PCP-analogue, the negative result should be interpreted cautiously; the bioisosteric properties are not perfect. Even in the case of the PCP-analogues, which are considered to be the superior mimics from a structural standpoint, the replacement of an oxygen atom with a methylene group leads to a reduction in acidity and higher pKa values; at physiological pH, even a PCP analogue is expected to be less electronegative than the corresponding natural PP-InsP [36], which by itself may reduce affinity for a protein target. Thus, it is best to utilize the analogues in excess of the concentration of the natural PP-InsP that gives a biological effect. It has been noted that the pKa of a difluoromethylene-bisphosphonate group is closer to that of a diphosphate, but the incorporation of this fluorinated group into a PP-InsP analogue is a considerable synthetic challenge [34,36].
An unexpected development to emerge from experiments with stable PP-InsP analogues has been the discovery that, adjacent to the PPIP5K catalytic pocket, there is a second ligand-binding site that resides on the surface of the protein [37]. The latter has been described as a ‘substrate capture site.’ As its name implies, it enhances ‘capture’ of substrate from the bulk phase, following which the ligand is transferred into the catalytic site – a ‘catchand-pass’ catalytic mechanism [37]. The capture site was originally identified in a structural analysis [37]. In that study, either 5-PA-InsP5 or another substrate analogue, 2,5-di-O-benzyl-inositol-1,3,4,6-tetrakisphosphate (di-Bn-InsP4), were separately soaked into crystals of the PPIP5K2 kinase domain. It was the electron density maps of the two analogues that revealed that the kinase has two ligand-binding sites. In contrast, when a natural substrate, 5-InsP7, was soaked into the crystals, its electron density was restricted to the catalytic site alone [37]. Wang et al. [37] have interpreted these observations as indicating that natural substrates only transiently occupy the capture site during the catalytic cycle. More recent structural work with a PA analogue of InsP8 has shown that the kinase reaction product also binds to the capture site during its egress from the catalytic site [31].
The path to inhibitors of IP6Ks and PPIP5Ks
Selective, cell-permeant inhibitors of IP6Ks and PPIP5Ks – ‘probes’ [38] – could be a useful complement to genetic intervention in PP-InsP signalling processes; there is always the possibility that a particular phenotype that results from gene deletion is complicated by secondary genetic changes. One observation that is particularly pertinent to this topic is the altered degree of transcription of over 900 genes (≥2-fold change in expression), following the deletion of vip1 [39]. This raises ample opportunity for off-target, secondary genetic changes to yield confounding data. The extent of this genetic penetration may reflect functional promiscuity, as a consequence of an inositol phosphate kinase having scaffolding roles separate from its catalytic roles (as is the case, for example, for the inositol pentakisphosphate kinase [40] and the inositol polyphosphate multikinase [41]). A genetic approach can distinguish between catalytic and scaffolding functions. For example, in yeast the wild-type Vip1 can be replaced with a version hosting a point mutation that renders it kinase-dead. However, even then, the degree of transcription of more than 250 genes is still modified [39]. One genetic complication of particular relevance is the reduction in vip1Δ cells of the expression of Pho81 [39], an inhibitor of Pho80-Pho85 cyclin/cyclin-dependent kinase activity [18]. This could complicate genetic research into the mechanism by which Vip1 regulates cyclin kinase activity during phosphate starvation [18]. In contrast, short-term application of a cell-permeant inhibitor of PP-InsP synthesis is an experimental approach that is not entangled by secondary genetic changes and also leaves scaffolding functions intact.
One of the features of PP-IPs that render them amenable to perturbation by defined reagents is the rapidity of their metabolic flux through futile cycles [42] (Figure 1). Indeed, the very demonstration of these fluxes arose from the first reported pharmacological intervention in PP-InsP turnover: the use of fluoride as a diphosphoinositol polyphosphate phosphohydrolase (DIPP) inhibitor, which rapidly (within minutes) brought about manyfold elevations in PP-InsP levels [42,43]. More recent work introduced a cell-permeant pan-IP6K inhibitor, N2-(m-(trifluoromethyl)benzyl) N6-(p-nitrobenzyl) purine (TNP) [44]. This and subsequent studies showed that within 2 h treatment of various cell types with 10–30 µMTNP, levels of InsP7 fell by 60–90%[15,22,44,45]. Naturally (Figure 1), IP6K inhibition does not just affect 5-InsP7 levels; InsP8 synthesis is also compromised [15]. Another collateral impact of TNP addition to intact cells is to elevate InsP6 levels by as much as 40%; deletion of the IP6K2 gene has a similar effect [15,22]. That outcome may in part reflect InsP6 levels normally being curbed through their dephosphorylation by the InsP6/ADP phosphotransferase activity of IP6Ks [45].
TNP has been used in several studies to explore biological functions of IP6Ks [15,22,45–49], so it seems appropriate to assess its specificity profile. In a counter-screen, TNP did not significantly affect a panel of 71 protein kinases [44], although they represent less than 15% of the human kinome. The catalytic site of the IP6K family is structurally related to that of two other groups of inositol phosphate kinases: the IPMKs and the IP3Ks [50]. Nevertheless, TNP is considered a competitive inhibitor of ATP binding and the IP6Ks have around 100-fold lower affinity for ATP than do IPMKs and IP3Ks [44]. Thus, the IC50 for inhibition of IP3K by TNP (18 µM) is almost 40-fold higher than the corresponding value for inhibition of IP6K (0.47 µM). Yet, TNP was originally developed as a cell-permeant inhibitor of IP3K, which is accomplished by using treatment protocols similar to those now used to block IP6K activity [51]. For example, within a couple of minutes of the addition of 5–10 µM TNP to HL60 cells, Ca2+ levels were reported to be slightly elevated [51]. Perhaps because intracellular stores of Zn2+ and Ca2+ are closely coupled [52], cells treated with 20 µM TNP show increased cytosolic Zn2+ levels – a potential off-target cell-signalling response [53]. Furthermore, 20–40 µM TNP relieves the inhibition by IP3 kinase of neurite outgrowth from PC12 cells [54]. In the same cells, as little as 15 min treatment with 40 µM TNP enhances the degree of stimulation of extracellular signal-regulated kinase (ERK) phosphorylation by nerve growth factor [54]. A separate study with human microvascular endothelial cells described inhibition of receptor-dependent ERK phosphorylation after 4 h of TNP treatment [55]. Since IP6K does not appear to regulate ERK phosphorylation [22], the effects upon that kinase of TNP should be considered off-target. Thus, it would be wise not to consider TNP as a substitute for a molecular biology approach to studying IP6K biology.
Not only would it be useful to develop alternate IP6K inhibitors with an improved specificity profile, there could also be great utility in a drug that specifically targets an individual isoform of IP6Ks, particularly as certain biological functions of these kinases are not redundant. For example, mice null for either IP6K1 [56] or IP6K2 [57] each exhibit specific phenotypes. Only the IP6K1(−/−) mice show lower circulating levels of insulin, reduced male fertility and lower body weight. It also appears that the pro-apoptotic effects of IP6K are predominantly exerted by the type 2 isoform [7,8]. The N-terminal ‘glycine loop’ [58] in IP6Ks, that may contribute to binding the gamma phosphate of ATP [50], offers possibilities for development of an isoform-specific drug; residues on either side of this loop do vary a little between IP6K isoforms.
As for the PPIP5Ks, currently there are no known, cell-permeant inhibitors of this group of kinases; the development of such reagents is one of our laboratory’s goals. Lead inhibitors could be identified by high-throughput screening (HTS) of drug libraries. In general, small molecule kinases offer their catalytic pocket as a potential target, most likely to substrate analogues. In the case of the PPIP5Ks, the ‘druggability’ [59] of substrate analogues is doubtful; polyphosphates are highly charged and therefore inherently membrane-impermeant. It remains to be seen whether or not these problems can be overcome with cell-permeant, bioactivatable analogues [60,61] or drug encapsulation technologies [62]. However, the substrate capture site of PPIP5Ks (see above) may be a more viable drug target. Being on the surface of the kinase, it is much less sterically constrained. Additionally, the capture site can accept analogues that are more hydrophobic than natural substrate [75].
It can be a challenge to select an appropriate drug library for screening purposes that might include a suitable inhibitor; as has been noted [63], millions of molecules are commercially available and trillions more could be synthesized. To avoid HTS being a tedious, expensive and ultimately frustrating exercise, focused libraries have been prepared, such as those that are dominated by natural products [63]. Another approach has been to curate a library of approximately 5000 molecules that are either known or predicted to target nucleotide binding [64,65]. In addition, such molecules have been pre-selected to be membrane-permeant, by imposing admission criteria based on lipophilicity, hydrogen-bond formation and size [66]. This particular library has a proven track record, already generating useful reagents from previous screens of protein kinase and lipid kinase activities [65,67]. We aim to deploy this library in our own, future studies. In the case of the ATP-driven kinases that synthesize PP-InsPs, assays of changes in nucleotide levels represent a HTS-compatible assay. This is the approach that we are taking, but in an unusual manner that takes advantage of the ‘highenergy’ of InsP8 hydrolysis, and some unique properties of PPIP5K: its Vmax in the ‘reverse’ direction of ATP hydrolysis is significantly higher than the Vmax in the forward direction [68]. These unusual kinetic characteristics in the ‘reverse-kinase’ direction can be exploited to increase the sensitivity of an HTS. As for preparing pure enzyme for these assays, full-length recombinant versions are difficult to express and purify in large quantities. Fortunately, the kinase activity of PPIP5Ks is self-contained in the N-terminally-located catalytic domain that is readily expressed in Escherichia coli [69–71].
Fragment-based screening offers another approach [72]. Fragments, being low-complexity molecules, interact with proteins weakly but in ideal conformations. They sample chemical space exponentially more effectively than drug-sized molecules, to the extent that a diverse set of 1000 fragments has been calculated to represent chemical space as effectively as would 10 trillion diverse drug-sized molecules [72]. Chemists can then assimilate these different molecular components into more complex drug leads. Since the structure of the PPIP5K kinase domain is available [73], drug development can be assisted by computational methods such as virtual screening and rational design [74,75].
Conclusions
Beyond the use of chemical probes as research tools there is the holy grail: development of therapeutically-beneficial drugs that improve human health. The desire for new anti-inflammatory reagents, and more importantly, improved anti-cancer drugs, is what has driven pharmacological interest in another group of inositide-kinases, the PI3Ks [76]. Indeed, a disrupted PI3K signalling pathway has been associated with 30–50% of human cancers [76]. Even though the lipid products have many functions, over 30 small molecule inhibitors of PI3K activity have progressed into clinical trials for cancer treatment [76]. Furthermore, some of these drugs exhibit good specificity against particular PI3K isoforms [76].
PP-InsPs may play roles in the development of diabetes [17], inflammation [20] and cancer [77]; these are three human health conditions that could conceivably benefit from inhibitors of PP-InsP synthesis or their biological actions. It is too soon to hypothesize if the potential for therapeutic intervention might be thwarted by too many off-target effects of drug treatment due, in particular, to PP-InsP multifunctionality (see above).
There appears to be an opening to develop reagents that might specifically target a particular isoform of IP6K (see above). The preparation of isoform-specific inhibitors of the two PPIP5Ks is also a worthwhile endeavour, but more challenging: the kinase domains of PPIP5K1 and PPIP5K2 are 84% identical, with all non-conserved residues lying away from the ATP-binding site, the catalytic pocket, and even the substrate capture site. On the other hand, we can be sure there remains much to learn about PP-InsP function and metabolism; new opportunities for pharmacological intervention in PP-InsP signalling will surely reveal themselves during future studies.
Acknowledgments
I would like to thank Dr Dorothea Fiedler for her helpful comments.
Funding
This work was supported by the Intramural Research Program of the NIH/National Institute of Environmental Health Sciences.
Abbreviations
- CK2
casein kinase 2
- DIPP
diphosphoinositol polyphosphate phosphohydrolase
- ERK
extracellular signal-regulated kinase
- HTS
high-throughput screening
- IPMK
inositol polyphosphate multikinase
- IP3K
inositol-1,4,5-trisphosphate 3-kinase
- IP6K
inositol hexakisphosphate kinase
- PA
α-phosphonoacetic acid esters
- PCP
methylenebisphosphonates
- PI3K
phosphoinositide 3-kinase
- PP-IP
inositol pyrophosphate
- PPIP5K
diphosphoinositol pentakisphophate kinase
- PtdIns(3,4,5)P3
phosphatidylinositol-3,4,5-trisphosphate
- TNP
N2-(m-(trifluoromethyl)benzyl) N6-(p-nitrobenzyl)purine.
References
- 1.Shears SB. Inositol pyrophosphates: why so many phosphates? Adv. Biol. Regul. 2015;57:203–216. doi: 10.1016/j.jbior.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson MS, Livermore TM, Saiardi A. Inositol pyrophosphates: between signalling and metabolism. Biochem. J. 2013;452:369–379. doi: 10.1042/BJ20130118. [DOI] [PubMed] [Google Scholar]
- 3.York SJ, Armbruster BN, Greenwell P, Petes TD, York JD. Inositol diphosphate signaling regulates telomere length. J. Biol. Chem. 2005;280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- 4.Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. Inositol pyrophosphates regulate cell death and telomere length via PI3K-related protein kinases. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saiardi A, Caffrey JJ, Snyder SH, Shears SB. The inositol hexakisphosphate kinase family: catalytic flexibility, and function in yeast vacuole biogenesis. J. Biol. Chem. 2000;275:24686–24692. doi: 10.1074/jbc.M002750200. [DOI] [PubMed] [Google Scholar]
- 6.Saiardi A, Sciambi C, McCaffery JM, Wendland B, Snyder SH. Inositol pyrophosphates regulate endocytic trafficking. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14206–14211. doi: 10.1073/pnas.212527899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata E, Luo HR, Saiardi A, Bae BI, Suzuki N, Snyder SH. Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J. Biol. Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- 8.Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ. Inositol hexakisphosphate kinase 2 mediates growth suppressive and apoptotic effects of interferon-b in ovarian carcinoma cells. J. Biol. Chem. 2001;276:24965–24970. doi: 10.1074/jbc.M101161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata E, Saiardi A, Tsukamoto H, Satoh T, Itoh Y, Itoh J, Shibata M, Takizawa S, Takagi S. Inositol hexakisphosphate kinases promote autophagy. Int. J. Biochem. Cell Biol. 2010;42:2065–2071. doi: 10.1016/j.biocel.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Jadav RS, Chanduri MV, Sengupta S, Bhandari R. Inositol pyrophosphate synthesis by inositol hexakisphosphate kinase 1 is required for homologous recombination repair. J. Biol. Chem. 2013;288:3312–3321. doi: 10.1074/jbc.M112.396556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo HR, Saiardi A, Yu H, Nagata E, Ye K, Snyder SH. Inositol pyrophosphates are required for DNA hyperrecombination in protein kinase C1 mutant yeast. Biochemistry. 2002;41:2509–2515. doi: 10.1021/bi0118153. [DOI] [PubMed] [Google Scholar]
- 12.Szijgyarto Z, Garedew A, Azevedo C, Saiardi A. Influence of inositol pyrophosphates on cellular energy dynamics. Science. 2011;334:802–805. doi: 10.1126/science.1211908. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S, Shukla D, Suman K, Lakshmi BJ, Manorama R, Kumar S, Bhandari R. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood. 2013;122:1478–1486. doi: 10.1182/blood-2013-01-481549. [DOI] [PubMed] [Google Scholar]
- 14.Prasad A, Jia Y, Chakraborty A, Li Y, Jain SK, Zhong J, Roy SG, Loison F, Mondal S, Sakai J, et al. Inositol hexakisphosphate kinase 1 regulates neutrophil function in innate immunity by inhibiting phosphatidylinositol-(3,4,5)-trisphosphate signaling. Nat. Immunol. 2011;12:752–760. doi: 10.1038/ni.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarmah B, Wente SR. Inositol hexakisphosphate kinase-2 acts as an effector of the vertebrate Hedgehog pathway. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19921–19926. doi: 10.1073/pnas.1007256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton A, Azevedo C, Andreassi C, Riccio A, Saiardi A. Inositol pyrophosphates regulate JMJD2C-dependent histone demethylation. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18970–18975. doi: 10.1073/pnas.1309699110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illies C, Gromada J, Fiume R, Leibiger B, Yu J, Juhl K, Yang S-N, Barma DK, Falck JR, Saiardi A, et al. Inositol pyrophosphates determine exocytic capacity. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, Mulugu S, York JD, O’Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pohlmann J, Risse C, Seidel C, Pohlmann T, Jakopec V, Walla E, Ramrath P, Takeshita N, Baumann S, Feldbrugge M, et al. The vip1 inositol polyphosphate kinase family regulates polarized growth and modulates the microtubule cytoskeleton in fungi. PLoS. Genet. 2014;10:e1004586. doi: 10.1371/journal.pgen.1004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulloor NK, Nair S, Kostic AD, Bist P, Weaver JD, Tyagi R, Uchil PD, York JD, Snyder SH, Garcia-Sastre A, et al. Human genome-wide RNAi screen identifies an essential role for inositol pyrophosphates in Type-I interferon response. PLoS Pathog. 2014;10:e1003981. doi: 10.1371/journal.ppat.1003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YS, Huang K, Quiocho FA, O’Shea EK. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat. Chem. Biol. 2008;4:25–32. doi: 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, et al. Inositol pyrophosphates inhibit akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K, Huang Y, Nagata E, Devreotes P, Snyder SH. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 24.Gokhale NA, Zaremba A, Janoshazi AK, Weaver JD, Shears SB. PPIP5K1 modulates ligand competition between diphosphoinositol polyphosphates and PtdIns(3,4,5)P3 for polyphosphoinositide-binding domains. Biochem. J. 2013;453:413–426. doi: 10.1042/BJ20121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ, Molina H, Pandey A, Werner JK, Jr, Juluri KR, et al. Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiardi A, Bhandari A, Resnick R, Cain A, Snowman AM, Snyder SH. Inositol pyrophosphate: physiologic phosphorylation of proteins. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 27.Marmelstein AM, Yates LM, Conway JH, Fiedler D. Chemical pyrophosphorylation of functionally diverse peptides. J. Am. Chem. Soc. 2014;136:108–111. doi: 10.1021/ja411737c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azevedo C, Burton A, Ruiz-Mateos E, Marsh M, Saiardi A. Inositol pyrophosphate mediated pyrophosphorylation of AP3B1 regulates HIV-1 Gag release. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21161–21166. doi: 10.1073/pnas.0909176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shears SB, Gokhale NA, Wang H, Zaremba A. Diphosphoinositol polyphosphates: what are the mechanisms? Adv. Enzyme Regul. 2011;51:13–25. doi: 10.1016/j.advenzreg.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best MD, Zhang H, Prestwich GD. Inositol polyphosphates, diphosphoinositol polyphosphates and phosphatidylinositol polyphosphate lipids: structure, synthesis, and development of probes for studying biological activity. Nat. Prod. Rep. 2010;27:1403–1430. doi: 10.1039/b923844c. [DOI] [PubMed] [Google Scholar]
- 31.Riley AM, Wang H, Shears SB, BV LP. Synthetic tools for studying the chemical biology of InsP8. Chem. Commun. 2015;51:12605–12608. doi: 10.1039/c5cc05017k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley AM, Wang H, Weaver JD, Shears SB, Potter BVL. First synthetic analogues of diphosphoinositol polyphosphates: interaction with PPIP5 kinase. Chem. Commun. 2012;48:11292–11294. doi: 10.1039/c2cc36044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M, Chong LS, Capolicchio S, Jessen HJ, Resnick AC, Fiedler D. Elucidating diphosphoinositol polyphosphate function with nonhydrolyzable analogues. Angew. Chem. Int. Ed. Engl. 2014;53:9508–9511. doi: 10.1002/anie.201402905. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, Dul BE, Trevisan AJ, Fiedler D. Synthesis and characterization of non-hydrolysable diphosphoinositol polyphosphate second messengers. Chem. Sci. 2013;4:405–410. doi: 10.1039/C2SC21553E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao F, Cha J, Xu J, Xu R, Vandiver MS, Tyagi R, Tokhunts R, Koldobskiy MA, Fu C, Barrow R, et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol. Cell. 2014;54:119–132. doi: 10.1016/j.molcel.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliott TS, Slowey A, Ye Y, Conway SJ. The use of phosphate bioisosteres in medicinal chemistry and chemical biology. Med. Chem. Commun. 2012;3:735–751. [Google Scholar]
- 37.Wang H, Godage HY, Riley AM, Weaver JD, Shears SB, Potter BVL. Synthetic inositol phosphate analogs reveal that PPIP5K2 has a surface-mounted substrate capture site that is a target for drug discovery. Chem. Biol. 2014;21:689–699. doi: 10.1016/j.chembiol.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frye SV. The art of the chemical probe. Nat. Chem. Biol. 2010;6:159–161. doi: 10.1038/nchembio.296. [DOI] [PubMed] [Google Scholar]
- 39.Worley J, Luo X, Capaldi AP. Inositol pyrophosphates regulate cell growth and the environmental stress response by activating the HDAC Rpd3L. Cell Rep. 2013;3:1476–1482. doi: 10.1016/j.celrep.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brehm MA, Wundenberg T, Williams J, Mayr GW, Shears SB. A non-catalytic role for inositol 1,3,4,5,6-pentakisphosphate 2-kinase in the synthesis of ribosomal RNA. J. Cell Sci. 2013;126:437–444. doi: 10.1242/jcs.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, Kim SF, Maag D, Maxwell MJ, Resnick AC, Juluri KR, Chakraborty A, Koldobskiy MA, Cha SH, Barrow R, et al. Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase. Cell Metab. 2011;13:215–221. doi: 10.1016/j.cmet.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menniti FS, Miller RN, Putney JW, Jr, Shears SB. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J. Biol. Chem. 1993;268:3850–3856. [PubMed] [Google Scholar]
- 43.Safrany ST, Caffrey JJ, Yang X, Bembenek ME, Moyer MB, Burkhart WA, Shears SB. A novel context for the “MutT” module, a guardian of cell integrity, in a diphosphoinositol polyphosphate phosphohydrolase. EMBO J. 1998;17:6599–6607. doi: 10.1093/emboj/17.22.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padmanabhan U, Dollins DE, Fridy PC, York JD, Downes CP. Characterization of a selective inhibitor of inositol hexakisphosphate kinases: use in defining biological roles and metabolic relationships of inositol pyrophosphates. J. Biol. Chem. 2009;284:10571–10582. doi: 10.1074/jbc.M900752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wundenberg T, Grabinski N, Lin H, Mayr GW. Discovery of InsP6-kinases as InsP6-dephosphorylating enzymes provides a new mechanism of cytosolic InsP6 degradation driven by the cellular ATP/ADP ratio. Biochem. J. 2014;462:173–184. doi: 10.1042/BJ20130992. [DOI] [PubMed] [Google Scholar]
- 46.Rao F, Xu J, Khan AB, Gadalla MM, Cha JY, Xu R, Tyagi R, Dang Y, Chakraborty A, Snyder SH. Inositol hexakisphosphate kinase-1 mediates assembly/disassembly of the CRL4-signalosome complex to regulate DNA repair and cell death. Proc. Natl. Acad. Sci. U.S.A. 2014;111:16005–16010. doi: 10.1073/pnas.1417900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Li H, Bajrami B, Kwak H, Cao S, Liu P, Zhou J, Zhou Y, Zhu H, Ye K, Luo HR. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. U.S.A. 2013;110:7726–7731. doi: 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Liang D, Gao X, Zhao C, Qin X, Xu Y, Su T, Sun D, Li W, Wang H, et al. Selective inhibition of inositol hexakisphosphate kinases (IP6Ks) enhances mesenchymal stem cell engraftment and improves therapeutic efficacy for myocardial infarction. Basic Res. Cardiol. 2014;109:417. doi: 10.1007/s00395-014-0417-x. [DOI] [PubMed] [Google Scholar]
- 49.Sun D, Li S, Wu H, Zhang M, Zhang X, Wei L, Qin X, Gao E. Oncostatin M (OSM) protects against cardiac ischaemia/reperfusion injury in diabetic mice by regulating apoptosis, mitochondrial biogenesis and insulin sensitivity. J. Cell Mol. Med. 2015;19:1296–1307. doi: 10.1111/jcmm.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, DeRose EF, London RE, Shears SB. IP6K structure and the molecular determinants of catalytic specificity in an inositol phosphate kinase family. Nat. Commun. 2014;5:4178. doi: 10.1038/ncomms5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang YT, Choi G, Bae YS, Burdett M, Moon HS, Lee JW, Gray NS, Schultz PG, Meijer L, Chung SK, et al. Purine-based inhibitors of inositol-1,4,5-trisphosphate-3-kinase. Chembiochem. 2002;3:897–901. doi: 10.1002/1439-7633(20020902)3:9<897::AID-CBIC897>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 52.Pitt SJ, Stewart AJ. Examining a new role for zinc in regulating calcium release in cardiac muscle. Biochem. Soc. Trans. 2015;43:359–363. doi: 10.1042/BST20140285. [DOI] [PubMed] [Google Scholar]
- 53.Stork CJ, Li YV. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J. Mol. Signal. 2010;5:5. doi: 10.1186/1750-2187-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eva R, Bouyoucef-Cherchalli D, Patel K, Cullen PJ, Banting G. IP3 3-kinase opposes NGF driven neurite outgrowth. PLoS. One. 2012;7:e32386. doi: 10.1371/journal.pone.0032386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekar MC, Shahiwala K, Leloup L, Wells A. Modulation of epidermal growth factor stimulated ERK phosphorylation and cell motility by inositol trisphosphate kinase. J. Pharm. Sci. Pharmacol. 2014;1:160–164. doi: 10.1166/jpsp.2014.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhandari R, Juluri KR, Resnick AC, Snyder SH. Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2439–2353. doi: 10.1073/pnas.0712227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison BH, Haney R, Lamarre E, Drazba J, Prestwich GD, Lindner DJ. Gene deletion of inositol hexakisphosphate kinase 2 predisposes to aerodigestive tract carcinoma. Oncogene. 2009;28:2383–2392. doi: 10.1038/onc.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez B, Schell MJ, Letcher AJ, Veprintsev DB, Irvine RF, Williams RL. Structure of a human inositol 1,4,5-trisphosphate 3-kinase; substrate binding reveals why it is not a phosphoinositide 3-kinase. Mol. Cell. 2004;15:689–701. doi: 10.1016/j.molcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Brown D, Superti-Furga G. Rediscovering the sweet spot in drug discovery. Drug Discov. Today. 2003;8:1067–1077. doi: 10.1016/s1359-6446(03)02902-7. [DOI] [PubMed] [Google Scholar]
- 60.Vajanaphanich M, Schultz C, Rudolf MT, Wasserman M, Enyedi P, Craxton A, Shears SB, Tsien RY, Barrett KE, Traynor-Kaplan AE. Long-term uncoupling of chloride secretion from intracellular calcium levels by Ins(3,4,5,6)P4. Nature. 1994;371:711–714. doi: 10.1038/371711a0. [DOI] [PubMed] [Google Scholar]
- 61.Pavlovic I, Thakor DT, Bigler L, Wilson MS, Laha D, Schaaf G, Saiardi A, Jessen HJ. Prometabolites of 5-diphospho-myo-inositol pentakisphosphate. Angew. Chem. Int. Ed. Engl. 2015;54:9622–9626. doi: 10.1002/anie.201503094. [DOI] [PubMed] [Google Scholar]
- 62.Kong F, Zhang X, Hai M. Microfluidics fabrication of monodisperse biocompatible phospholipid vesicles for encapsulation and delivery of hydrophilic drug or active compound. Langmuir. 2014;30:3905–3912. doi: 10.1021/la404201m. [DOI] [PubMed] [Google Scholar]
- 63.Drewry DH, Macarron R. Enhancements of screening collections to address areas of unmet medical need: an industry perspective. Curr. Opin. Chem. Biol. 2010;14:289–298. doi: 10.1016/j.cbpa.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 64.Janzen WP. Screening technologies for small molecule discovery: the state of the art. Chem. Biol. 2014;21:1162–1170. doi: 10.1016/j.chembiol.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Hutti JE, Porter MA, Cheely AW, Cantley LC, Wang X, Kireev D, Baldwin AS, Janzen WP. Development of a high-throughput assay for identifying inhibitors of TBK1 and IKKepsilon. PLoS One. 2012;7:e41494. doi: 10.1371/journal.pone.0041494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 67.Wright BD, Loo L, Street SE, Ma A, Taylor-Blake B, Stashko MA, Jin J, Janzen WP, Frye SV, Zylka MJ. The lipid kinase PIP5K1C regulates pain signaling and sensitization. Neuron. 2014;82:836–847. doi: 10.1016/j.neuron.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weaver JD, Wang H, Shears SB. The kinetic properties of a human PPIP5K reveal that its kinase activities are protected against the consequences of a deteriorating cellular bioenergetic environment. Biosci. Rep. 2013;33:228–241. doi: 10.1042/BSR20120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fridy PC, Otto JC, Dollins DE, York JD. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J. Biol. Chem. 2007;282:30754–30762. doi: 10.1074/jbc.M704656200. [DOI] [PubMed] [Google Scholar]
- 70.Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, Haystead TA, Ribeiro AA, York JD. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 71.Choi JH, Williams J, Cho J, Falck JR, Shears SB. Purification, sequencing, and molecular identification of a mammalian PP-InsP5 kinase that is activated when cells are exposed to hyperosmotic stress. J. Biol. Chem. 2007;282:30763–30775. doi: 10.1074/jbc.M704655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edfeldt FN, Folmer RH, Breeze AL. Fragment screening to predict druggability (ligandability) and lead discovery success. Drug Discov. Today. 2011;16:284–287. doi: 10.1016/j.drudis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Falck JR, Hall TM, Shears SB. Structural basis for an inositol pyrophosphate kinase surmounting phosphate crowding. Nat. Chem. Biol. 2012;8:111–116. doi: 10.1038/nchembio.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lionta E, Spyrou G, Vassilatis D, Cournia Z. Structure-based virtual screening for drug discovery: principles, applications and recent advances. Curr. Top. Med. Chem. 2014;14:1923–1938. doi: 10.2174/1568026614666140929124445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergner A, Parel SP. Hit expansion approaches using multiple similarity methods and virtualized query structures. J. Chem. Inf. Model. 2013;53:1057–1066. doi: 10.1021/ci400059p. [DOI] [PubMed] [Google Scholar]
- 76.Yap TA, Bjerke L, Clarke PA, Workman P. Drugging PI3K in cancer: refining targets and therapeutic strategies. Curr. Opin. Pharmacol. 2015;23:98–107. doi: 10.1016/j.coph.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao F, Xu J, Fu C, Cha JY, Gadalla MM, Xu R, Barrow JC, Snyder SH. Inositol pyrophosphates promote tumor growth and metastasis by antagonizing liver kinase B1. Proc. Natl. Acad. Sci. U.S.A. 2015;112:1773–1778. doi: 10.1073/pnas.1424642112. [DOI] [PMC free article] [PubMed] [Google Scholar]