Abstract
The present study was cherished to investigate in vitro thrombolytic, membrane stabilizing and antibacterial activities of Allamanda neriifolia and Aegialitis rotundifolia. Different types methanolic extracts of these two medicinal plants were tested for determining membrane stabilizing activity at a hypotonic solution and heat induce condition by comparing with reference standard acetyl salicylic acid (0.10 mg/mL), where thrombolytic activity assessment was done by employing Streptokinase as standard drug. Finally, antibacterial activity was performed against Staphylococcus aureus as a Gram-positive (+ve) and Salmonella typhi, Escherichia coli and Pseudomonas aeruginosa as Gram negative (−ve) bacteria by using disc diffusion method. In case of membrane stabilizing studies, crude methanolic extracts of A. neriifolia at 10 mg/ml concentration, more importantly, showed 45.80% & 23.52% whereas 10 mg/ml concentration of A. rotundifolia more significantly (p < 0.01) produced 38.40% and 27.04% inhibition of hemolysis for both experimental conditions. Dose-dependently increased activity was found in the thrombolytic study where 10 mg/ml concentration of both A. neriifolia and A. rotundifolia more significantly (p < 0.01) showed 41.91% and 32.76% lysis of clot respectively by in vitro clot lysis assay method. Crude methanolic extracts of A. rotundifolia did not show any suitable antibacterial property against the test bacteria. However, the gram positive (+ve) bacteria also seemed resistant against A. neriifolia extract but this crude methanolic extracts was found to generate moderate antibacterial action against gram-negative (−ve) bacteria. The obtained results confirmed the presence of thrombolytic, membrane stabilizing activity for both plant extract along with moderate antibacterial activity for A. neriifolia.
Keywords: Allamanda neriifolia, Aegialitis rotundifolia, Thrombolytic, Membrane stabilizing, Antibacterial
Graphical abstract
1. Introduction
Medicinal plants have always been considered a healthy source of life for the recuperation of various diseases.1 Therapeutic properties of medicinal plants are very useful in healing various diseases and the advantages what are they providing is being 100% natural. Since ancient times, herbal preparations have been used for the treatment of several diseases. Herbal products are often perceived as safe because they are “natural”.2 Botanical medicine or phytomedicine refers to the use of any plant's seeds, berries, roots, leaves, bark, or flowers for medicinal purposes.3 Even among prescription drugs, minimum 25% contain at least one compound derived from plants. The percentage might be higher if we include over-the-counter (OTC) drugs.4 In developing countries, about 75% of the world populations rely on traditional medicine for their primary healthcare.5 Like other developing countries, thromboembolic disorders are one of the main causes of morbidity and mortality in Bangladesh.6 Most commonly used thrombolytic agents for dissolving clots are alteplase, anistreplase, streptokinase, urokinase, and tissue plasminogen activator.7 Almost each and every thrombolytic agent have significant shortcomings, including limited fibrin specificity, bleeding tendency, and required large doses to have a maximum therapeutic effect. Epidemiologic studies have provided evidence that foods with experimentally proven anti-thrombotic effect could reduce the risk of thrombosis. Thus, herbs showing thrombolytic activity have been studied, and a few significant observations have been reported.8 Another important pathological disorder is inflammation. This is the non-specific immune response that occurs in any type of bodily injury or sometimes manifested due to the complex biological response of vascular tissues to harmful stimuli.9, 10 From ancient time mans were used plants to treat common infectious diseases and even long before mankind discovered the existence of microbes; the idea that certain plants had healing potential was well accepted.11 A large number of bacteria can cause infections resulting in mild to life-threatening illnesses. Bacterial infections include: urinary tract, surgical wound infections, respiratory infections, skin infections, and gastrointestinal infections. The proceeding of resistance of pathogens against antibiotics has become a difficult issue, in the recent years caused by the baffling use of antibiotics.12, 13, 14, 15, 16, 17, 18 Advances in phytochemistry and identification of plant compounds that are effective in curing certain diseases have renewed interest in herbal medicines. Allamanda neriifolia and Aegialitis rotundifolia are the medicinal plants belong to the family Apocynaceae and Plumbaginaceae respectively. A. neriifolia, locally known as yellow bell which is native to the old world tropics, but now pan-tropical.19 It was possibly domesticated in India and southern China and is now found naturalized in almost all tropical and subtropical regions. It is an important market vegetable in southern and eastern Asia, e.g. India, Sri Lanka, Vietnam, Thailand, Malaysia, the Philippines and southern China.20 Local cultivars originally from Asia are cultivated on a small scale in tropical America, and bitter gourd is also cultivated in the southern part of the United States for the Asiatic kitchen. It is a common cucurbit in the wild flora of Africa, occurring almost throughout tropical Africa. It is only occasionally collected from the wild as a vegetable or medicinal plant.19 A. rotundifolia have a discontinuous distribution which mainly occurs in the Lesser Sunda Islands, the Moluccas, New Guinea and northern Australia, in low mangroves on sandy and the rocky substrate and also found in the further west. It is locally known as banrua and is small trees or woody mangrove shrubs that grow up to 2–3 m tall.21, 22, 23 The deciduous species have leafy stems with leathery leaves, the flowers are organized in terminal cymose racemous inflorescences forms and fruits are dehiscent and have a spongy mesocarp.18 Locally it is pounded with oil and applied to relief insect bites pain but there is no scientific evidence to support this therapeutic use. Both the plant species are not native in Bangladesh and they are found here as cultivated forms. The objective of the present study was to investigate the possible in-vitro thrombolytic, anti-inflammatory and antimicrobial activities of the crude methanolic extracts of both A. neriifolia and A. rotundifolia.
2. Materials and methods
2.1. Drugs and chemicals
All chemicals and reagents used in this study were of analytical grade. Methanol (99.5%), which was used for extraction, was procured from Sigma-Aldrich (Hamburg, Germany). Standard streptokinase and acetyl salicylic acid were purchased from Beximco Pharmaceuticals Limited, Bangladesh. Again during conducting an antibacterial assay of plant extracts Ampicillin, Cefoxitin, Penicillin, and Imipenem was procured from Square Pharmaceuticals Limited, Bangladesh (Kaliakoir, Gazipur, Bangladesh).
2.2. Plant materials
For this present investigation, the whole plant of A. neriifolia was collected from Noakhali, Bangladesh and was identified by the expert of National Herbarium Institute, Mirpur, Dhaka, Bangladesh. (Accession number: DACB-38329). The other plant, A. rotundifolia (whole plant) was collected from Sonadia Island, Bangladesh and was ascertained by the expert of National Herbarium Institute, Mirpur, Dhaka, Bangladesh. (Accession number: DACB-38310). Both of these plants were collected during January, 2016.
2.3. Extraction
After collection of whole plants of A. neriifolia and A. rotundifolia were thoroughly washed with water. Then the collected plant materials were chopped, dried, and powdered. About 500 g of the powdered materials of each plant were soaked in 1.5 L of methanol at room temperature for two weeks. Then the solution was filtered using filter cloth and Whatman filter paper no. 1 (Sargent, Welch, USA) and concentrated with a rotary evaporator (RE-52AA). It rendered a brown granular. The brown granular were designated as crude methanolic extracts.
2.4. Red blood cells (RBC) collection
Human RBCs were collected for conducting thrombolytic and membrane stabilizing assay. RBCs collected from the human was male, 65 kg, fair complexion and free from diseases (using a protocol approved by Institutional Ethics Committee). The collected RBCs were kept in a test tube with an anticoagulant EDTA under standard conditions of temperature 23 ± 2 °C and relative humidity 55 ± 10%.
2.5. Thrombolytic activity
The thrombolytic activity of all extracts was evaluated by the method developed by Daginawala (2006)24 and modified by Kawsar et al. (2011)25 using streptokinase (SK) as the standard.
2.5.1. Specimen
Five different test solutions for this two respective plant were used to evaluate the thrombolytic activity of the plant extract. The extract of both plants was dissolved in methanol and shaken vigorously on a vortex mixer to prepare different concentrations (2, 4, 6, 8 and 10 mg/ml respectively) of the test samples. The suspension was kept overnight and decanted to remove the soluble supernatant, which was filtered through a 0.22-micron syringe filter. 100 μl of the methanolic preparations of the plant were added to the microcentrifuge tube containing the clots to check thrombolytic activity,24, 25 where streptokinase was employed as positive control and methanol was employed as negative control.
2.5.2. Thrombolytic activity
During this study 7 ml of venous blood was drawn from healthy volunteers (n = 3) and transferred to different pre-weighed sterilized microcentrifuge tube (1 ml/tube). The microcentrifuge tubes were exposed to incubation at 37 °C for 45 min. After the formation of a clot, serum was completely discarded from the tubes (carried out without disturbing the clot formed) and each tube having clot was again weighed to determine the weight of the clot (clot weight = weight of clot containing tube – weight of tube alone). Each micro-centrifuge tube containing clot was appropriately labeled and 100 μl of the plant extract with various concentrations (2, 4, 6, 8 and 10 mg/ml respectively) was added to the tubes accordingly. As a positive control, 100 μl of streptokinase and as a negative non thrombolytic control, 100 μl of methanol were distinctly added to the control tubes numbered. After that, the tubes were incubated again at 37 °C for 90 min and observed for clot lysis. After the following incubation, the obtained fluid was discarded from the tubes and they were again weighed to observe the difference in weight after clot disruption.24, 25 Finally, difference obtained in weight was calculated and the result was expressed as percentage of clot lysis following the under beneath equation
2.6. Membrane stabilizing activity
In vitro membrane stabilizing the activity of the extractives was performed by using hypotonic solution and heat-induced hemolysis of erythrocyte membrane by the method developed by Shinde et al. (1999)26 and modified by Sikder et al. (2011).27
2.6.1. Hypotonic solution-induced hemolysis
The experiments were carried out with a hypotonic solution where extraction solvent at 1 mg/ml is used as a control. The test sample consisted of stock erythrocyte (RBC) suspension (0.50 mL) with 5 ml of hypotonic solution (50 mM NaCl) in 10 mM sodium phosphate buffer saline (pH 7.4) containing either the different methanolic extract (1.0 mg/mL) or Acetyl Salicylic Acid (0.10 mg/mL). The Acetyl Salicylic Acid was used as a reference standard and methanol was employed as control. The mixtures were incubated for 10 min at room temperature, centrifuged for 10 min at 3000 g and the absorbance (O.D.) of the supernatant was measured at 540 nm using Shimadzu UV spectrophotometer.
The percentage inhibition of either hemolysis or membrane stabilization was calculated using the following equation:
Where,
OD1 = Optical density of hypotonic-buffered saline solution alone (control) and
OD2 = Optical density of test sample in hypotonic solution.
2.6.2. Heat induced hemolysis
Aliquots (5 ml) of the isotonic buffer, containing 1.0 mg/ml of different extracts of the plant were put into two duplicate sets of centrifuge tubes 16. The vehicle methanol, in the same amount, was added to another tube as a control. Erythrocyte suspension (30 μL) was added to each tube and mixed gently by inversion. One pair of the tubes was incubated at 54 °C for 20 min in a water bath. The other pair was maintained at 0–5 °C in an ice bath. The reaction mixture was centrifuged for 3 min at 1300 g and the absorbance of the supernatant was measured at 540 nm using UV spectrometer. The percentage inhibition or acceleration of hemolysis in tests and was calculated using the following equation:
Where,
OD1 = test sample unheated,
OD2 = test sample heated and,
OD3 = control sample heated
2.7. Antibacterial activity
2.7.1. Test organisms
Gram-positive bacteria (Staphylococcus aureus), Gram negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi). The bacterial strains used for the experiment were collected from pure cultures from the Institute of Chittagong veterinary and animal sciences university. Antibacterial activities of both A. neriifolia and A. rotundifolia were determined according to Kirby-Bauer's Disc diffusion method.28
2.7.2. Procedure
The antimicrobial screening which is the first stage of antimicrobial drug research is performed by several ways, but we used Kirby-Bauer's Disc diffusion method which is widely accepted method for in-vitro antimicrobial screening.28 Previously sterilized four petri dishes were flooded with Mueller-Hinton Agar media and after solidification of the media desired culture of micro-organism was inoculated on the plate through cotton burg stick which is already soaked into the desired microbial culture broth. Sample was prepared by dissolving 1 mg extract in 1 ml methanol (1 mg/ml) and loaded into dried and sterilized filter paper discs with different μl/disc (50 μl/disc, 75 μl/disc, and 100 μl/disc respectively) using micropipette. These discs were transferred to each petri dish carefully using a sterile forceps and allowed to dry for 24 h at room temperature. The dishes were then incubated at 37 °C for 24 h to allow optimal growth of microorganism. Then the dishes were observed for bacterial growth, which can be expressed in term of zone of inhibition (in millimeter) by using antibiotic zone scale. Different antibiotics (Ampicillin, Imipenem) and sterile filter paper disc with respective solvent (methanol) of 25 μl were used as positive and negative control respectively. If the test sample possesses any antimicrobial activity, it will reduce the growth of the microorganisms and a clear, distinct zone of inhibition will be appeared surrounding the medium. The antimicrobial activity of the test sample was calculated by measuring the diameter of zone of inhibition expressed in centimeter.
2.8. Statistical analysis
All the above assays were conducted in triplicate and repeated threes for consistency of results and statistical purpose. The data were expressed as Mean ± SD and analyzed by one-way analysis of variance (ANOVA) followed by Dunnett ‘t’ test using SPSS software of 10 version. p < 0.05 was considered statistically significant.
3. Results
3.1. Thrombolytic activity
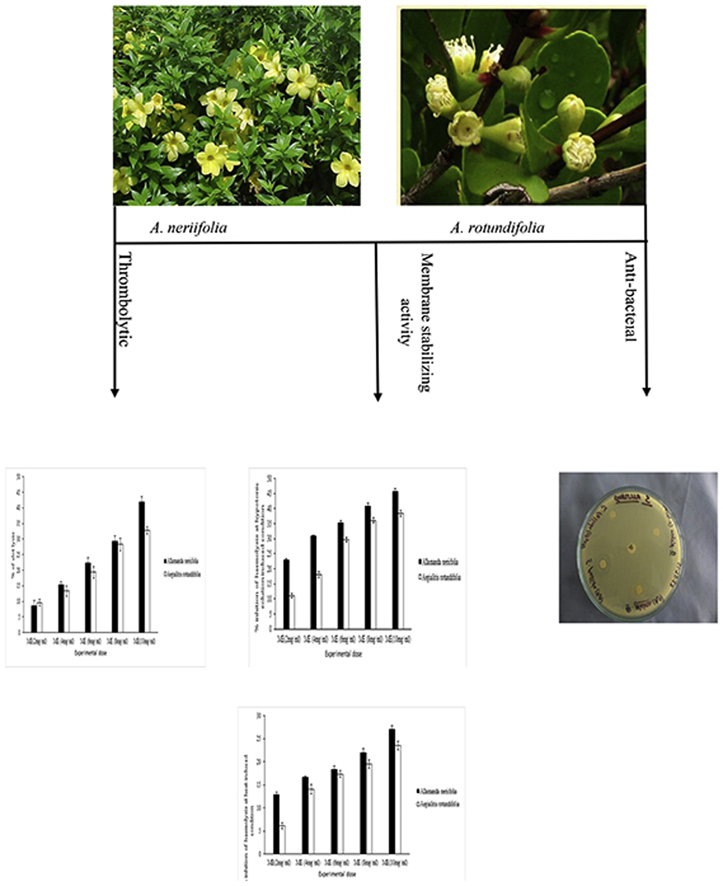
The effects of both A. neriifolia and A. rotundifolia on in-vitro clot lysis are tabulated in Table 1. From Table 1, it is evident that the percentage of clot lysis was 58.49% when 100 μl of streptokinase (30,000 I.U.) was used as a positive control, while in the case of methanol (negative control) the percentage of clot lysis was negligible (1.32%). The percentage of clot lysis were dose-dependently increased for both of this plant extract, whereas 10 mg/ml concentration showed more significant clot lysis that was 41.91% and 32.76% respectively (p < 0.01) respectively for A. neriifolia and A. rotundifolia. While their clot lysis potential is compared with each other than A. neriifolia found to dominate over clot lysis activity of A. rotundifolia.
Table 1.
Effects of different concentration of methanolic extracts of both A. neriifolia and A. rotundifolia on in-vitro clot lysis.
| Treatment | % of clot lysis of A. neriifolia (mean ± SD) | % of clot lysis of A. rotundifolia Roxb. (mean ± SD) |
|---|---|---|
| ME (2 mg/ml) | 8.45 ± 1.82 | 9.57 ± 1.06 |
| ME (4 mg/ml) | 15.34 ± 1.02 | 13.35 ± 1.67 |
| ME (6 mg/ml) | 22.31 ± 1.68 | 19.35 ± 1.84 |
| ME (8 mg/ml) | 29.28 ± 1.76* | 28.23 ± 1.97* |
| ME(10 mg/ml) |
41.91 ± 1.65** |
32.76 ± 1.22** |
| Blank (water) and Streptokinase |
||
| Treatment | % of clot lysis | |
| Streptokinase | 58.49 ± 0.68*** | |
| Blank | 1.32 ± 0.77 |
Here, ME stands for methanolic extract and all the value expressed as mean ± SD where, level of significance sated at *** for p < 0.001, ** for p < 0.01, * for p < 0.05. Distilled water was employed as negative control and streptokinase was employed as positive control.
3.2. Membrane stabilizing activity
The crude methanolic extracts of both A. neriifolia and A. rotundifolia were also subjected to analyze membrane stabilizing activity using standard protocol and the acquired results were expressed statistically in Table 2, Table 3. Here, crude methanol extracts (ME) of different concentration showed different effect with a value of various inhibition of hemolysis caused by hypotonic solution and heat-induced hemolysis. In the case of hypotonic solution induced hemolysis, 10 mg/ml concentration of both plant extracts showed maximum % inhibition of hemolysis respectively 45.80% and 38.40% whereas the standard Acetylsalicylic acid (0.10 mg/mL) revealed 63.81% inhibition of hemolysis. Otherwise, in the case of heat-induced hemolysis, 10 mg/ml concentration of both plant extracts displayed 23.52% and 27.04% inhibition of hemolysis respectively while the standard showed 49.93% inhibition of hemolysis. Moreover, in membrane stabilizing potentiality of A. neriifolia showed higher value while comparing with A. rotundifolia.
Table 2.
Effects of crude methanolic extracts of both A. neriifolia and A. rotundifolia on hypotonic solution-induced hemolysis of erythrocyte membrane.
| Treatment | % inhibition of hemolysis of A. neriifolia | % inhibition of hemolysis of A. rotundifolia Roxb. |
|---|---|---|
| Blank | ||
| ME (2 mg/ml) | 22.80 ± 0.49 | 11.02 ± 0.68 |
| ME (4 mg/ml) | 30.80 ± 0.36 | 18.10 ± 0.99 |
| ME (6 mg/ml) | 35.30 ± 0.74 | 29.60 ± 0.73 |
| ME (8 mg/ml) | 40.80 ± 0.89* | 36.07 ± 0.89* |
| ME (10 mg/ml) | 45.80 ± 0.77** | 38.40 ± 0.94** |
| Acetyl salicylic acid (0.10 mg/ml) | 63.81 ± 0.84*** | 63.81 ± 0.84*** |
Here, Methanol was employed as control, ME stands for methanolic extract and all the value expressed as mean ± SD where, level of significance sated at *** for p < 0.001, ** for p < 0.01, * for p < 0.05.
Table 3.
Effects of crude methanolic extracts of both A. neriifolia and A. rotundifolia on heat induced hemolysis of erythrocyte membrane.
| Treatment | % inhibition of Hemolysis A. neriifolia | % inhibition of Hemolysis A. rotundifolia Roxb. |
|---|---|---|
| Blank | ||
| ME (2 mg/ml) | 12.94 ± 0.87 | 6.11 ± 0.67 |
| ME (4 mg/ml) | 16.60 ± 0.56 | 14.06 ± 0.61 |
| ME (6 mg/ml) | 18.33 ± 0.47 | 17.32 ± 0.51 |
| ME (8 mg/ml) | 21.94 ± 0.77* | 19.48 ± 0.83* |
| ME (10 mg/ml) | 23.52 ± 0.99** | 27.04 ± 0.94** |
| Acetyl Salicylic Acid (0.10 mg/ml) | 49.93 ± 0.76*** | |
Here, methanol was employed as control, ME stands for methanolic extract and all the value expressed as mean ± SD where, level of significance sated at *** for p < 0.001, ** for p < 0.01, * for p < 0.05.
3.3. Antibacterial activity
The effects of crude methanolic extracts of both A. neriifolia and A. rotundifolia on antibacterial screening have been represented in Table 4, Table 5. Crude methanolic extracts of A. rotundifolia did not show any considerable antibacterial activity against the test bacteria. As we didn't find any anti-microbial activity in gram negative (−ve) spectrum and also even in gram positive (+ve) spectrum. So, we can say that the test sample is resistant against both the gram positive and gram negative bacteria. The crude methanolic extracts of A. neriifolia were also resistant against gram positive (+ve) bacteria but it produced moderate antibacterial activity against gram-negative (−ve) bacteria such as: S. typhi and E. coli.
Table 4.
The statistical analysis of antibacterial activities of A. neriifolia.
| Test bacteria | Zone of inhibition of extract in various conc. | Zone of inhibition of Std. | ||||
|---|---|---|---|---|---|---|
| Gram positive (+ve) | Staphylococcus aureus | 25 μl | 50 μl | 75 μl | 100 μl | Ampicillin (10 μl) |
| _ | _ | _ | _ | +++ | ||
| Gram negative (−ve) | Salmonella typhi | _ | + | ++ 1 cm |
++ 1.1 cm |
Cefoxitin (30 μl) |
| +++ | ||||||
| E. coli | _ | _ | _ | ++ 1 cm |
Penicillin (10 μl) | |
| +++ | ||||||
| Pseudomonas aeruginosa | _ | _ | _ | _ | Imipenem (10 μl) | |
| +++ | ||||||
Here, (+++) = highly active; (++) = moderately active; (+) = slightly active; (−) = not active against microorganism.
Table 5.
The statistical analysis of antibacterial activities of A. rotundifolia.
| Test bacteria | Zone of inhibition of extract in various conc. | Zone of inhibition of Standard | ||||
|---|---|---|---|---|---|---|
| Gram positive (+ve) | Staphylococcus aureus | 25 μl | 50 μl | 75 μl | 100 μl | Ampicillin (10 μl) |
| _ | _ | _ | _ | +++ | ||
| Gram negative (−ve) | Salmonella typhi | _ | _ | _ | _ | Cefoxitin (30 μl) |
| +++ | ||||||
| E. coli | _ | _ | _ | _ | Penicillin (10 μl) | |
| +++ | ||||||
| Pseudomonas aeruginosa | _ | _ | _ | _ | Imipenem (10 μl) | |
| +++ | ||||||
Here, (+++) = highly active; (++) = moderately active; (+) = slightly active; (−) = not active against microorganism.
4. Discussion
WHO reported that almost 80% of world's population uses medicinal plant extracts or their constituents as folk medicine in traditional therapies.14 Our present study was an attempt to compare the clot lysis potentiality of methanolic extracts of A. neriifolia and A. rotundifolia. The comparison of the positive control (streptokinase) with negative control clearly demonstrated that clot dissolution does not occur when methanol was added to the clot. Encouraged by the result of the positive control, we compared five different concentrations of the test sample of two different plants in the same manner with the negative control and observed significant thrombolytic activity, where A. neriifolia dominated over A. rotundifolia. Nowadays, cell surface or blood vessel are blocked by the deposition of platelets, tissue factor, and fibrin through thrombosis or blood clot formation has become a critical event.29 During this biological process platelets are playing the major role in the process of thrombosis is initiated when the activated platelets form platelets to platelets bonds. Finally, a complex process of plaque formation and growth activated platelets is generated. Then activated platelets bind to the leucocytes.30 Most of the thrombolytic agents lyse clot by disrupting the fibrinogen and fibrin contained in a clot. Among all of them, plasmin is one of the natural anti-thrombotic agents, which is itself activated from cell surface bound plasminogen which is then ultimately leads to fibrinolysis.31 Very recently large number of research works have been undertaken to discover antithrombotic agents (anticoagulant and antiplatelet) from plants and natural food sources in order to the prevention of coronary events and stroke.32 At our present finding, we tried to find whether the herbal preparations of methanolic extract of A. neriifolia and A. rotundifolia. possess clot lysis potentiality or not, while we also compare their clot lysis potentiality to find out the better alternatives in place of a synthetic drug with untoward side effects. When we compared the result of positive control (streptokinase) with that of negative control (methanol), we found that there was a negligible amount of clot disruption when methanol was added to the clot. This prominent result encouraged us to compare five different test samples of two different plants in the same manner against the negative control and observe significant thrombolytic activity. It was reported that phytochemicals like saponin, alkaloids, and tannin are responsible for the thrombolytic activity.33 As the methanolic extract of the both of this plant possess saponin, alkaloids, therefore may be the probable reason for demonstrating the thrombolytic activity of both these plants.18, 20, 22
The present study is also an evidence that the methanolic extract of A. neriifolia and A. rotundifolia dose-dependently protect the human erythrocyte membrane against both hypotonic solution induced and heat induced condition, where A. neriifolia also dominated over membrane stabilizing potentiality of A. rotundifolia. During inflammation, phagocytes release many lysosomal enzymes and hydrolytic components to the extracellular space, which assists a variety of disorders by inducing damages of the surrounding organelles and tissues.34 Studies also evidence that non-steroidal anti-inflammatory drugs act through stabilization of lysosomal membranes by inhibiting these lysosomal enzymes. Again, lysis of the RBC membranes accompanied by the oxidation when exposed to harmful substances such as hypotonic medium, heat, etc through lysis of hemoglobin.35 Thus the mechanism of anti-inflammatory activity of the plant extract is assessed by considering their potentials in inhibition of hypotonicity and heat induced RBC membrane lysis because human RBC membranes are considered similar to lysosomal membrane components.36 One can also assume that the possible mode of action of the extract and standard anti-inflammatory drugs may be connected with binding to the erythrocyte membranes through consequent alteration of surface charges of cells. Some research works were able to reveal the name of some responsible chemical components present in the extracts, which are well known for their anti-inflammatory activity.37 Both in-vitro and in vivo studies in experimental animals showed that the flavonoids exert stabilizing effects largely on lysosomes,38 while tannin and saponins are also capable of stabilizing the erythrocyte membrane with an ability to bind with cations and other biomolecules.39 As both plant extracts possessed all of those respective phytochemicals which may be the predictable cause for generating membrane stabilizing activity.18, 40 Our present research reveals that plant methanolic extracts of A. neriifolia and A. rotundifolia showed potent RBC membrane stabilization activity with a good protection against both hypotonic solution and heat-induced lysis. Antibacterial properties of medicinal plants are being increasingly reported from different parts of the world. In the present work, the extracts obtained from A. rotundifolia show moderate activity against gram-negative bacterial strains. The results were compared with standard antibiotic drugs penicillin, cefoxitine, imipenem, and ampicillin. In this present screening, extracts of A. rotundifolia were found to not active against both gram-positive and gram-negative bacterial strains. This finding is supported by some previous reports done by the several workers.41, 42, 43, 44 A. rotundifolia possessed tannin, alkaloids and steroids might account for this and the antibacterial activity may be indicative of the presence of some metabolic toxins or broad-spectrum antibiotic compounds. In conclusion, the obtained results confirmed the presence of anti-microbial properties of A. rotundifolia while A. neriifolia missed, which supports the traditional use of this plant in various diseases caused by pathogenic microorganisms. Bioactivity-guided isolation can be carried out to separate bioactive metabolites.
5. Conclusions
In conclusion, the obtained results confirmed the presence of thrombolytic, membrane stabilizing activity of both plant extract and moderate antibacterial activity of A. rotundifolia which supports the traditional use of this plant in various diseases caused by pathogenic microorganisms and act as a basis for cardiovascular and anti-inflammatory drug screening from a natural source.
Contribution statement
Imam Hasan, Md. Shalahuddin Millat, and Md. Saddam Hussain were directly related to conducting this research work. Niloy Sen, Md. Abdur Rahman, Md. Atikur Rahman, and Md. Shafiqul Islam contributed during data generation, manuscript preparation, and collection of the plant part. All of these authors are acknowledged about the publication of this research work.
Conflict of interest
The authors declare they have no competing interests.
Acknowledgement
The authors would like to express their heartfelt gratitude, indebtedness, profound appreciation to Department of Pharmacy of Noakhali Science and Technology University for their continuous support, untiring inspiration, scholastic supervision, constructive criticism, affectionate feeling and optimistic counseling throughout the project work. Authors are also grateful to Department of Microbiology, Noakhali Science and Technology University, Sonapur-3814, Noakhali, Bangladesh for their utmost support during the preparation of bacterial cell culture.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Md. Saddam Hussain, Email: shussain070591@gmail.com.
Md. Mizanur Rahman Moghal, Email: mizanpharmbstu@gmail.com.
References
- 1.Sen N., Bulbul L., Hussain F., Amin M.T. Assessment of thrombolytic, membrane stabilizing potential and total phenolic content of Typha elephantina Roxb. J Med Plants Res. 2016;10:669–675. [Google Scholar]
- 2.Demrow H.S., Slane P.R., Folts J.D. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–1188. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- 3.Barrett B., Kiefer D., Rabago D. Assessing the risks and benefits of herbal medicine: an overview of scientific evidence. Altern Ther Health Med. 1999;5:40–49. [PubMed] [Google Scholar]
- 4.Duke J.A. Promising phytomedicinals. In: Janick J., Simon J.E., editors. Advances in New Crops. Timber Press; Portland: 1990. pp. 491–498. [Google Scholar]
- 5.Matu E.N., Staden J.V. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. J Ethnopharmacol. 2003;87:35–41. doi: 10.1016/s0378-8741(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 6.Islam A.K., Majumder A.A. Coronary artery disease in Bangladesh: a review. Indian Heart J. 2013;65:424–435. doi: 10.1016/j.ihj.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collen D. Coronary thrombolysis: streptokinase or recombinant tissue-type plasminogen activator? Ann Intern Med. 1990;112:529–538. doi: 10.7326/0003-4819-112-7-529. [DOI] [PubMed] [Google Scholar]
- 8.Basta G., Lupi C., Lazzerini G., Chiarelli P., L'Abbate A., Rovai D. Therapeutic effect of diagnostic ultrasound on enzymatic thrombolysis. An in vitro study on blood of normal subjects and patients with coronary artery disease. Thromb Haemost. 2004;91:1078–1083. doi: 10.1160/TH03-11-0684. [DOI] [PubMed] [Google Scholar]
- 9.Perianayagam J.B., Sharma S.K., Pillai S.K. Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J Ethnopharmacol. 2006;104:410–414. doi: 10.1016/j.jep.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 10.Anosike C.A., Obidoa O., Ezeanyika L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum) DARU J Pharm Sci. 2012;20:76. doi: 10.1186/2008-2231-20-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan A., Rahman M., Islam S. Antibacterial, antifungal and cyto-toxic activities of tuberous roots of Amorphophallus campanulatus. Turk J Biol. 2007;31:167–172. [Google Scholar]
- 12.Kunin C.M. Resistance to antimicrobial drugs – a worldwide calanity. Ann Intern Med. 1993;118:557–561. doi: 10.7326/0003-4819-118-7-199304010-00011. [DOI] [PubMed] [Google Scholar]
- 13.Kunin C.M. Antibiotic resistance – a world health problem we cannot ignore (Editorial) Ann Intern Med. 1983;99:859–860. doi: 10.7326/0003-4819-99-6-859. [DOI] [PubMed] [Google Scholar]
- 14.Bhalodia N.R., Shukla V.J. Antibacterial and antifungal activities from leaf extracts of Cassia fistula: an ethnomedicinal plant. J Adv Pharm Technol Res. 2011;2(2):104–109. doi: 10.4103/2231-4040.82956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke J.P., Levy S.B. Summary report of worldwide antibiotic resistance international task forces on antibiotic use. Rev Infect Dis. 1985;7:560–564. doi: 10.1093/clinids/7.4.560. [DOI] [PubMed] [Google Scholar]
- 16.Kunin C.M., Lipton H.L., Tupasi T., Sacks T., Scheckler W.F., Jivani A. Social, behavioral, and practical factors affecting antibiotic use worldwide: report of Task Force 4. Rev Infect Dis. 1987;9:270–285. doi: 10.1093/clinids/9.supplement_3.s270. [DOI] [PubMed] [Google Scholar]
- 17.Cohen M.L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 18.Neu H.C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 19.Sumathi R., Anuradha R. Phytochemical screening and in vitro antioxidant activity of methanolic extract of flowers of Allamanda neriifolia Hook. IJPPR. 2016;8(7):1111–1117. [Google Scholar]
- 20.Gilman E.F. University of Florida; Gainesville: 1999. Allamanda neriifolia. Institute of food and agricultural sciences Cooperative Extension Service, Institute of Food and Agricultural Sciences. [Google Scholar]
- 21.Blasco F., Aizpuru M., Gers C. Depletion of the mangroves of continental Asia. Wetl Ecol Manag. 2001;9:245–256. [Google Scholar]
- 22.Behera D.P., Nayak L. Floral diversity of Bhitarkanika, East coast of India and its potential uses. J Chem Biol Phys Sci. 2013;3(3):1863–1874. [Google Scholar]
- 23.Weber-El G., Magda O. The systematic relationships of Aegialitis (Plumbaginaceae) as revealed by pollen morphology. Plant Syst Evol. 1984;144(1):53–58. [Google Scholar]
- 24.Daginawala H.F., Prasad S., Kashyap R.S., Deopujari J.Y., Purohit H.J., Taori G.M. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb J. 2006;4:14. doi: 10.1186/1477-9560-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawsar M.H., Sikder M.A., Rana M.S., Nimmi I., Rashid M.A. Studies of thrombolytic and cytotoxic properties of two asteraceous plants of Bangladesh. Bang Pharm J. 2011;14(2):103–106. [Google Scholar]
- 26.Shinde U.A., Phadke A.S., Nair A.M., Mungantiwar A.A., Dikshit V.J., Saraf M.N. Membrane stabilizing activity – a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70:251–257. [Google Scholar]
- 27.Sikder M.A., Rahman M.A., Kaisar M.A., Rahman M.S., Hasan C.M., Rashid M.A. In vitro antioxidant, reducing power, free radical scavenging and membrane stabilizing activities of seeds of Syzygium cumini L. Lat Am J Pharm. 2011;30:781–785. [Google Scholar]
- 28.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by standardized single method. Am J Clin Path. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 29.Furie B., Furie B.C. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 30.Das A., Dewan S.M.R., Ali M.R., Debnath P.C., Billah M.M. Investigation of in vitro thrombolytic potential of ethanolic extract of Momordica charantia fruits: an anti-diabetic medicinal plant. Der Pharm Sin. 2013;4:104–108. [Google Scholar]
- 31.Pantzar M., Ljungh A., Wadström T. Plasminogen binding and activation at the surface of Helicobacter pylori CCUG 17874. Infect Immun. 1998;66:4976–4980. doi: 10.1128/iai.66.10.4976-4980.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain M.S., Hossain M.S., Amin M.T., Millat M.S. In vitro thrombolytic potentials of methanolic extract of Vigna unguiculata Linn (seed) J Pharmacog Phytochem. 2016;5(3):129–131. [Google Scholar]
- 33.Dewan S.M.R., Das A. Investigation of in vitro thrombolytic potential and phytochemical nature of Crinum latifolium L. leaves growing in coastal region of Bangladesh. Int J Bio Pharm Res. 2013;4:1–7. [Google Scholar]
- 34.Ackerman N.R., Beebe J.B. Release of lysosomal enzymes by alveolar mononuclear cells. Nature. 1974;247:475–477. doi: 10.1038/247475a0. [DOI] [PubMed] [Google Scholar]
- 35.Feirrali M., Signormi C., Ciccolili L., Comporti M. Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenylhydrazine, devicene and iso-uranil. Biochem J. 1992;285:295–301. doi: 10.1042/bj2850295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mounnissamy V.M., Kavimani S., Balu V., Drlin Q.S. Evaluation of anti-inflammatory and membrane stabilizing properties of ethanol extract of Canjera rehedi. Iran J Pharmacol Ther. 2007;6:235–237. [Google Scholar]
- 37.Vinod R.K., Chandrasekhar J., Sudhakar K., Rajeswar T., Sandhya S.K., Venkatramana K.R. Membrane stabilizing potency of two Tephrosia species. J Phytol. 2010;2(17):42–46. [Google Scholar]
- 38.Van-Cangeghen P. Influence of some hydrosolube substances with vitamin P activity on the fragility of lysosomes in vitro. Biochem Toxicol. 1972;11:1543–1548. doi: 10.1016/0006-2952(72)90303-6. [DOI] [PubMed] [Google Scholar]
- 39.Khan I., Nisar M., Ebad F. Anti-inflammatory activities of Sieboldogenin from Smilax china Linn. Experimental and computational studies. J Ethnopharmacol. 2009;121(1):175–177. doi: 10.1016/j.jep.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Raju G.S., Moghal M.M.R., Hossain M.S. Assessment of pharmacological activities of two medicinal plant of Bangladesh: Launaea sarmentosa and Aegialitis rotundifolia roxb in the management of pain, pyrexia and inflammation. Biol Res. 2014;47(1):55. doi: 10.1186/0717-6287-47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buwa L.V., van Staden J. Antibacterial and antifungal activity of traditional medicinal plants used against venereal diseases in South Africa. J Ethnopharmacol. 2006;103:139–142. doi: 10.1016/j.jep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Valsaraj R., Pushpangadan P., Smitt U.W., Adsersen A., Nyman U. Antimicrobial screening of selected medicinal plants from India. J Ethnopharmacol. 1997;58:75–83. doi: 10.1016/s0378-8741(97)00085-8. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan D., Nathan S., Suresh T., Lakshmanaperumalsamy P. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol. 2001;74:217–220. doi: 10.1016/s0378-8741(00)00345-7. [DOI] [PubMed] [Google Scholar]
- 44.Samy R.P., Ignacimuthu S. Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India. J Ethnopharmacol. 2000;69:63–71. doi: 10.1016/s0378-8741(98)00156-1. [DOI] [PubMed] [Google Scholar]


