Abstract
Platelets play a key role in thrombosis and cardiovascular diseases. Medicinal plants could be one of the most important factors that influence risks for platelet activation. Buddleja globosa (known as “matico”) is a medicinal plant with many biological activities. The high content of polyphenols suggest that matico could have antiplatelet activity. The present study was aimed at evaluating mechanisms of antiplatelet action of an extract of matico. We demonstrated that matico extract at low concentrations and in a concentration dependent manner (0.05–1 mg/mL) was a potent inhibitor of platelet aggregation in response to collagen, convulsion and ADP (IC50 values was 61 μg/mL, 72 μg/mL and 290 μg/mL, respectively). In this sense matico extract exerted the greatest antiaggregant activity induced by collagen. Similarly, matico showed a decrease in % of positive platelet for P-selectina (vehicle, 0.01, 0.05, 0.1, 0.5 and 1 mg/mL were 32 ± 2%, 29 ± 2 (p < 0.05), 19 ± 1 (p < 0.01), 15 ± 2 (p < 0.01), 10 ± 1% (p < 0.01) and 7 ± 2% (p < 0.01), respectively) and PAC-1 binding (vehicle, 0.01, 0.05, 0.1, 0.5 and 1 mg/mL were 59 ± 1, 58 ± 3 (n.s), 55 ± 2 (p < 0.05), 50 ± 2 (p < 0.01), 38 ± 1 (p < 0.01), 36 ± 2 (p < 0.01). The cellular mechanism for the antiplatelet activity of matico might be mediated by the inhibition of phospholipase C-gamma 2 and protein kinase C phosphorylation. This beneficial property of matico may be of importance in thrombosis, in which platelet activation and aggregation are important determinants of thrombus initiation and development, and may contribute to the beneficial effects of matico intake in the prevention of cardiovascular diseases.
Keywords: Buddleja globosa, Matico, Platelet, Collagen, Phospholipase C-gamma 2
Graphical abstract
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death throughout the world and in recent years has increased in frequency.1 Platelets play an important role in thrombosis and CVD; where a state of hyper-aggregation is associated with the presence of cardiovascular risk factors.2, 3
Patients with CVD using antiplatelet drugs present adverse effects,4 for this reason the search for new strategies to modulate platelet activity are needed. Different extracts have been shown to be relevant in the prevention of CVD, probably due to their phytoconstituents.5, 6
Buddleja globosa, known as “matico”, is a medicinal plant that mainly grows in the central zone of Chile, but also in Bolivia, Peru and Argentina. Matico is a plant often used in Mapuche culture applied for the treatment of wounds, intestinal and liver problems.7 Matico has bioactive compounds such as flavonoids (luteolin, luteolin 7-glucoside, linarina and caffeic acid derivatives), phenylethanoids, sterols, phenolic fatty acid ester and terpenes.8, 9, 10, 11, 12
Matico extract may be used topically or also as an infusion.11, 13 It has been reported that matico has effects on healing and wound closure modulating inflammation and oxidative stress.12 In this regard, a study in which mice consumed an extract of matico for 12 days showed that intake did not alter the blood count and biochemical parameters of the animals under study.14 In mice, the topical application of matico favors wound healing, correlating with decreased histological expression of ciclooxigenase (COX)-2 enzyme.14 In addition, antinociceptive effects of matico have been attributed to β-sitosterol, α and β-amyrins, luteolin 7-glucoside and verbascoside.11, 13
The use of matico extract for the treatment of gastric ulcers and other gastric disorders has been suggested.15 Furthermore, the protective effects in hepatocyte cultures exposed to cytotoxic compounds have been described. These effects are primarily mediated by flavonoids from matico.16
Studies evaluating the biological effects of matico are guided by empirical knowledge; however, no anti-thrombotic effects have been documented. Matico has a high content of compounds with anti-platelet effects, such as luteonin and verbascoside. This fact suggests that it could have effects on hemostasis. In this sense, other species of the genus Buddleja, such as Buddleja crispa17 and Buddleja thyrsoides18 have shown antiplatelet action. The present study aimed to determine the antiplatelet effects of B. globosa and evaluate possible mechanisms of action.
2. Materials and methods
2.1. Reagents
Adenosine 5′- diphosphate (ADP), collagen and prostaglandin E1 (PGE-1) were obtained from Sigma-Aldrich (St. Louis, Missouri/MO, U.S.A). Convulxin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Sodium chloride (p.a.) was obtained from Arquimed (Santiago, Chile). Antiphospho (S660)-PKC-β2 and antiphospho (Tyr753)-PLC-γ2 antibodies were obtained from Santa Cruz (Biotechnology, CA, USA). Anti γ-tubulin monoclonal antibody (4D11) was obtained from Thermo Scientific (Thermo Scientific, Pierce, Rockford, IL, USA). Antibodies (anti-CD62P-PE, anti-CD61-FITC, anti-GPIIb/IIIa-FITC PAC-1 and anti-CD61-PE) were obtained from BD Pharmingen (BD Biosciences, San Diego, CA, USA).
2.2. Matico recollection and authentication
B. globbosa leaves were collected from Linares city, Maule region, Chile, in January (summer) in 2016 and identified by an agricultural engineer. A voucher specimen (PRL-V03) was stored in our laboratory.
2.3. Preparation of extract from matico
The air-dried leaves of matico (50 g) were ground and successively extracted at room temperature with ethanol and after concentration under reduced pressure 1.4 g of extract was obtained (yield 2.8%). Then the extract was lyophilized and stored at −70 °C until use. Matico extract was dissolved in DMSO. The final concentration of DMSO used required as diluent was 0.2% (v/v), which does not affect platelet function.
2.4. Human platelet
The samples were taken from young healthy volunteers in 3.2% sodium. Platelet-rich plasma (PRP) was obtained by centrifugation at 240 g (DCS-16 Centrifugal Presvac RV) for 10 min and adjusted to 200 × 109 platelets/L with platelet-poor plasma (PPP). Samples for washing platelet were collected with ACD-A containing 50 ng/mL of PGE-1 (sample/anticoagulant ratio 9:1). Platelet was pellet with 50 ng/mL PGE-1 by centrifugation at 750 g for 5 min, resuspended in solution (CaCl2, MgCl2 1, 0.1% dextrose, pH 7.4) and adjusted to 200 × 109 platelets/L. Hematologic counter (Bayer Advia 60 Hematology System, Tarrytown, NY, USA) was used for platelet counts. Protocol was approved by the ethics committee of the Universidad de Talca.
2.5. Flow cytometry study
Expression of P-selectin and glycoprotein (GP)IIb/IIIa on platelet surface were analyzed by double-label flow cytometry by method previously described by Fuentes et al.19 480 μL of PRP (200 × 109 platelets/L) were incubated with concentrations of matico ranged from 0.01 to 1 mg/mL for 3 min. Then, each sample was treated for 6 min at 37 °C with ADP 8 μmol/L. An aliquot of 50 μL was taken and mixed with saturated concentrations of anti-CD62P-PE and anti-CD61-FITC for P-selectin expression, or incubated with anti-GPIIb/IIIa antibody PAC-1 and anti-CD61-PE for GPIIb/IIIa activation. The sample was incubated for 25 min in the dark. Platelet populations were gated on cell size using CD61 positivity and forward scatter (FSC) vs. side scatter (SSC), and analyzed over 5000 events in Accuri C6 flow cytometer (BD, Biosciences, USA). The results represent the mean and standard deviation of three independent determinations.
2.6. Platelet aggregation study
Effects of matico on platelet aggregation were evaluated using a lumi-aggregometer (Chrono-Log, Havertown, PA, USA). Matico (final concentration 0.01–1 mg/mL) was added to 480 μL of PRP (200 × 109 platelets/L) and incubated 3 min at 37 °C in constant agitation before addition of agonist collagen (1.5 μg/mL), convulxin (20 ng/mL) or ADP (8 μmol/L). The effect of matico on platelet aggregation was followed for 6 min and compared with saline control. Maximal amplitude (%) was measured by AGGRO/LINK software (Chrono-Log, Havertown, PA, USA).
The results represent the mean and standard deviation of three independent determinations.
2.7. Western blotting study
The samples were treated as in aggregation assay and these were activates with collagen or ADP (1.5 μg/mL or 8 μmol/L, respectively). When the reaction was stopped the samples were centrifuged and lised with lysis bufferr (50 mM Tris-HCl, 50 mM NaCl, 1 mM MgCl2, 1 mM EDTA, 0.1% Triton® X-100 at pH 7.4). 20 μg of protein from each samples were subjected to SDS/PAGE, immunoblotted and probed with antiphospho (Tyr753)- PLC-γ2, antiphospho (S660)-PKC-β2 or an anti-γ-tubulin antibody overnight at 4 °C. Membranes were incubated with a goat anti-rabbit secondary antibody linked to horseradish peroxidase and phosphoproteins were detected using an enhanced chemiluminescence method. Analysis of individual protein was quantified by densitometry with ImageJ software. The results represent the mean and standard deviation of three independent determinations.
2.8. Statistical analysis
Data were expressed as mean ± standard deviation (SD) and analyzed using Prism 6.0 software (GraphPad Inc., San Diego CA, USA). All measurements were performed from six separate platelet donors. Results were expressed as percentage of vehicle (as 100%). Fifty-percent inhibitory concentration (IC50) of matico extract against agonist-induced platelet aggregation was calculated from the dose-response curves. Differences between groups were analyzed by nonparametric Kruskal–Wallis statistical test and the Mann–Whitney as post-test for cases where there were significant differences. P values < 0.05 were considered significant.
3. Results
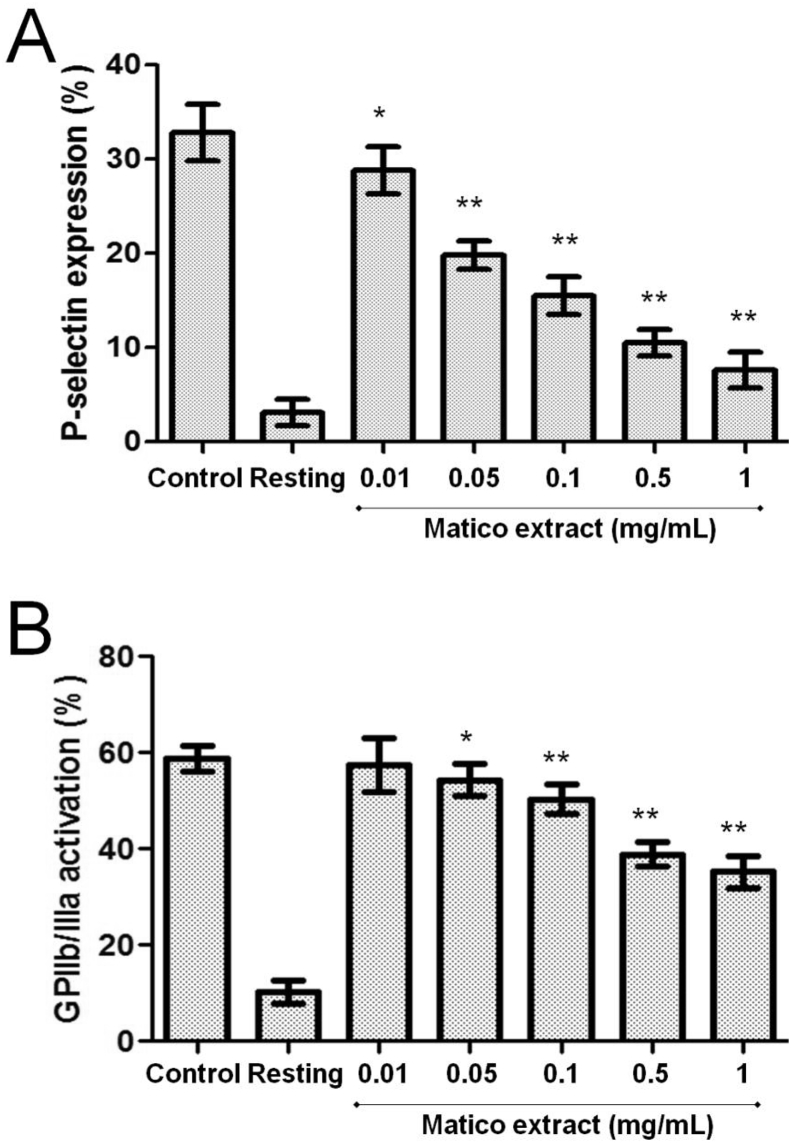
3.1. Matico inhibits platelet P-selectin expression in human platelets
Platelet P-selectin expression is a thrombo-inflammatory protein involved in platelet activation and thrombus formation. In this study, we used matico extract at 0.01, 0.05, 0.1, 0.5 and 1 mg/mL to evaluate its inhibitory activity on platelet P-selectin expression ADP-induced using flow cytometry. As shown in Fig. 1A, ADP 8 μmol/L induced platelet P-selectin expression was inhibited by different concentrations of matico extract. Thus P-selectin expression in presence of matico extract at 0.01, 0.05, 0.1, 0.5 and 1 mg/mL was inhibited from 32 ± 2% in the control group to 29 ± 2 (p < 0.05), 19 ± 1 (p < 0.01), 15 ± 2 (p < 0.01), 10 ± 1% (p < 0.01) and 7 ± 2% (p < 0.01), respectively.
Fig. 1.
Effect of matico extract on platelet activation. Platelet activation (P-selectin expression and GPIIb/IIIa activation) was determined by flow cytometry. Bar Graphs expressed as mean ± SD, n = 6. *p < 0.05 and **p < 0.01 compared with control analyzed by Mann–Whitney t-test.
3.2. Matico inhibits GPIIb/llla activation in human platelets
The GPIIb/IIIa is an integrin complex found on platelet membrane. GPIIb/IIIa activation represents the first phase of the platelet-aggregation process. In this assay we used PAC-1 that reacts with activation-induced conformational epitope of the GPIIb/IIIa complex. Matico extract (0.01, 0.05, 0.1, 0.5 and 1 mg/mL) concentration-dependently attenuated ADP induced platelet GPIIb/IIIa activation at 3 min after ADP stimulation (Fig. 1B). The above results indicate that inhibition of GPIIb/IIIa activation may contribute to matico extract action in activated platelets.
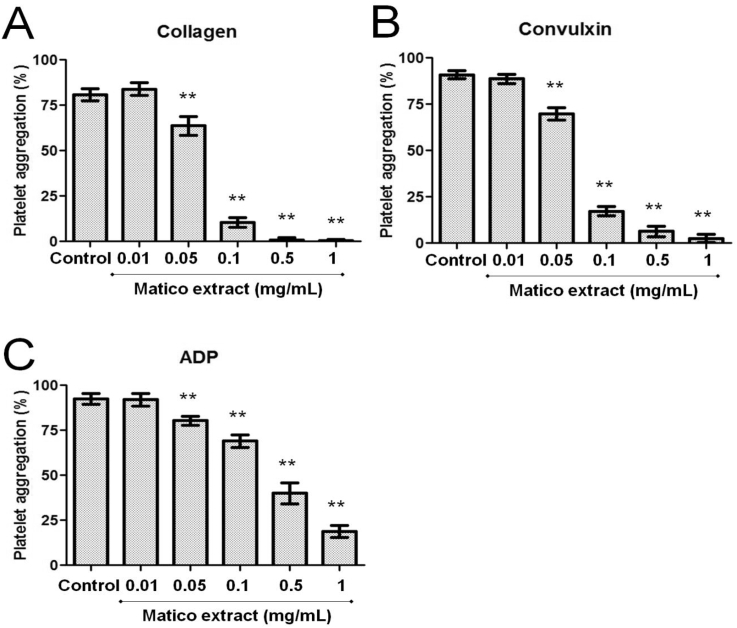
3.3. Matico inhibits human platelet aggregation
We determined whether matico extract affected platelet aggregation induced by collagen 1.5 μg/mL, convulxin 20 ng/mL or ADP 8 μmol/L. Matico extract (0.01–1 mg/mL) exhibited potent activity of inhibiting platelet aggregation stimulated by collagen 1.5 μg/mL, convulxin 20 ng/mL and ADP 8 μmol/L (Fig. 2). The IC50 values of matico extract for platelet aggregation induced by collagen, convulxin and ADP were approximately 61 μg/mL, 72 μg/mL and 290 μg/mL, respectively.
Fig. 2.
Effect of matico on human platelet aggregation induced by different agonists (collagen, convulxin and ADP). Results (bar graphs) were expressed as mean ± SD, n = 6. **p < 0.01 compared with control analyzed by Mann–Whitney t-test.
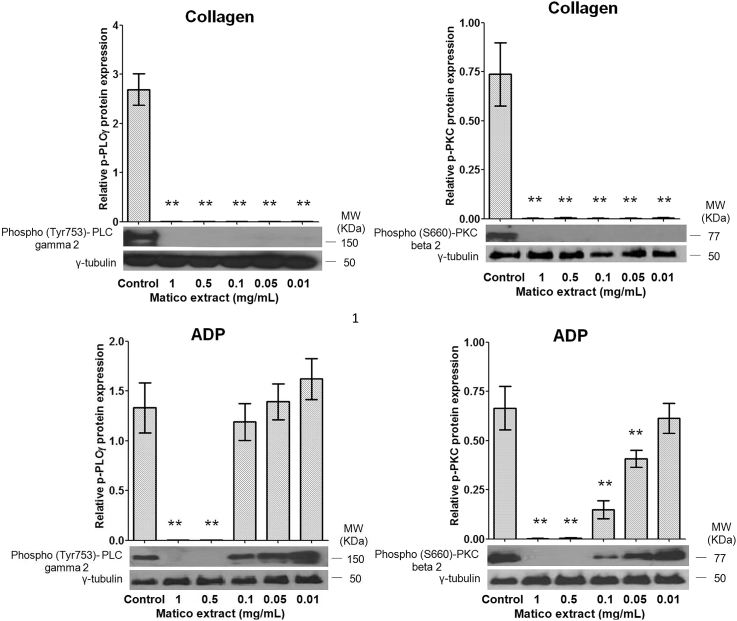
3.4. Matico inhibits PLC-γ2 and PKC-β2 phosphorylation in human platelets
Phosphorylation of PLC-γ2 and PKC-β2 is one of the key mechanisms regulating the activity of activated platelet in human platelets. In order to elucidate the mechanism underlying the effects of matico extract on platelets, we further examined the phosphorylation of downstream signaling protein of collagen including PLC-γ2 and PKC-β2. Our immunoblot study revealed that phosphorylation of PLC gamma 2 and PKC beta 2 was almost completely blocked by pre-incubation with different concentrations of matico extract (Fig. 3).
Fig. 3.
Effect of matico on protein phosphorylation of PLC-γ2 and PKC-β2 in human platelets. Results (bar graphs) were expressed as mean ± SD, n = 6. **p < 0.01 compared with control analyzed by Mann–Whitney t-test.
4. Discussion
In this study we demonstrated for first time that matico extract (B. globosa), at a low concentration and in a concentration dependent manner, was a potent inhibitor of platelet aggregation in response to collagen, convulsion and ADP. In this sense matico extract exerted the greatest antiaggregant activity induced by collagen. Similarly, matico showed a decrease of P-selectin expression from α-granules and GPIIb/IIIa expression. The cellular mechanism for the antiplatelet activity of matico might be mediated by the inhibition of PLC-γ2 and PKC-β2 phosphorylation.
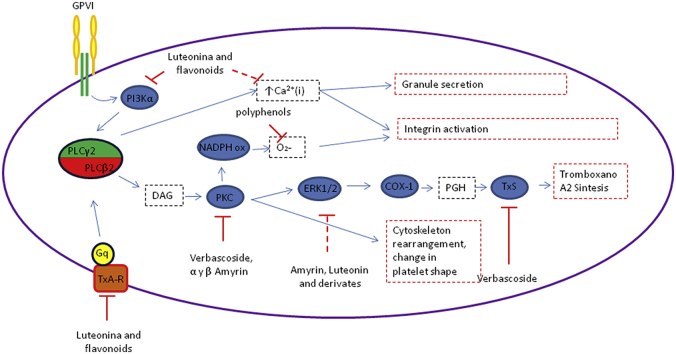
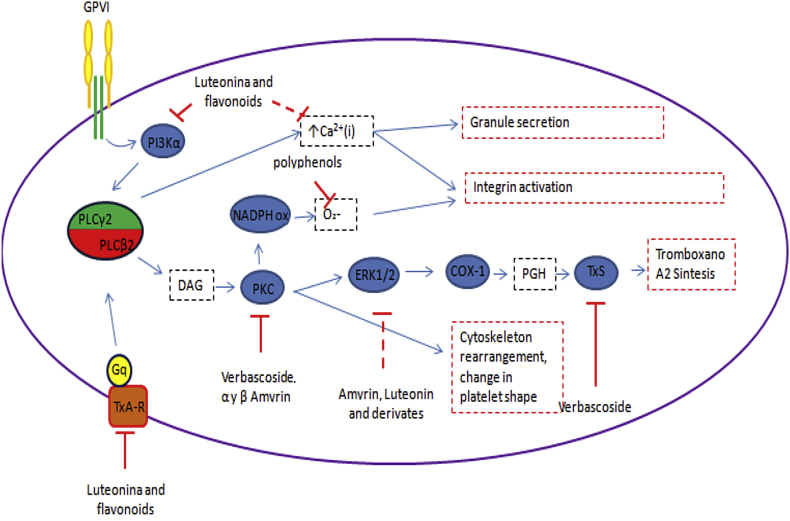
Matico extract is rich in polyphenols and flavonoids compound (Table 1) with high anti-oxidant an anti-inflammatory activity.20 This is able to modulate platelet via a common mechanism. Reactive oxygen species (ROS) are produced in platelet mostly by NAPDH oxidase enzyme. ROS are important in platelet activity. Polyphenols can counter them acting as scavengers and also modulating NADPH oxidase activity.21, 22 Certain flavonoids are able to bind and cause antagonism in thromboxane receptors, among them luteolin, the principal flavonoid present in B. globose, can inhibit TxA2-R mediated activation, impair release of Ca2+ and ERK 1/2 phosphorilation.23 TxA2-R is important in collagen-induced activation. Luteolin, among other flavonoids, reduces collagen activation acting on TxA2-R.23 Furthermore, luteolin is able to cause inhibition of PI3Kα, a pivotal enzyme in collagen-induced activation.24
Table 1.
Compound's detected in Matico.
| Compound | mg/g | Solvent extract | Type compound | Plant part | Place | Reference |
|---|---|---|---|---|---|---|
| Luteolin | 3.3 | Water | Flavonoids | Fresh leaves | London garden | 9 |
| 6-hydroxyluteolin | 2.5 | Water | Flavonoids | Fresh leaves | London garden | 9 |
| Linarin | 58 | Water | Flavonoids | Fresh leaves | London garden | 9 |
| Echinacoside | 75 | Water | Phenylethanoids | Fresh leaves | London garden | 9 |
| α-amyrins | 4.5 | Hexane | Triterpenes | Dried leaves | Temuco, IX Region, Chile | 11 |
| β-amyrins | 6.7 | Hexane | Triterpenes | Dried leaves | Temuco, IX Region, Chile | 11 |
| β-sitosterol glucoside | 6.5 | CH2Cl2 | Steroid | Dried leaves | Temuco, IX Region, Chile | 11 |
| β-sitosterol glucoside | 1 | Metanol | Steroid | Dried leaves | Temuco, IX Region, Chile | 11 |
| Luteolin 7-O-glucoside | 11 | Metanol | Flavonoids | Dried leaves | Temuco, IX Region, Chile | 11 |
| Apigenin 7-O-glucoside | 0.25 | Metanol | Flavonoids | Dried leaves | Temuco, IX Region, Chile | 11 |
| Buddledin A | 47 | CHCl3 | Sequiterpenos | Roots | London garden | 12 |
| Buddledin B | 1.3 | CHCl3 | Sequiterpenos | Roots | London garden | 12 |
| Buddledin C | 1.5 | CHCl3 | Sequiterpenos | Roots | London garden | 12 |
| Zerumbone | 1 | CHCl3 | Sequiterpenos | Roots | London garden | 12 |
| Dihydrobuddledin A | 3.5 | CHCl3 | Terpenoid | Roots | London garden | 12 |
| Buddledone A | 0.4 | CHCl3 | Diterpenoid | Roots | London garden | 12 |
| Buddledone B | 0.4 | CHCl3 | Diterpenoid | Roots | London garden | 12 |
| Verbascoside | 92 | Water | Phenylethanoids | Fresh leaves | London garden | 9 |
| 1.2 | Ethanol | Phenylethanoids | Dried leaves | Cunco, IX Region, Chile | 8 | |
| 17.5 | Metanol | Phenylethanoids | Dried leaves | Temuco, IX Region, Chile | 11 |
Recent studies have shown that verbascoside possesses antiplatelet effects, inhibiting the aggregation induced by ADP and AA in vitro,25 as well as in patients with high cardiovascular risk.26 Verbascoside acts on the arachidonic acid pathway, particularly in the synthesis of TxA2, probably interfering with the enzyme thromboxane synthase.27 Its role as a PKC inhibitor has also been described. Verboscoside inhibits the activity of this enzyme in a concentration-dependent manner by competing with ATP.28
Alpha and beta amyrin: in vitro assays have shown that amyrins inhibited platelet aggregation induced by different agonists,29 in particular β-amyrin inhibited collagen induced aggregation with IC50 lower than Aspirin.30 α-Amyrin, has also shown to be a competitive inhibitor of PKC.31 In another model, Amyrins disrupted both MAPK and AA metabolisms related to PKC upstream inhibition.32 Mechanism of platelet inhibition of compounds present in matico is summarized in Fig. 4.
Fig. 4.
Possible antiplatelet mechanism of compounds from matico.
In platelet aggregation study, matico inhibited collagen and convulxin-mediated platelet aggregation in a concentration-dependent manner, and the IC50 value for collagen and convulxin was more potent than that for ADP. In addition, matico plays an important role in the inhibition of GPIIb/IIIa in activated platelets. Accordingly the granule secretion, which was determined as the level of platelet P-selectin expression, was inhibited by matico in the same pattern as inhibition of platelet aggregation. Because the collagen-mediated platelet activation is believed to be caused by activation of PLC-γ2 and PKC-β2, we further examined the effect of matico extract on PLC-γ2 and PKC-β2 activation.
Convulxin, a potent platelet aggregating protein from snake venom of the Crotalus durissus terrificus is known to bind to the platelet collagen receptor, GPVI.33 The downstream signaling pathway of collagen and convulxin induces platelet activation of GPVI through a tyrosine kinase-based signaling pathway that involves the kinase Syk and PLC-γ2, which results in PKC-β2 activation and (Ca2+)i increase. In fact, the activation of this signaling pathway results in shape change and granule release (expression of P-selectin), and platelet adhesion and aggregation (activation of GPIIb/IIIa).34 In this sense, collagen-induced time-dependent increase of PLC-γ2 and PKC-β2 phosphorylation was completely abrogated after treatment of matico extract. These results suggest that inhibition of PLC-γ2 phosphorylation may be important in the inhibition of matico on collagen-mediated platelet aggregation.
In summary, our study showed that matico was able to inhibit the phosphorylation of PLC-γ2 and PKC-β2, both are downstream signaling pathways of collagen induced platelet activation. This suggests that B. globosa may be useful in CVD prevention, since platelet Hyperreactivity is an important determinant of thrombosis related diseases.
This beneficial property of matico must be tested by in vivo assays. Studies that have evaluated the in vivo anti-inflammatory and anti-nociceptive effects of B. globosa use a range from 200 to 600 mg/kg.11, 13 Future studies on the antiplatelet effects of matico would consider this range of concentrations. In clinical trials, different plant extracts have shown to induce protection against CVD development.35 In view of our results, B. globosa consumption could induce cardioprotective effects in addition to their known properties. Unfortunately, to date, the biological effects are not supported by any rigorous clinical studies. Only one study has focused on evaluating the healing effects of topical use of this plant in patients with wounds, however results are not yet available.36 Our results suggest a potential caridioprotective effect not previously described, supporting the evaluation of these effects on people. It is also necessary to contrast empirical knowledge and evaluate toxicological effects.
Conflict of interest
The authors report no conflict of interest.
Acknowledgements
This work was funded by grant no. No 11140142 (FONDECYT Initiation from Eduardo Fuentes), CONICYT REGIONAL/GORE MAULE/CEAP/R09I2001 and Interdisciplinary Excellence Research Program on Healthy Aging (PIEI-ES).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Iván Palomo, Email: ipalomo@utalca.cl.
Eduardo Fuentes, Email: edfuentes@utalca.cl.
References
- 1.Palomo G.I., Icaza N.G., Mujica E.V. Prevalence of cardiovascular risk factors in adult from Talca, Chile. Rev Med Chil. 2007;135:904–912. doi: 10.4067/s0034-98872007000700011. [DOI] [PubMed] [Google Scholar]
- 2.Willoughby S., Holmes A., Loscalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nurs. 2002;1:273–288. doi: 10.1016/s1474-5151(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri Z.M. Mechanisms initiating platelet thrombus formation. Thromb Haemost. 1997;78:611–616. [PubMed] [Google Scholar]
- 4.Kim A.J., Lim H.J., Ro H. Low-dose aspirin for prevention of cardiovascular disease in patients with chronic kidney disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwivedi S., Chopra D. Revisiting Terminalia arjuna – an ancient cardiovascular drug. J Tradit Complement Med. 2014;4:224–231. doi: 10.4103/2225-4110.139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin H.H., Charles A.L., Hsieh C.W., Lee Y.C., Ciou J.Y. Antioxidant effects of 14 Chinese traditional medicinal herbs against human low-density lipoprotein oxidation. J Tradit Complement Med. 2014;5:51–55. doi: 10.1016/j.jtcme.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estomba D., Ladio A., Lozada M. Medicinal wild plant knowledge and gathering patterns in a Mapuche community from North-western Patagonia. J Ethnopharmacol. 2006;103:109–119. doi: 10.1016/j.jep.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Pardo F., Perich F., Villarroel L., Torres R. Isolation of verbascoside, an antimicrobial constituent of Buddleja globosa leaves. J Ethnopharmacol. 1993;39:221–222. doi: 10.1016/0378-8741(93)90041-3. [DOI] [PubMed] [Google Scholar]
- 9.Mensah A., Sampson J., Houghton P. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J Ethnopharmacol. 2001;77:219–226. doi: 10.1016/s0378-8741(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 10.Houghton P.J., Mensah A.Y. Springer; 1999. Biologically Active Compounds from Buddleja Species. Phytochemicals in Human Health Protection, Nutrition, and Plant Defense; pp. 343–368. [Google Scholar]
- 11.Backhouse N., Rosales L., Apablaza C. Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa, Buddlejaceae. J Ethnopharmacol. 2008;116:263–269. doi: 10.1016/j.jep.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Liao Y.-H., Houghton P.J., Hoult J. Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J Nat Prod. 1999;62:1241–1245. doi: 10.1021/np990092+. [DOI] [PubMed] [Google Scholar]
- 13.Backhouse N., Delporte C., Apablaza C. Antinociceptive activity of Buddleja globosa (matico) in several models of pain. J Ethnopharmacol. 2008;119:160–165. doi: 10.1016/j.jep.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Letelier M.E., Jones R., López C. Safety profile and wound healing properties of a standardized Buddleja globosa hope (matico) extract in sprague-dawley rats. Rev Farmacol Chile. 2012;5:13–19. [Google Scholar]
- 15.Munoz M.E.L., Salas E.A.O. Use of a standardised dry extract of leaves of Buddleja globosa hope, BG-126, for the treatment and prevention of gastrointestinal disorders caused by treatment with nitrofurantoin and other antimicrobials. Google Pat. 2014:1–19. Publication number US8852654 B2. [Google Scholar]
- 16.Houghton P.J., Hikino H. Anti-hepatotoxic activity of extracts and constituents of Buddleja species. Planta Medica. 1989;55:123–126. doi: 10.1055/s-2006-961903. [DOI] [PubMed] [Google Scholar]
- 17.Bukhari I.A., Gilani A.H., Meo S.A., Saeed A. Analgesic, anti-inflammatory and anti-platelet activities of Buddleja crispa. BMC Complement Altern Med. 2016;16:016–1021. doi: 10.1186/s12906-016-1021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahlke J.D., Boligon A.A., Machado M.M., Athayde M.L. In vitro toxicity, antiplatelet and acetylcholinesterase inhibition of Buddleja thyrsoides Lam. leaves. Nat Prod Res. 2012;26:2223–2226. doi: 10.1080/14786419.2011.643884. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes E., Badimon L., Caballero J. Protective mechanisms of adenosine 5′-monophosphate in platelet activation and thrombus formation. Thromb Haemost. 2014;111:491–507. doi: 10.1160/TH13-05-0386. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H.L., Zhang L.J., Liang Y.H. Antiinflammatory and antioxidant flavonoids and phenols from Cardiospermum halicacabum (Dao Di Ling) J Tradit Complement Med. 2013;3:33–40. doi: 10.4103/2225-4110.106541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pignatelli P., Di Santo S., Buchetti B., Sanguigni V., Brunelli A., Violi F. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. Faseb J. 2006;20:1082–1089. doi: 10.1096/fj.05-5269com. [DOI] [PubMed] [Google Scholar]
- 22.Carnevale R., Loffredo L., Pignatelli P. Dark chocolate inhibits platelet isoprostanes via NOX2 down-regulation in smokers. J Thromb Haemost. 2012;10:125–132. doi: 10.1111/j.1538-7836.2011.04558.x. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero J.A., Lozano M.L., Castillo J., Benavente-Garcia O., Vicente V., Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost. 2005;3:369–376. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- 24.Agullo G., Gamet-Payrastre L., Manenti S. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53:1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- 25.Campo G., Marchesini J., Bristot L. The in vitro effects of verbascoside on human platelet aggregation. J Thromb Thrombolysis. 2012;34:318–325. doi: 10.1007/s11239-012-0757-z. [DOI] [PubMed] [Google Scholar]
- 26.Campo G., Pavasini R., Biscaglia S. Platelet aggregation values in patients with cardiovascular risk factors are reduced by verbascoside treatment. A randomized study. Pharmacol Res. 2015;97:1–6. doi: 10.1016/j.phrs.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Diaz A.M., Abad M.J., Fernandez L., Silvan A.M., De Santos J., Bermejo P. Phenylpropanoid glycosides from Scrophularia scorodonia: in vitro anti-inflammatory activity. Life Sci. 2004;74:2515–2526. doi: 10.1016/j.lfs.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Herbert J.M., Maffrand J.P., Taoubi K., Augereau J.M., Fouraste I., Gleye J. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J Nat Prod. 1991;54:1595–1600. doi: 10.1021/np50078a016. [DOI] [PubMed] [Google Scholar]
- 29.Aragão G.F., Carneiro L.M., Júnior A.P., Bandeira P.N., Lemos T.L., Viana G.S.D.B. Antiplatelet activity of α.-and β.-amyrin, isomeric mixture from Protium heptaphyllum. Pharm Biol. 2007;45:343–349. [Google Scholar]
- 30.Ching J., Chua T.K., Chin L.C. Beta-amyrin from Ardisia elliptica Thunb. is more potent than aspirin in inhibiting collagen-induced platelet aggregation. Indian J Exp Biol. 2010;48:275–279. [PubMed] [Google Scholar]
- 31.Hasmeda M., Kweifio-Okai G., Macrides T., Polya G.M. Selective inhibition of eukaryote protein kinases by anti-inflammatory triterpenoids. Planta Med. 1999;65:14–18. doi: 10.1055/s-1999-13954. [DOI] [PubMed] [Google Scholar]
- 32.Medeiros R., Otuki M.F., Avellar M.C., Calixto J.B. Mechanisms underlying the inhibitory actions of the pentacyclic triterpene alpha-amyrin in the mouse skin inflammation induced by phorbol ester 12-O-tetradecanoylphorbol-13-acetate. Eur J Pharmacol. 2007;559:227–235. doi: 10.1016/j.ejphar.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Niedergang F., Alcover A., Knight C.G. Convulxin binding to platelet receptor GPVI: competition with collagen related peptides. Biochem Biophys Res Commun. 2000;273:246–250. doi: 10.1006/bbrc.2000.2940. [DOI] [PubMed] [Google Scholar]
- 34.Lee J.Y., Woo E.-R., Kang K.W. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J Ethnopharmacol. 2005;97:561–566. doi: 10.1016/j.jep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Waltenberger B., Mocan A., Smejkal K., Heiss E.H., Atanasov A.G. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules. 2016;21 doi: 10.3390/molecules21060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bustamante S., Álvareza N. Fundamentación preclínica del uso etnomédico de matico (Buddleja globosa Hope) Rev Fitoter. 2015;15:37–51. [Google Scholar]