Abstract
Clerodendrum is a genus of ca. 500 species in the family Lamiaceae and widely distributed throughout the whole world. Up to now, many species of this genus have been described in various indigenous systems of medicine and are used in preparation of folklore medicines for the treatment of various life-threatening diseases, and more than eleven species of the Clerodendrum genus have been very well studied for their chemical constituents and biological activities, and 283 compounds, including monoterpene and its derivatives, sesquiterpene, diterpenoids, triterpenoids, flavonoid and flavonoid glycosides, phenylethanoid glycosides, steroids and steroid glycosides, cyclohexylethanoids, anthraquinones, cyanogenic glycosides, and others have been isolated and identified. Pharmacological studies have shown that these compounds and extracts from the Clerodendrum genus have extensive activities, such as anti-inflammatory and anti-nociceptive, anti-oxidant, anti-hypertensive, anticancer, antimicrobial, anti-diarrheal, hepatoprotective, hypoglycemic and hypolipidemic, memory enhancing and neuroprotective, and other activities. In this review, we attempt to highlight over phytochemical progress and list the phytoconstituents isolated from the genus Clerodendrum reported so far. The biological activities of this genus are also covered.
Keywords: Clerodendrum, Diterpenoids, Triterpenoids, Flavonoids, Phenylethanoid glycosides, Biological activity
Graphical abstract
1. Introduction
Clerodendrum is a genus of flowering plants in the family Lamiaceae (Verbenaceae).1 Its common names include glorybower, bagflower, and bleeding-heart. Estimates of the number of species in Clerodendrum vary widely, from about 1502 to about 500,1 and is native to tropical and warm temperate regions of the world, with most of the species occurring in tropical Africa and southern Asia, but with a few in the tropical Americas and northern Australasia, and a few extending north into the temperate zone in eastern Asia.3 Clerodendrum is a genus of small trees, shrubs, lianas, and sub herbaceousperennials. There are 40 species in mainland China, mainly spread in southern and southwest regions, including Clerodendrum serratum, Clerodendrum inerme, Clerodendrum bungei, Clerodendrum phlomidis, C. serratum var. amplexifolium, Clerodendron infortunatum, Clerodendrum trichotomum, Clerodendrum chinense, Clerodendrum petasites, Clerodendrum grayi, Clerodendrum indicum, and so on. C. trichotomum is a common ornamental in warmer parts of the world.3 Eight other species are also grown in the tropics for their abundant and attractive flowers.4 Both butterflies and hummingbirds are often attracted by blooming Clerodendrum.
Plants belonging to genus Clerodendrum are well known for their pesticidal properties,5 and various Clerodendrum species like C. indicum, C. phlomidis, C. serratum var. amplexifolium, C. trichotomum, C. chinense, C. petasites, etc. have been historically used as folk and traditional medicine to treat many kinds of diseases, such as cold, hyperpyrexia, asthma, furunculosis, hypertension, rheumatism, dysentery, mammitis, toothache, anorexia, leucoderma, leprosy, arthrophlogosis, and other inflammatory disease in various parts of the world such as India, China, Korea, Japan, Thailand, and Africa.6, 7, 8, 9 The traditional or ethnomedical claims of the species have also been evaluated. The biological activities of these species described in ancient literature have been reported to be associated with the chemical constituents present in the species.
A variety of constituents have been isolated and characterized from this genus, including: monoterpene and its derivatives,10 sesquiterpene,11 diterpenoids,12, 13 triterpenoids,14, 15 flavonoid and flavonoid glycosides,16 phenylethanoid glycosides,17, 18 steroids and steroid glycosides,19 cyclohexylethanoids,20 anthraquinones,21 cyanogenic glycosides,22 and others. Some of these constituents have been evaluated with a number of biological properties, mainly including anti-inflammatory and anti-nociceptive, anti-oxidant, anti-hypertensive, anticancer, antimicrobial, anti-diarrheal, hepatoprotective, hypoglycemic and hypolipidemic, memory enhancing and neuroprotective, and other activities.
In this review, we will summary all identified chemical constituents and biological activities from the genus Clerodendrum over the past few decades. It will provide a basis for the development of therapeutic agents and utilization of these plants in forthcoming studies.
2. Phytochemistry
To the best of our knowledge, over 280 chemical constituents have been isolated and identified from different species of the genus Clerodendrum, These compounds could be divided into: 27 monoterpene and its derivatives, 3 sesquiterpene, 58 diterpenoids, 31 triterpenoids, 43 flavonoid and flavonoid glycosides, 40 phenylethanoid glycosides, 43 steroids and steroid glycosides, 13 cyclohexylethanoids, 4 anthraquinones, 2 cyanogenic glycosides, and 19 others (Table 1). With respect to isolated phytochemicals of the genus, aerial parts, roots and leaves were the most common targets of investigation for bioactive principles and most of these compounds were reported from C. serratum, C. inerme, C. bungei, Clerodendrum incisum, C. infortunatum, and C. trichotomum. Diterpenoids, flavonoids, phenylethanoid glycosides, and steroids are abundant and major bioactive principles of this genus.
Table 1.
The phytochemicals obtained from the Clerodendrum genus plants.
| No. | Phytochemicals | Plant parts | Source | Ref. |
|---|---|---|---|---|
| Monoterpene and its derivatives | ||||
| 1 | Serratumin A | Aerial parts | C. serratum | 23 |
| 2 | Serratoside A | Aerial parts | C. serratum | 24 |
| 3 | Serratoside B | Aerial parts | C. serratum | 24 |
| 4 | 7-O-p-couma-royloxyugandoside | Aerial parts | C. serratum | 25 |
| 5 | Monomelittoside | Aerial parts | C. inerme | 26 |
| 6 | Melittoside | Aerial parts | C. inerme | 27 |
| 7 | Sammangaoside C | Aerial parts | C. inerme | 28 |
| 8 | Inerminosides A | Leaves | C. inerme | 10 |
| 9 | Inerminosides C | Leaves | C. inerme | 10 |
| 10 | Inerminosides D | Leaves | C. inerme | 10 |
| 11 | Inerminoside C heptaacetate | Aerial parts | C. inerme | 29 |
| 12 | Inerminoside A | Aerial parts | C. inerme | 29 |
| 13 | Inerminoside A hexaacetate | Aerial parts | C. inerme | 29 |
| 14 | Inerminoside B | Aerial parts | C. inerme | 29 |
| 15 | Inerminoside B heptaacetate | Aerial parts | C. inerme | 29 |
| 16 | 8-O-foliamenthoyleuphroside | Roots | C. incisum | 30 |
| 17 | 2′-O,8-O-difoliamenthoyleuphroside | Roots | C. incisum | 30 |
| 18 | Euphroside | Roots | C. incisum | 30 |
| 19 | Plantarenaloside | Roots | C. incisum | 30 |
| 20 | Aucubin | Whole plants | C. thomsonae | 27 |
| 21 | 8-O-acetylharpagide | Whole plants | C. thomsonae | 27 |
| 22 | Harpagide | Whole plants | C. thomsonae | 27 |
| 23 | Ajugoside | Leaves | C. thomsonae | 27 |
| 24 | 8-O-acetylmioporoside | Whole plants | C. thomsonae | 27 |
| 25 | Reptoside | Whole plants | C. thomsonae | 27 |
| 26 | Ugandoside | Whole plants | C. ugandense | 27 |
| 27 | 5-O-β-glucopyranosyl-harpagide | Aerial parts | C. chinense | 31 |
| Sesquiterpene | ||||
| 28 | Sammangaoside A | Aerial parts | C. inerme | 28 |
| 29 | Sammangaoside B | Aerial parts | C. inerme | 28 |
| 30 | 2-{(2S,5R)-5-[(1E)-4-hydroxy-4-methylhexa-1,5-dien-1-yl]-5-methyltetrahydrofuran-2-yl}propan-2-yl-β-d-glucopyranoside | Roots | C. bungei | 32 |
| Diterpenoids | ||||
| 31 | Mandarone A | Stems | C. mandarinorum | 33 |
| 32 | Mandarone B | Stems | C. mandarinorum | 33 |
| 33 | Mandarone C | Stems | C. mandarinorum | 33 |
| 34 | Crolerodendrum A | Whole plants | C. philippinum | 25 |
| 35 | Bungone A | Stems | C. bungei | 34 |
| 36 | Bungone B | Stems | C. bungei | 34 |
| 37 | Inerme A | Leaves | C. inerme | 35 |
| 38 | Inerme B | Leaves | C. inerme | 35 |
| 39 | 14,15-dihydro-15β-methoxy-3-epicaryoptin | Leaves | C. inerme | 35 |
| 40 | 14,15-dihydro-15-hydroxy-3-epicaryoptin | Leaves | C. inerme | 35 |
| 41 | Clerodermic acid | Whole plants | C. inerme | 36 |
| 42 | Cleroinermin | Whole plants | C. inerme | 37 |
| 43 | 3-epicaryoptin | Whole plants | C. paniculatum | 38 |
| 44 | Clerodin | Whole plants | C. paniculatum | 38 |
| 45 | Uncinatone | Stems | C. trichotomum | 39 |
| Roots | C. bungei | 40 | ||
| Roots | C. trichotomum | 41 | ||
| Aerial parts | C. inerme | 42 | ||
| 46 | 2-acetoxyclerodendrin B | Whole plants | C. infortunatum | 25 |
| 47 | Clerodendrin A | Whole plants | C. trichotomum | 38 |
| 48 | Clerodendrin B | Whole plants | C. trichotomum | 38 |
| 49 | Clerodendrin C | Whole plants | C. trichotomum | 38 |
| 50 | Clerodendrin D | Whole plants | C. trichotomum | 38 |
| 51 | Clerodendrin E | Whole plants | C. trichotomum | 38 |
| 52 | Clerodendrin F | Whole plants | C. trichotomum | 38 |
| 53 | Clerodendrin G | Whole plants | C. trichotomum | 38 |
| 54 | Clerodendrin H | Whole plants | C. trichotomum | 38 |
| 55 | Trichotomone | Roots | C. trichotomum | 43 |
| 56 | Sugiol | Stems | C. trichotomum | 39 |
| 57 | Teuvincenone A | Stems | C. trichotomum | 39 |
| 58 | Teuvincenone B | Stems | C. trichotomum | 39 |
| 59 | Teuvincenone F | Stems | C. trichotomum | 39 |
| Roots | C. bungei | 40 | ||
| Roots | C. trichotomum | 41 | ||
| 60 | Teuvincenone H | Stems | C. trichotomum | 39 |
| 61 | Cyrtophyllone B | Stems | C. trichotomum | 39 |
| 62 | Bungnate A | Roots | C. bungei | 40 |
| 63 | Bungnate B | Roots | C. bungei | 40 |
| 64 | 15-dehydrocyrtophyllone A | Roots | C. bungei | 40 |
| 65 | 15-dehydro-17-hydroxycyrtophyllone A | Roots | C. bungei | 40 |
| 66 | 12,16-epoxy-11,14,17-trihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13-tetraene-7-one | Roots | C. bungei | 40 |
| 67 | Cyrtophyllone A | Roots | C. bungei | 40 |
| 68 | Villosin C | Roots | C. bungei | 40 |
| Roots | C. trichotomum | 41 | ||
| 69 | 19-hydroxyteuvincenone F | Roots | C. bungei | 40 |
| 70 | Mandarone E | Roots | C. bungei | 40 |
| Roots | C. trichotomum | 41 | ||
| 71 | 12,16-epoxy-11,14-dihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13,15-pentaene-3,7-dione | Roots | C. bungei | 40 |
| Roots | C. trichotomum | 41 | ||
| 72 | 12-O-β-d-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene | Roots | C. bungei | 40 |
| 73 | 6-methoxyvillosin C | Roots | C. trichotomum | 41 |
| 74 | 18-hydroxy-6-methoxyvillosin C | Roots | C. trichotomum | 41 |
| 75 | (10R,16S)-12,16-epoxy-11,14-dihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13-tetraene-3,7-dione | Roots | C. trichotomum | 41 |
| 76 | (10R,16S)-12,16-epoxy-11,14-dihydroxy-18-oxo-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13-pentaene-7-one | Roots | C. trichotomum | 41 |
| 77 | (10R,16R)-12,16-epoxy-11,14,17-trihydroxy-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13-pentaene-2,7-dione | Roots | C. trichotomum | 41 |
| 78 | (3S,4R,10R,16S)-3,4:12,16-diepoxy-11,14-dihydroxy-17(15→16),18(4→3)-diabeo-abieta-5,8,11,13-tetraene-7-one | Roots | C. trichotomum | 41 |
| 79 | 12,16-epoxy-11,14-dihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13,15-pentaene-3,7-dione | Roots | C. trichotomum | 41 |
| 80 | Formidiol | Roots | C. trichotomum | 41 |
| 81 | Teuvincenone E | Roots | C. trichotomum | 41 |
| 82 | 12,16-epoxy-17(15→16),18(4→3)-diabeo-abieta-3,5,8,12,15-pentaene-7,11,14-trione | Roots | C. trichotomum | 41 |
| 83 | 3β-(β-d-glucopyranosyl)isopimara-7,15-diene-11α,12α-diol | Roots | C. bungei | 44 |
| 84 | 16-O-β-d-glucopyranosyl-3β-20-epoxy-3-hydroxyabieta-8,11,13-triene | Roots | C. bungei | 44 |
| 85 | Coleon U | Whole plants | C. canescens | 45 |
| 86 | Coleon U-12-methyl ether | Whole plants | C. canescens | 45 |
| 87 | Cleroserroside A | Aerial parts | C. serrartum | 46 |
| 88 | Cleroserroside B | Aerial parts | C. serrartum | 46 |
| Triterpenoids | ||||
| 89 | 3-O-acetyloleanolicacid | Aerial parts | C. inerme | 42 |
| 90 | 3-O-acetyloleanolicaldehyde | Aerial parts | C. inerme | 42 |
| 91 | Glutinol | Aerial parts | C. inerme | 42 |
| 92 | Friedelin | Leaves | C. trichotomum | 47 |
| Aerial parts | C. inerme | 48 | ||
| 93 | Taraxerol | Roots | C. indicum | 49 |
| Whole plants | C. bungei | 50 | ||
| Leaves | C. trichotomum | 47 | ||
| 94 | Clerodone | Whole plants | C. bungei | 51 |
| 95 | α-amyrin | Whole plants | C. bungei | 51 |
| 96 | Glochidone | Whole plants | C. bungei | 50 |
| 97 | Glochidonol | Whole plants | C. bungei | 50 |
| 98 | Glochidiol | Whole plants | C. bungei | 50 |
| 99 | Lupeol | Roots | C. indicum, C. villosum | 49, 52 |
| Leaves | C. trichotomum | 47 | ||
| Whole plants | C. canescens | 51 | ||
| Aerial parts | C. inerme | 42 | ||
| 100 | α-amyrin 3-undecanotate | Whole plants | C. canescens | 51 |
| 101 | Lupeol acetate | Whole plants | C. canescens | 51 |
| 102 | Lupeol 3-palmitate | Whole plants | C. canescens | 51, 52 |
| 103 | Melastomic acid | Whole plants | C. canescens | 51 |
| 104 | β-amyrin acetate | Whole plants | C. canescens | 51 |
| 105 | Betulinic acid | Roots | C. villosum | 49, 52 |
| Aerial parts | C. inerme | 53 | ||
| Leaves | C. trichotomum | 47 | ||
| Whole plants | C. canescens | 51 | ||
| 106 | Magnificol | Aerial parts | C. inerme | 42 |
| 107 | Glutinone | Aerial parts | C. inerme | 42 |
| 108 | Mi-saponin | Roots | C. wildii | 54 |
| 109 | Basic acid | Roots | C. wildii | 54 |
| 110 | Protobassic | Roots | C. wildii | 54 |
| 111 | Mi-glycoside I | Roots | C. wildii | 54 |
| 112 | Ursolic acid | Roots | C. japonicum | 55 |
| 113 | 3β-hydroxy-D:B-friedo-olean-5-ene | Roots | C. indicum, C. villosum | 49 |
| 114 | Oleanolic acid | Whole plants | C. serratum | 56 |
| 115 | Oleanolic acid-3-acetate | Roots | C. indicum | 49 |
| 116 | Taraxerol-3β-yloctacosanoate | Roots, stems | C. philippinum | 57 |
| 117 | Se-saponin | Aerial parts | C. serratum | 58 |
| 118 | Lup-1,5,20(29)-trien-3-O-d-glucopyranoside | Leaves | C. inerme | 59 |
| 119 | Clerodendrumic acid | Leaves | C. glabrum | 60 |
| Flavonoid and flavonoid glycosides | ||||
| 120 | 5,7,8,4′-tetrahydroxy-6-methoxy-flavone | Aerial parts | C. serratum | 23 |
| 121 | 5,6,7-trihydroxy-4′-methoxyflavone 7-glucopyranoside | Aerial parts | C. serratum | 23 |
| 122 | 5, 7, 4′-trihydroxy-3′-methoxyflavone | Whole plants | C. serratum | 25 |
| 123 | Astragalin | Whole plants | C. philippinum | 61 |
| 124 | Apigenin | Aerial parts | C. inerme | 48 |
| 125 | Tricin | Whole plants | C. japonicum | 25 |
| 126 | Hispidulin | Roots | C. indicum | 62 |
| 127 | Hispidulin-glucuronide | Whole plants | C. infortunatum | 63 |
| 128 | Eupafolin | Whole plants | C. infortunatum | 63 |
| 129 | Scutellarin | Whole plants | C. infortunatum | 63 |
| 130 | Scutellarein | Whole plants | C. serratum | 64 |
| 131 | Pectolinarigenin | Aerial parts | C. inerme | 65 |
| 132 | 7-hydroxyflavone | Flowers | C. phlomidis | 66 |
| 133 | 7-hydroxyflavanone 7-O-glucoside | Flowers | C. phlomidis | 66 |
| 134 | Luteolin | Whole plants | C. serratum | 64 |
| 135 | Chalcone glycoside | Flowers | C. phlomidis | 66 |
| 136 | α-l-Rhamnopyranosyl-(1→2)-α-D-Glu-copyranosyl-7-O-naringin-4-d-glucopyranoside-5-methylether | Whole plants | C. phlomidis | 25 |
| 137 | 4,2′,4′-trihydroxy-6′-methoxy ehalcone-4,4′-α-D-diglucoside | Whole plants | C. phlomidis | 25 |
| 138 | 7-hydroxyflavonone | Flowers | C. phlomidis | 66 |
| 139 | Kaempferol | Whole plants | C. fragrans | 67 |
| 140 | 5,4′-dihydroxy-kaempferol-7-O-β-rutinoside | Whole plants | C. fragrans | 67 |
| 141 | 6-hydroxyflavone | Flowers | C. phlomidis | 66 |
| 142 | 4′-methyl scutellarein | Aerial parts | C. inerme | 65 |
| 143 | Apigenin-7-O-glucuronide | Roots | C. serratum | 68 |
| 144 | 5-hydroxy-4′, 7-dimethoxymethyl flavone | Whole plants | C. inerme | 25 |
| 145 | Salvigenin | Aerial parts | C. inerme | 65 |
| 146 | Acacetin | Leaves | C. inerme | 69 |
| Aerial parts | C. inerme | 48 | ||
| 147 | Cynaroside | Aerial parts | C. inerme | 13 |
| 148 | 2′,4,4′-trihydroxy-6′-methylchalcone | Flowers | C. phlomidis | 66 |
| 149 | Cirsimaritin | Aerial parts | C. petasites | 70 |
| 150 | Cirsimaritin-4′-glucoside | Aerial parts | C. mandarinorum | 71 |
| 151 | Quercetin-3′-methyl | Aerial parts | C. mandarinorum | 71 |
| 152 | Pectolinarigenin | Roots | C. indicum | 49 |
| 153 | 5-hydroxy-6,7,4′-trimethoxyflavone | Aerial parts | C. inerme | 53 |
| 154 | 5,7,4′-trihydroxy-flavone | Leaves | C. trichotomum | 72 |
| Whole plants | C .serratum | 56 | ||
| 155 | 5,7,4′-trihydroxy-3′-methoxyflavone | Whole plants | c. serratum | 73 |
| 156 | 3,2′,3′-trihydroxy-4′-methoxychalcone | Seeds | C. phlomidis | 74 |
| 157 | 3,2'-dihydroxy-4′,6′-dimethoxychalcone | Seeds | C. phlomidis | 74 |
| 158 | 5-hydroxy-7-methoxyflavanone | Seeds | C. phlomidis | 74 |
| 159 | 5-hydroxy-7-methoxyflavone | Seeds | C. phlomidis | 74 |
| 160 | Kaempferol-3-O-α-l-rhamnopyranoside | Seeds | C. phlomidis | 74 |
| 161 | Hispidulin7-O-glucuronide | Aerial parts | C. infortunatum | 63 |
| 162 | Naringin-4′- O-α- glucopyranoside | Flowers | C. phlomidis | 66 |
| Phenylethanoid glycosides | ||||
| 163 | Decaffeoylverbascoside | Aerial parts | C. inerme | 75 |
| 164 | Darendoside B | Roots | C. bungei | 40 |
| 165 | Salidroside | Aerial parts | C. inerme | 25 |
| 166 | Verbascoside | Roots | C. bungei | 40 |
| Roots | C. villosum | 49 | ||
| Aerial parts | C. inerme | 75 | ||
| 167 | Isoverbascoside | Aerial parts | C. inerme | 75 |
| 168 | Campneoside I | Aerial parts | C. bungei | 76 |
| Aerial parts | C. inerme | 75 | ||
| 169 | Cistanoside E | Aerial parts | C. inerme | 75 |
| 170 | Purpureaside B | Aerial parts | C. inerme | 75 |
| 171 | 2-phenylethyl-3-O-(6-dexoy-α-l-mannopyranosyl)-β-d-glucopyranoside | Roots | C. bungei | 32 |
| 172 | Campneoside II | Aerial parts | C. bungei | 76 |
| 173 | Martynoside | Whole plants | C. japonicum | 55 |
| 174 | Jionoside D | Aerial parts | C. trichotomum | 77 |
| 175 | Clerodendronoside | Aerial parts | C. bungei | 76 |
| 176 | Cistanoside C | Aerial parts | C. bungei | 76 |
| 177 | Jionoside C | Aerial parts | C. bungei | 76 |
| 178 | Leucosceptoside A | Roots | C. bungei | 40 |
| Aerial parts | C. bungei | 76 | ||
| 179 | Cistanoside D | Aerial parts | C. bungei | 76 |
| 180 | Cistanoside F | Aerial parts | C. bungei | 76 |
| 181 | Bungein A | Aerial parts | C. bungei | 78 |
| 182 | Monoacetylmartinoside | Whole plants | C. japonicum | 55 |
| 183 | Clerodenoside A | Whole plants | C. japonicum | 55 |
| 184 | 3,4-dihydroxyphenylethanol | Whole plants | C. indicum | 25 |
| 185 | Isomartynoside | Roots | C. bungei | 40 |
| 186 | Serratumoside A | Aerial parts | C. serratum | 79 |
| 187 | Bunginoside A | Roots | C. bungei | 40 |
| 188 | 3″,4″-di-O-acetylmartynoside | Roots | C. bungei | 40 |
| 189 | Acetylmartynoside A | Roots | C. bungei | 40 |
| 190 | Acetylmartynoside B | Roots | C. bungei | 40 |
| 191 | 3″-O-acetylmartynoside | Roots | C. bungei | 40 |
| 192 | 2″-O-acetylmartynoside | Roots | C. bungei | 40 |
| 193 | Martynoside | Roots | C. bungei | 40 |
| 194 | Trichotomoside | Roots | C. bungei | 40 |
| 195 | O-2-(3-hydroxy-4-methoxyphenyl)-ethyl O-2,3-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-(4-O-cis-feruloyl)-β-d-glucopyranoside | Roots | C. bungei | 40 |
| 196 | Isoacteoside | Roots | C. bungei | 40 |
| Aerial parts | C. bungei | 76 | ||
| 197 | Darendoside A | Roots | C. bungei | 40 |
| 198 | Phlomisethanoside | Roots | C. bungei | 40 |
| 199 | Acteoside | Aerial parts | C. bungei | 76 |
| Whole plants | C. serratum | 56 | ||
| 200 | Markhamioside F | Aerial parts | C. inerme | 75 |
| 201 | Benzylglucoside | Aerial parts | C. inerme | 75 |
| 202 | Myricoside | Aerial parts | C. serratum | 79 |
| Steroids and steroid glycosides | ||||
| 203 | Stigmasterol | Roots | C. indicum | 49 |
| Leaves | C. trichotomum | 47 | ||
| Whole plants | C. serratum | 56 | ||
| 204 | α-spinasterol | Whole plants | C. serratum | 64 |
| 205 | Stigmasterol-3-O-β-d-glucopyranoside | Roots | C. indicum | 49 |
| Whole plants | C. serratum | 73 | ||
| 206 | Serratin | Whole plants | C. serratum | 80 |
| 207 | Clerosterol | Roots | C. indicum, C. villosum | 49 |
| Leaves | C. quadriloculare | 81 | ||
| Leaves | C. trichotomum | 47 | ||
| 208 | Bungesterol | Whole plants | C. bungei | 51 |
| 209 | 4α-methyl-24β-ethyl-5α-cholesta-14,25-dien-3β-ol | Aerial parts | C. inerme | 36 |
| 210 | 4α,24,24-trimethyl-5α-cholesta-7,25-dien-3β-ol | Whole plants | C. inerme | 62 |
| 211 | 4α-methyl-24β-ethyl-5α-cholesta-7,25-dien-3β-ol | Whole plants | C. inerme | 62 |
| 212 | Gramisterol | Whole plants | C. inerme | 62 |
| 213 | 4α-methyl-24α-ethyl-5α-cholest-7-en-3β-ol | Whole plants | C. inerme | 62 |
| 214 | Obtusifoliol | Whole plants | C. inerme | 62 |
| 215 | 24,24-dimethyl-5α-cholesta-7,25-dien-3β-ol | Whole plants | C. inerme | 62 |
| 216 | 22,23-dihydrostigmasterol | Whole plants | C. japonicum | 55 |
| 217 | 25,26-dehydrostigmasterol | Whole plants | C. japonicum | 55 |
| 218 | 22-dehydroclerosterol 3β-O-β-D-(6′-O-margaroyl)-glucopyranoside | Leaves | C. trichotomum | 82 |
| Whole plants | C. quadriloculare | 81 | ||
| 219 | Sitosterol | Leaves | C. trichotomum | 47 |
| 220 | Stigmasterol | Aerial parts | C. inerme | 48 |
| 221 | 24β-methylcholesta-5,22E,25-trien-3β-ol | Whole plants | C. fragrans | 83 |
| 222 | 24α-ethyl-5α-cholest-22E-en-3β-ol | Whole plants | C. fragrans | 83 |
| 223 | Colebrin A | Aerial parts | C. colebrookianum | 84 |
| 224 | Colebrin B | Aerial parts | C. colebrookianum | 84 |
| 225 | Colebrin C | Aerial parts | C. colebrookianum | 84 |
| 226 | Colebrin D | Aerial parts | C. colebrookianum | 84 |
| 227 | Colebrin E | Aerial parts | C. colebrookianum | 84 |
| 228 | Dehydropo-riferasterol | Aerial parts | C. splendens | 25 |
| 229 | Campesterol | Stems | C. phlomidis | 85 |
| 230 | Cholestanol | Stems | C. phlomidis | 85 |
| 231 | (22E)-stigmasta-4,22,25-trien-3-one | Roots | C. indicum | 49 |
| 232 | Stigmasta-4,25-dien-3-one | Roots | C. indicum | 49 |
| 233 | Stigmasta-4,22-dien-3-one | Roots | C. indicum | 49 |
| 234 | 22-dehydroclerosterol | Roots | C. indicum, C. villosum, | 49 |
| Leaves | C. quadriloculare | 81 | ||
| Leaves | C. trichotomum | 47 | ||
| 235 | β-sitosterol | Roots | C. villosum | 49 |
| Aerial parts | C. inerme | 53 | ||
| Whole plants | C. bungei | 50 | ||
| 236 | 22-dehydroclerosterol-3-O-β-d-glucopyranoside | Roots | C. indicum, C. villosum | 49 |
| 237 | Clerosterol-3-O-β-d-glucopyranoside | Roots | C. indicum, C. villosum | 49 |
| 238 | β-sitosterol-3-O-β-d-glucopyranoside | Roots | C. villosum | 49 |
| 239 | (22E,24R)-stigmasta-4,22,25-trien-3-one | Leaves | C. trichotomum | 82 |
| 240 | (20R,22E,24R)-3β-hydroxy-Stigmasta-5,22,25-trien-7-one | Leaves | C. trichotomum | 82 |
| 241 | (20R,22E,24R)-stigmasta-22,25-dien-3,6-dione | Leaves | C. trichotomum | 82 |
| 242 | (20R,22E,24R)-6β-hydroxy-Stigmasta-4,22,25-trien-3-one | Leaves | C. trichotomum | 82 |
| 243 | (20R,22E,24R)-stigmasta-5,22,25-trien-3β,7β-diol | Leaves | C. trichotomum | 82 |
| 244 | (20R,22E,24R)-stigmasta-22,25-dien-3β,6β,9α-triol | Leaves | C. trichotomum | 82 |
| 245 | Bis(2-ethylhexyl)phthalate | Whole plants | C. serratum | 56 |
| Cyclohexylethanoids | ||||
| 246 | 1-hydroxy-1-(8-palmitoyloxyethyl)cyclohexanone | Leaves | C. trichotomum | 20 |
| 247 | 5-O-butyl cleroindin D | Leaves | C. trichotomum | 20 |
| 248 | Rengyolone | Leaves | C. trichotomum | 20 |
| Aerial parts | C. bungei | 86 | ||
| 249 | Cleroindin C | Leaves | C. trichotomum | 20 |
| 250 | Cleroindin B | Leaves | C. trichotomum | 20 |
| 251 | Rengyol | Leaves | C. trichotomum | 20 |
| 252 | Clerobungin A(1a) | Aerial parts | C. bungei | 86 |
| 253 | Clerobungin A(1b) | Aerial parts | C. bungei | 86 |
| 254 | (+)-rengyolone | Aerial parts | C. bungei | 86 |
| 255 | Cleroindicin | Aerial parts | C. bungei | 86 |
| 256 | 5-O-ethylcleroindicin D | Aerial parts | C. bungei | 78 |
| 257 | 6″-O-[(E)-caffeoyl] rengyoside B | Roots | C. bungei | 32 |
| 258 | Clerodenone A | Roots | C. bungei | 32 |
| Anthraquinones | ||||
| 259 | Aloe-emodin | Stems | C. trichotomum | 39 |
| 260 | Emodin | Stems | C. trichotomum | 39 |
| 261 | Chrysophanol | Stems | C. trichotomum | 39 |
| 262 | 2,5-dimethoxybenzoquinone | Whole plants | C. serratum | 73 |
| Cyanogenic glycosides | ||||
| 263 | (R)-lucumin | Leaves | C. grayi | 87 |
| 264 | (R)-prunasin | Leaves | C. grayi | 87 |
| Others | ||||
| 265 | B-friedoolean-5-ene-3-β-ol | Aerial parts | C. inerme | 53 |
| 266 | Stigmasta-5,22,25-trien-3-β-ol (3) | Aerial parts | C. inerme | 53 |
| 267 | Spicatolignan B | Stems | C. trichotomum | 39 |
| 268 | Trans-phytol | Leaves | C. trichotomum | 47 |
| 269 | 1H-indole-3-carboxylic acid | Leaves | C. trichotomum | 47 |
| 270 | Palmitic acid | Leaves | C. trichotomum | 72 |
| 271 | Octadecanoic acid | Leaves | C. trichotomum | 72 |
| 272 | Cis-cinnamic acid | Aerial parts | C. serratum | 23 |
| 273 | Trans-cinnamic acid | Aerial parts | C. serratum | 23 |
| 274 | P-coumaric acid | Aerial parts | C. serratum | 23 |
| 275 | Syringic acid | Aerial parts | C. inerme | 48 |
| 276 | P-methoxybenzoic acid | Aerial parts | C. inerme | 48 |
| 277 | Daucosterol | Aerial parts | C. inerme | 48 |
| 278 | 2-({6-O-[(4-hydroxy-3-methoxyphenyl)carbonyl]-β-d-glucopyranosyl}oxy)-2-methylbutanoic acid | Roots | C. bungei | 32 |
| 279 | 24β-ethylcholesta-5,22E,25-triene-3β-ol | Aerial parts | C. phlomidis | 88 |
| 280 | Pentadecanoic acid β-D-glucoside | Aerial parts | C. inerme | 66 |
| 281 | Cryptojaponol | Aerial parts | C. kiangsiense | 89 |
| 282 | Fortuning E | Aerial parts | C. kiangsiense | 89 |
| 283 | 12-methoxy-6,11,14,16-tetrahydroxy-17(15→16)-abeo-5,8,11,13-abietatetraen-3,7-dione | Aerial parts | C. kiangsiense | 89 |
2.1. Monoterpene and its derivatives
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings. Most monoterpenes are fragrant and the main composition of essential oil. Twenty-seven monoterpenes and derivatives (1–27) were isolated from the roots, leaves, aerial parts of C. serratum, C. inerme, C. incisum, C. trichotomum, Clerodendrum ugandense, and C. chinense.
2.2. Sesquiterpenes
Sesquiterpenes are bitter substances and a class of terpenes that consist of three isoprene units and have the molecular formula C15H24. They often contain α, β-unsaturated-γ-lactone as a major structural feature. In recent studies, sesquiterpenes have been associated with anti-tumor, cytotoxic, and anti-microbial activities. But, only three sesquiterpenes (28–30) were obtained from the aerial parts and roots of C. inerme and C. bungei, respectively.
2.3. Diterpenoids
To date, fifty-eight diterpene compounds (31–88) have been isolated and identified from this genus, and all of them are labdane diterpenoids. These compounds can be sorted to five types based on the pentacyclic ring on C12: a furan ring, dihydrofuran ring, lactone ring, α,β-undersaturated lactone ring, and tetrahydrofuran ring. Many of these chemical compounds have shown remarkable bioactivities in vivo or in vitro study.
2.4. Triterpenoids
So far, a total of thirty-one triterpenoids (89–119), including 3-O-acetyloleanolicacid (89), 3-O-acetyloleanolicaldehyde (90), glutinol (91), friedelin (92), taraxerol (93), clerodone (94), α-amyrin (95), glochidone (96), glochidonol (97), glochidiol (98), lupeol (99), α-amyrin 3-undecanotate (100), lupeol acetate (101), lupeol 3-palmitate (102), melastomic acid (103), β-amyrin acetate (104), betulinic acid (105), magnificol (106), glutinone (107), etc. have been purified and characterized from the whole plants, roots, leaves, or aerial parts of C. inerme, C. trichotomum, C. indicum, C. bungei, Clerodendrum canescens, Clerodendrum villosum, Clerodendrum wildii, Clerodendrum japonicum, C. serratum, Clerodendrum philippinum, or Clerodendrum glabrum.
2.5. Flavonoid and flavonoid glycosides
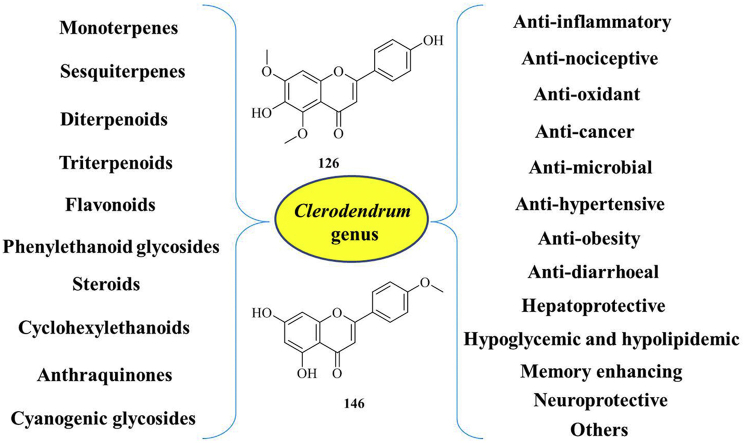
Flavonoids, important secondary metabolites, are widespread throughout the plant kingdom. Flavonoids and their derivatives are the main bioactive components of this genus, and receiving extreme attention. Up to now, forty-three flavonoid and flavonoid glycosides (120–162), including astragalin (123), apigenin (124), and tricin (125), hispidulin (126), hispidulin-glucuronide (127), eupafolin (128), scutellarin (129), scutellarein (130), pectolinarigenin (131), 7-hydroxyflavone (132), 7-hydroxyflavanone 7-O-glucoside (133), luteolin (134), chalcone glycoside (135), etc. have been isolated and identified from the roots, leaves, aerial parts of different Clerodendrum species.
2.6. Phenylethanoid glycosides
Phenylethanoid glycosides are another kind of characteristic compounds of the Clerodendrum species with antioxidant activity. To date, forty phenylethanoid glycosides (163–202) have been obtained from this genus and the structure contains three parts: sugar chain, phenylacetyl, and coffee-acyl or ferulic-acyl. The sugar chain is often composed of glucose, rhamnose, xylose or arabinose. The phenylacetyl is linked to C1-glucopyranose, and coffee-acyl or ferulic-acyl is often connected with the C4 or C6 of glucose.
2.7. Steroids and steroid glycosides
Steroids are terpenes based on the cyclopentane perhydroxy phenanthrene ring, but they are considered separately because of their chemical, biological and medicinal importance. Steroids are found in nature in free as well as in glycosidic form. There are many steroids reported from plants and they are termed phytosteroids. Total forty-three steroids and steroid glycosides (203–245) have been obtained and identified from Clerodendrum species, mainly from C. trichotomum, Clerodendrum colebrookianum, and C. bungei.
2.8. Cyclohexylethanoids
A series of cyclohexylethanoids (246–258), including two new compounds 1-hydroxy-1-(8-palmitoyloxyethyl) cyclohexanone (246) and 5-O-butyl cleroindin D (247), together with four known ones, rengyolone (248), cleroindin C (249), cleroindin B (250), rengyol (251), were isolated from the leaves of C. trichotomum, and the others (252–258) were obtained and identified from the aerial parts and roots of C. bungei.
2.9. Anthraquinones
Only four anthraquinones (259–262), aloe-emodin (259), emodin (260), chrysophanol (261) and 2,5-dimethoxybenzoquinone (262), have been isolated and identified from the stem of C. trichotomum and C. serratum.
2.10. Cyanogenic glycosides
Two cyanogenic glycosides (263–264), including (R)-lucumin (263) and (R)-prunasin (264) have been obtained and identified from the leaves of C. grayi.
2.11. Others
A range of other compounds (265–283) were isolated and identified from the aerial parts, stems, leaves and roots of C. inerme, C. trichotomum, C. serratum, C. bungei, C. phlomidis, and Clerodendrum kiangsiense.
3. Pharmacological properties
Wide clinical uses of traditional Chinese medicine of the genus Clerodendrum have inspired researchers to investigate its pharmacological properties and to validate the uses of different species as therapeutic remedy. More and more studies showed that extracts or active compounds isolated from Clerodendrum species exhibited a wide range of pharmacological activities (Table 2).
Table 2.
The pharmacological activities of extracts and compounds from the genus Clerodendrum.
| Pharmacological activities | Extract/Compound | Types | Testing subjects | Dose | Effects | Ref. |
|---|---|---|---|---|---|---|
| Anti-inflammatory and anti-nociceptive activity | 3-Hydroxy, 2-methoxy-sodium butanoate | In vivo | Carrageenan-induced inflammation and freund complete adjuvant (FCA)-induced arthritic rat models | 25, 50, 100 mg/kg, i.g. | Reduced the paw edema response, decrease lysosomal enzymes, protein-bound carbohydrates, and acute phase protein levels | 90 |
| Methanol extract from C. petasites | In vivo | Ethyl phenylpropiolate-induced ear edema and carrageenan-induced paw edema in rats | 1, 2, 4 mg/ear, i.g. | Inhibited prostaglandin synthesis | 91 | |
| Ethanol extract from C. laevifolium | in vitro | lipoxygenase | 10–1000 μg/ml | Displayed the greatest inhibition capacity with the IC50 value of 14.12 μg/ml | 92 | |
| Methanolic extract from C. inerme | In vivo | Formalin induced hind paw edema animals | 50, 100, 200 mg/kg, i.g. | Inhibited main inflammatory mediators | 53 | |
| Petroleum ether and chloroform extracts from C. paniculatum | In vitro | Human red blood cell membrane stabilization method | 1000 μg/ml | Showed 57.15% protection and 48.98% protection of HRBC in hypotonic solution, respectively | 93 | |
| Petroleum ether and chloroform extracts from C. paniculatum | in vivo | Carrageenan-induced rat paw edema model | 200 400 mg/kg, i.g. | Inhibited of the cyclooxygenase leading to inhibition of prostaglandin synthesis | 93 | |
| Hispidulin | In vitro | RAW 264.7 macrophage stimulated with LPS | 12.5, 25, 50, 100, and 200 μM | inhibited PGE2 production as well as iNOS and cyclooxygenase-2 expressions | 94 | |
| Methanolic extract from C. serratum | In vivo | Carrageenan and arachidonic acid induced hind paw edema in rats | 50, 100, 200 mg/kg, i.g. | Inhibition of synthesis and inflammatory mediators release | 97 | |
| n-Butyl extract from C. bungei | In vivo | acetic acid-induced writhing model | 1.0 g/kg, i.p. | prolonged the latency reaction, suppressed the prostaglandin production | 102 | |
| Aqueous extracts from C. bungei | In vivo | DNFB-induced hypersensitivity | 10 and 20 g/kg, i.p. | Restrained the phlogistic infiltration, improved the ear edema, reduced the writhes of abdominal cavity and the ear edema | 103 | |
| Methanolic extract of C. indicum | In vivo | Carrageenan and arachidonic acid induced hind paw edema in rats | 200 and 400 mg/kg, i.g. | Reduced the number of writhes with 62.57%, inhibited the acetic acid-induced writhing test with 70.76%, respectively | 104 | |
| Aqueous extract from C. inerme | In vivo | Milk-induced hyperpyrexia in rabbits | 100 and 200 mg/kg, p.o. | Raising the pain threshold at different time of observation | 105 | |
| Anti-oxidant activity | Ethanol extract from C. infortunatum | In vitro | DPPH-radicals | 250 μg/ml | Inhibited DPPH | 106 |
| Phenolic extracts from C. volubile | In vitro | DPPH-radicals, OH radicals | 0–100 μg/ml | Inhibited DPPH free radicals and OH radicals | 107 | |
| Monoacetylmartinoside | In vitro | DPPH-radicals | 25 μmol/l | Inhibited DPPH | 108 | |
| 3″,4″-O-acetylmartynoside | In vitro | DPPH-radicals | 37 μmol/l | Inhibited DPPH | 108 | |
| Acteoside | In vitro | DPPH-radicals | 60 μmol/l | Inhibited DPPH | 108 | |
| Methanolic extract from C. inerme | In vitro | DPPH-radicals | 100 μg/ml | Inhibited DPPH | 53 | |
| 5-Hydroxy-6,7,4′-trimethoxyflavone | In vitro | DPPH-radicals | 20 μM | Inhibited DPPH | 53 | |
| Ethanolic extract from C. serratum | In vitro | DPPH-radicals, FRAP, hydrogen peroxide radical | 50–250 μg/ml | Inhibited DPPH, FRAP, hydrogen peroxide radical | 109 | |
| Methanolic extract from C. serratum | In vitro | DPPH-radicals, ABTS-radicals | 0.125–1.0 mg/ml | Inhibited DPPH | 110 | |
| Methanolic extract from C. serratum | In vitro | DPPH-radicals | 200–1000 μg/ml | Inhibited DPPH | 111 | |
| Phenolic extracts from C. volubile | In vitro, in vivo | DPPH-radicals, lipid peroxidation assay | 0–312.60 μg/ml | Reduced the MDA content | 107 | |
| Methanolic extract from C. umbellatum | In vivo | Schistosoma mansoni-infected mice | 100, 200, and 400 mg/kg, i.g. | Decreased MDA level, increase CAT activity and GSH level | 113 | |
| Methanolic extracts from C. siphonanthus | In vitro | Thiocyanate method, DPPH-radicals | 0–120 mg/ml | Scavenging lipid peroxide (IC50 = 8 mg/ml) and DPPH radicals (IC50 = 7 mg/ml) | 114 | |
| Anti-cancer activity | Methanolic extract from C. serratum | In vivo | DMBA-induced skin tumorigenesis in male mice | 300, 600 and 900 mg/kg, i.g. | Curtailed tumor development | 115, 116 |
| Methanolic extract from C. serratum | In vivo | DLA cell model | 100 and 200 mg/kg | Reduced skin papilloma incidence and multiplicity | 117 | |
| Cryptojaponol, fortunin E, 12-methoxy-6,11,14,16-tetrahydroxy-17(15→16)-abeo-5,8,11,13-abietatetraen-3,7-dione | In vitro | HL-60, SMMC-7721, lA-549, MCF-7 cell lines | 1.8–5.0 μM | Exhibited cytotoxicity | 89 | |
| Compounds 45, 70, 76, 78, 81, and 82 | In vitro | BGC-823, Huh-7, KB, KE-97, and Jurkat | 0.83–50.99 μM | Exhibited cytotoxicity | 41 | |
| Total flavonoids from C. Bungei | In vitro | HepG2 | 0.025–250 μg/ml | Inhibited HepG2 cells proliferation | 119 | |
| Trichotomone | In vitro | A549, Jurkat, BGC-823 and 293T WT | 7.51–19.38 μM | Exhibited cytotoxicity | 43 | |
| Compounds 240 and 243 | In vitro | Hela cell | 28.92–35.67 μg/ml | Exhibited moderate cytotoxicity | 82 | |
| Anti-bacterial activity | Methanolic extract from C. siphonanthus | In vitro | Klebsiella pneumoniae, Proteus mirabilis, Salmonella typhi, Staphylococcus aureus, Escherichia coli, and Bacillus subtilis | 5 mg/disc | The inhibition zones were 30, 16, 16, 12, 11.5 and 10 mm, respectively | 114 |
| n-Butyl extract from C. bungei | In vitro | Staphylococcus aureus and Micrococcus pyogenes | 50 mg/ml | The MIC values were 50 mg/ml and 25 mg/ml, respectively | 120 | |
| Aqueous extract from C. bungei | In vitro | Rhizoctonia cerealis, Fusarium graminearum, Rhizoctonia solani, and Setosphaeria turrum | 50–400 mg/ml | Displayed the strong antibacterial action on Fusarium graminearum, and the MIC values 10 mg/ml | 121 | |
| Anti-fungal activity | Ethyl acetate extract from C. inerme | In vitro | Alternaria, Lasiodiplodia, Pestalotiopsis, Nigrospora, Diaporthe, and Phomopsis | 50 μg/disc | Inhibited the growth of most fungi | 122 |
| Ethyl acetate and chloroform extracts from C. infortunatum | In vitro | B. megaterium, S. typhi, K. pneumoniae and to fungi against A. niger and C. albicans | 1–512 μg/ml | Inhibited B. subtilis, K. pneumonia, S. aureus and E. coli growth | 123 | |
| Anti-plasmodial activity | Ethyl acetate, methanol and aqueous extracts from C. rotundifolium | In vitro | NF54 chloroquine sensitive and FCR3 chloroquine-resistant strains of Plasmodium falciparum | 5 μg/ml | Inhibited the growth of NF54 and FCR3 strains of Plasmodium falciparum | 124 |
| Insecticidal activity | Aqueous extract from C. chinense | In vitro | A. subpictus, A. albopictus, and C. tritaeniorhynchus | 647.05–6877.28 μg/ml | Reduced populations of vector mosquitoes without detrimental effects on predation rates of non-target aquatic organisms, such as D. indicus, A. bouvieri and G. affinis | 125 |
| Anti-hypertensive activity | Aqueous extract from C. colebrookianum | In vivo, in vitro | Fructose-induced hypertension model in rats and in isolated frog heart. | 50–100 mg/ml | The 100 mg/ml test samples were showed calcium antagonism in rat ileum and at 50 mg/ml and 75 mg/ml doses exhibited ROCK-II and PDE-5 inhibition respectively | 126 |
| Compounds 64, 166, 178, 196 | In vitro | ACE and a-glucosidase inhibitory activity assay | 0.1–0.7 mM | Inhibited ACE and a-glucosidase. | 123 | |
| Anti-obesity activity | Methanolic extract from C. phlomidis | In vivo | High fat diet induced obesity in female mice | 200–400 mg/kg, i.g. | Decreased food consumption, body weight, adiposity index, pancreatic lipase activity, adiposity diameter, glucose, insulin, SGOT, SGPT, TG, TC and LDL-c levels | 40 |
| Aqueous extract from C. glandulosum | In vivo | High fat diet induced obesity in C57BL/6J mice | 0–200 μg/ml | Decreased adipogenesis, TG accumulation, leptin release and G3PDH activity | 130 | |
| Anti-diarrheal activity | Methanolic extract and chloroform fraction from the C. indicum | In vitro | Castor oil-induced diarrhea testing | 400 mg/kg | Inhibited defecation | 104 |
| Methanolic extract from C. phlomidis | In vivo | castor oil induced diarrhea and PGE2 induced enteropooling in rats | 600–800 mg/kg, p.o. | Exhibited significant inhibitory activity | 131 | |
| Hepatoprotective activity | Ethanolic extract of C. inerme | In vivo | CCl4-induced liver damage in rats | 200 mg/kg, i.g. | Decreased the serum ALT, AST, ALP, TGL, TC, and increased the GSH level | 132 |
| Alcoholic extract from C. serratum | In vivo | CCl4-induced wistar rats | 20 mg/kg, i.g. | Reduced the level of serum bilirubin and liver function marker enzymes | 133 | |
| Alcoholic and aqueous extract from C. serratum | In vivo | CCl4-induced liver damage in rats | 200 mg/kg, i.g. | Restored AST, ALT, and ALP level | 134 | |
| Methanolic extract from C. umbellatum | In vivo | Schistosoma mansoni-infected mice | 100, 200 and 400 mg/kg, i.g. | Reduced ALT activity and increase total protein level | 113 | |
| Hypoglycemic and hypolipidemic activities | Aqueous extract from C. capitatum | In vivo | High fat diet fed rats | 100, 400 and 800 mg/kg, i.g. | Reduced the mean fasting plasma glucose concentration, TC, VLDL-c and LDL-c | 136 |
| Aqueous extract from C. glandulosum | In vivo | High fat diet fed rats | 200, 400 and 800 mg/kg, i.g. | Suppressed the HMG CoA reductase and cholesterol ester synthase activity, increased the plasma lecithin cholesterol acyl transferase and lipoprotein lipase levels | 137 | |
| Memory enhancing effects | Methanolic extract from C. infortunatum | In vivo | Rectangular maze and Y maze (interoceptive behavioral models) | 100 and 200 mg/kg, i.g. | 138 | |
| Neuroprotective effects | Compound 46 | In vivo | Rat hippocampal nerve terminals (synaptosomes) | 10 and 50 mg/kg, i.p. | Inhibited depolarization-evoked glutamate release and cytosolic free Ca2+ concentration in the hippocampal nerve terminals, inhibited glutamate release | 69 |
| Other activities | Ethanolic extract from C. petasites | In vitro | Isolated guinea-pig | 2.25–9 mg/ml | Exhibited significantly tracheal smooth muscle relaxant activity | 9 |
| Methanolic extract from C. phlomidis | In vivo | Phenobarbitone sodium-induced sleeping time | 200, 400 and 600 mg/kg, i.g. | Reduced spontaneous activity, decreased exploratory behavioral profiles | 139 | |
| Ethanol extract from C. inerme | In vivo | Spontaneous locomotor activity or performance in the rotarod test | 100 mg/kg, i.p. | Reduced methamphetamine-induced hyperlocomotion in mice | 62 |
3.1. Anti-inflammatory and anti-nociceptive activities
Many studies have provided data on anti-inflammatory effects of C. phlomidis, C. petasites, Clerodendrum laevifolium, C. inerme, C. bungei, and C. serratum extracts of aerial parts, roots, leaves and stems. Of these, lots of studies have provided data on anti-inflammatory effects of C. serratum (Bharangi) extracts of aerial parts, roots and stems. An aqueous extract of roots reported significant anti-inflammatory effects at high dose (180 mg/kg, p.o.) in granuloma pouch model in rats. Roots in low dose (90 mg/kg, p.o.) and stems in high dose (180 mg/kg, p.o.) showed significant preventive effects in comparison with dexamethasone (a standard anti-inflammatory agent). Thus, it can be postulated that roots are more effective than stems and it would be useful as antiallergic and antiinflammatory drug for disease like asthma.95, 96 The methanolic extract of the aerial parts of C. serratum was demonstrated dual inhibitory effects on arachidonic acid metabolism or an inhibitor of phospholipase A2 when studied in ethyl phenylpropiolate-induced ear edema and in carrageenan and arachidonic acid induced hind paw edema in rats, and the extract exerted an inhibitory activity on the acute phase of inflammation due to an inhibition of synthesis and inflammatory mediators release through cyclooxygenase and lipoxygenase pathways.97 In contrast, the alcoholic root extract of C. serratum showed a potent antiinflammatory effect by reducing paw edema (acute) and cotton-pellet granuloma (chronic) in inflammation models.98 Apigenin-7-glucoside isolated from C. serratum roots has been demonstrated for anti-inflammatory effects in rats.99 The hydro-alcoholic extract (50, 200 and 500 mg/kg dose) of Bharangyadi preparation showed inhibition of carrageenan induced inflammation due to the inhibition of the enzyme cyclooxygenase and subsequent inhibition of prostaglandin synthesis which rationalizes traditional use of this plant in bronchial asthma and related inflammatory conditions.100 This anti-inflammatory effect of C. serratum might be observed due to flavonoids and saponins, but other active substances might also be responsible leading to synergistic effects.
Prakash et al reported that the monomer compound 3-hydroxy, 2-methoxy-sodium butanoate (HMSB, at doses of 25, 50, 100 mg/kg, i.g.) isolated from the leaves of C. phlomidis displayed anti-inflammatory and anti-arthritic effects on carrageenan-induced inflammation and freund complete adjuvant (FCA)-induced arthritic rat models. The results showed that HMSB could significantly reduce the paw edema response, decrease lysosomal enzymes, protein-bound carbohydrates, and acute phase protein levels. In addition, HMSB could significantly down-regulate pro-inflammatory cytokines TNF, IL-1 and IL-6 protein levels and mRNA expression in the joints with a dose-dependent manner.90 These results indicated that the HMSB possess considerable potency in anti-inflammatory action and has a prominent anti-arthritic effect. Panthong et al evaluated the anti-inflammatory and antipyretic activities of the methanol extract (at doses of 1.0, 2.0, 4.0 mg/ear, i.g.) from C. petasites. The results proved that the extract possessed moderate inhibitory activity on acute phase of inflammation in a dose-related manner on ethyl phenylpropiolate-induced ear edema (ED50 = 2.34 mg/ear) as well as carrageenan-induced paw edema (ED30 = 420.41 mg/kg) in rats, and also reduced the alkaline phosphatase activity in serum. Moreover, the extract exhibited an excellent antipyretic effect in yeast-induced hyperthermic rats.91 The anti-inflammatory and antipyretic effects of the methanol extract may be caused by the inhibition of the prostaglandin synthesis. The ethanol extract from the leaves of C. laevifolium exhibited the greatest anti-inflammatory activity against lipoxygenase with the IC50 of 14.12 μg/ml in vitro study.92 In addition, the methanolic extract from the aerial parts of C. inerme exhibited anti-inflammatory activity at doses of 50, 100 and 200 mg/kg in formalin induced hind paw edema animals.53 The anti-inflammatory activity of petroleum ether, chloroform, ethyl acetate, alcohol, and aqueous extracts of fresh leaves from Clerodendrum paniculatum Linn was evaluated by in vitro (human red blood cell membrane stabilization method) and in vivo methods (0.1 ml of 1% w/v carrageenan-induced rat paw edema model). Petroleum ether and chloroform extracts which showed, best in vitro anti-inflammatory activity also showed a dose dependent (200 and 400 mg/kg) significant reduction in paw edema when compared to the control (indomethacin, 10 mg/kg).93
Srisook et al found that two flavones, hispidulin (126) and acacetin (146) isolated from the ethyl acetate (EA) extracts from the leaves of C. inerme exhibit the most potent inhibitory activity on nitric oxide (NO) production in RAW 264.7 macrophage stimulated with lipopolysaccharide (LPS). Furthermore, IC50 values of hispidulin and acacetin were 43.7 ± 4.0 and 43.5 ± 6.4 μM, respectively. Hispidulin also inhibited prostaglandin E2 (PGE2) production as well as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 expressions via the blockade of nuclear factor kappa B (NF-κB) DNA binding activity and the c-Jun NH2-terminal protein kinase (JNK) way.94
Narayanan et al (1999) studied anti-nociceptive effects of an alcoholic extract of C. serratum roots (50, 100 and 200 mg/kg) in acetic acid induced writhing (200 mg/kg) and hot plate method (100 and 200 mg/kg).98 A reduction in the number of abdominal constrictions in acetic acid induced writhing in mice indicated the anti-nociceptive effect of C. serratum which has further been supported by the findings of hot plate method where a significant increase in area under curve was observed. However, the response was much less when compared to morphine and exact mechanism remains to be investigated in detail. The authors have also indicated significant antipyretic activity of alcoholic extract (100 and 200 mg/kg) of C. serratum roots in rabbit model through a dose dependent reduction in pyrexia after administration of C. serratum.97 The ethanolic extract of C. serratum leaves has been found to produce considerable centrally acting analgesic activity in tail flick test at 250 mg/kg dose and peripherally acting analgesic activity in acetic acid induced writhing test at 500 mg/kg dose which was found comparable with diclofenac sodium. Blockade of capillary permeability or release of endogenous substances like prostaglandins might be a postulated mechanism.101 In another study, the author has established a potent analgesic effect of methanolic extract of the aerial parts of C. serratum when injected subcutaneously into the right dorsal hind paw of the mice via an inhibition of peripherally and centrally mediated nociception in early as well as in late phase.97
The n-butyl extract (at dose of 1.0 g/kg, i.p.) from the roots of the C. bungei displayed a significant anti-nociceptive effect in an acetic acid-induced writhing model, prolonged the latency reaction in the hot-plate test in 15, 30, 60 and 90 min in mice. Moreover, the extracts administered in combination with naloxone significantly prolonged the latency reaction, and indicating that naloxone did not revert the action of the extract effect. Also, the extracts notably suppressed the production of prostaglandin (PG) in a dose-dependent manner.102 The extracts from the roots of C. bungei significantly restrained the phlogistic infiltration, improved the ear edema and reduced the writhes of abdominal cavity and the ear edema induced by 2,4-dinitro-1-fluorobenzene (DNFB)-induced hypersensitivity.103 The methanolic extract of C. indicum at doses of 200 and 400 mg/kg showed a significant (P < 0.001) and dose-dependent reduction in the number of writhes with 62.57% and 70.76% of inhibition in the acetic acid-induced writhing test, respectively.104 Thirumal et al reported that the aqueous extract obtained from C. inerme leaves (at doses of 100 and 200 mg/kg, p.o.) displayed significant analgesic effect by raising the pain threshold at different time of observation (0–120 min).105
The combination of antiinflammatory, anti-nociceptive and antipyretic effects of the Clerodendrum genus indicated a prospect of intervention with prostaglandin synthesis, as prostaglandins have been established as a common mediator in all these responses. However, this possibility remains to be investigated thoroughly. Advanced studies can be undertaken in the direction of purification of the chemical constituents of the leaves and investigation of the biochemical pathways for the development of a potent analgesic agent with a low toxicity and better therapeutic index.
3.2. Antioxidant activity
Gouthamchandra et al have demonstrated the antioxidant activity of the ethanol extract of leaves of C. infortunatum with the highest scavenging activity in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay (IC50 values 250 μg/ml). Moreover, the ethanol extract at 250 μg/ml concentration displayed significantly radical scavenging activity in hydroxyl, superoxide anion, and nitric oxide radical in vitro, and the scavenging ratio were 68.58%, 62.06%, and 52.65%, respectively.106 Adefegha et al reported that the phenolic (free and bound) extracts from the leaves of Clerodendrum volubile scavenging DPPH free radicals and OH radicals in a concentration dependent manner. Interestingly, the IC50 values revealed that the free soluble phenolic extract (IC50 = 89.18 μg/ml and 924.90 μg/ml) have a significantly higher scavenging ability against DPPH free radicals and OH radicals than the bound phenolic extracts (IC50 = 133.40 μg/ml and 1224.0 μg/ml), respectively.107 Three phenylethanoid glycosides monoacetylmartinoside (182), 3″,4″-O-acetylmartynoside (188) and acteoside (199) isolated from the roots of Clerodendrum lindleyi exhibited significant in vitro antioxidant activity in DPPH assay, and the radical scavenging rate were 25, 37, 60 μmol/l, respectively.108 The methanolic extract and 5-hydroxy-6,7,4′-trimethoxyflavone (153) isolated from the aerial parts of C. inerme showed notably scavenging activity with maximum inhibition of 61.84% for the methanolic extract (100 μg/ml) and 37.19% for 5-hydroxy-6,7,4′-trimethoxyflavone (20 μM), respectively, using DPPH assay.53
Bhujbal et al have demonstrated in-vitro antioxidant effects of ethanolic root extract of C. serratum (50–250 μg/ml) at various concentrations in the DPPH radical scavenging assay (IC50 value 175 μg/ml); FRAP (ferric reducing antioxidant power) assay and hydrogen peroxide radical scavenging assay (IC50 value 85 μg/ml) and suggested the role of polyphenols and flavonoids for the observed antioxidant effects in the extract.109 The antioxidant potential of methanolic extract of leaves of C. serratum was found more potent (EC50 value 0.51 μg/ml) due to higher polyphenolic content than other extracts (petroleum ether, chloroform and water) when evaluated in trolox equivalent antioxidant capacity (TEAC) in DPPH and 2,20-azinobis-(3-ethylbenzothiazoneline-6-sulfonic acid) diammonium salt (ABTS) assays.110 Antioxidant potential of methanolic extract (200–1000 μg/ml) from the leaves of C. serratum was further supported by additional reports on DPPH assay, reducing power assay and total antioxidant activity assay.111
Feng et al reported that the flavonoid compound from C. bungei exhibited strong scavenging capability on nitrite, superoxide anion free radicals and hydroxyl free radicals, and also showed stronger antioxidant effect on pork fat than vitamin C.112 Also, the phenolic extracts (free and bound) from the C. volubile leaf were able to significantly reduce the MDA content in a dose dependent manner (0–312.60 μg/ml). The free soluble phenolic extracts (192.30–77.90%) had a significantly higher concentration dependent inhibition of MDA compared with that of the bound phenolic extract (192.30–91.30%).107 Jatsa et al reported that the methanolic extract (at doses of 100, 200, and 400 mg/kg, i.g.) of Clerodendrum umbellatum significantly decrease malondialdehyde (MDA) level, increase catalase (CAT) activity and glutathione level.113 The methanolic extracts of leaves of Clerodendrum siphonanthus displayed extremely effective in scavenging lipid peroxide (IC50 = 8 mg/ml) and DPPH radicals (IC50 = 7 mg/ml).114
3.3. Anticancer activity
Chinchali et al reported that administration of methanolic extract of C. serratum leaves significantly reduced tumor development in 7,12-dimethylbenz[α] anthracene (DMBA) induced skin carcinogenicity in testis, liver and kidney of mice.115, 116 The researchers have further demonstrated that flavonoids and phenolics can effectively reduce the incidence and multiplicity of skin papilloma, many investigators have confirmed anti-cancer property of C. serratum by various in vivo and in-vitro studies.117, 118 The methanolic extract of roots of C. serratum exhibited notably in vivo anticancer activity using DLA cell model at the dose 100 and 200 mg/kg body weight.117
Xu et al reported that diterpenoids cryptojaponol (281), fortunin E (282), 12-methoxy-6,11,14,16-tetrahydroxy-17(15→16)-abeo-5,8,11,13-abietatetraen-3,7-dione (283) isolated from the hydroalcoholic extract of the herb of C. kiangsiense exhibited significant cytotoxicity against human myeloid leukemia (HL-60), hepatocellular carcinoma (SMMC-7721), lung cancer (A-549) and breast cancer (MCF-7) cell lines, and the range of IC50 values was 1.8–5.0 μM.89 The results suggested that these compounds might have promising potential to be anticancer agents.
Compounds 45, 70, 76, 78, 81, and 82 isolated and identified from the roots of C. trichotomum displayed remarkable in vitro cytotoxicity activity against five human cancer cell lines (BGC-823, Huh-7, KB, KE-97, and Jurkat) by using the CellTiter Glo™ Luminescent cell viability assay method with the IC50 values ranging from 0.83 to 50.99 μM. Among of them, teuvincenone E (81) exhibited the most potent activity against these five cell lines with the IC50 values of 3.95, 5.37, 1.18, 1.27, and 0.83 μM, respectively.41 The total flavonoids isolated from the C. Bungei significantly inhibited the human hepatoma HepG2 cells proliferation at concentrations of 0.025, 0.25, 2.5, 25, 250 μg/ml in vitro, and the inhibition rates were 5.55%, 12.73%, 14.84%, 62.44%, and 76.81%, respectively.119 A dimeric diterpene trichotomone (55) isolated from the roots of the C. trichotomum exhibited strong in vitro cytotoxicities against several human cancer cell lines (A549, Jurkat, BGC-823 and 293T WT) with IC50 values ranged from 7.51 to 19.38 μM.43 Two steroids, (20R,22E,24R)-3β-hydroxy-stigmasta-5,22,25-trien-7-one (240), and (20R,22E,24R)-stigmasta-5,22,25-trien-3β,7β-diol (243) isolated from the leaves of C. trichotomum exhibited moderate cytotoxicity against Hela cell with IC50 values at 35.67 and 28.92 μg/ml, respectively.82
3.4. Antimicrobial activity
3.4.1. Antibacterial activity
Arokiyaraj et al reported that the methanolic extract of leaves of C. siphonanthus exhibited significant antibacterial effect against Klebsiella pneumoniae, Proteus mirabilis, Salmonella typhi, Staphylococcus aureus, Escherichia coli, and Bacillus subtilis, and the inhibition zones were 30, 16, 16, 12, 11.5 and 10 mm, respectively.114 Liu et al reported that the n-butyl extract from the roots of C. bungei displayed prominent antibacterial effect against Staphylococcus aureus and Micrococcus pyogenes, and the minimal inhibitory concentration (MIC) values were 50 mg/ml and 25 mg/ml, respectively.120 Moreover, the aqueous extracts from the roots of C. bungei have notably antibacterial action on Rhizoctonia cerealis, Fusarium graminearum, Rhizoctonia solani, and Setosphaeria turrum, especially the aqueous extract exhibited strongest antibacterial action on Fusarium graminearum, and the MIC values 10 mg/ml.121 The methanolic extract, and chloroform fraction of C. indicum showed moderate activity against the tested microorganisms in terms of both zones of inhibition (ranged from 9 to 13 mm, 10 to 13 mm and 10 to 13 mm, respectively, at a concentration of 400 μg/disc) and spectrum of activity.104
3.4.2. Antifungal activity
Gong et al firstly found that the crude ethyl acetate extract of endophytes from the stems of C. inerme exhibit broad in vitro antifungal activity against a number of fungal pathogens, including Alternaria, Lasiodiplodia, Pestalotiopsis, Nigrospora, Diaporthe, and Phomopsis, and inhibit the growth of most fungi.122 The ethyl acetate and chloroform extracts of root, leaf, and stem of the C. infortunatum showed significant inhibitory activity over the bacteria and fungus comparable to the standard drug tetracycline and fluconazole. The maximum average diameter zone of inhibition was recorded to bacterial strains against Bacillus megaterium, S. typhi, K. pneumoniae and to fungi against Anisops niger and Clerodendrum albicans. The MIC values of ethyl acetate and chloroform root extract were determined as 64 μg/ml to B. subtilis and K. pneumoniae; to S.-β-haemolyticus and S. typhi for ethyl acetate extracts, 128 μg/ml to S. aureus, and E. coli for both ethyl acetate and chloroform root extracts but only S. typhi and S.-β-haemolyticus for chloroform extract.123
3.4.3. Antiplasmodial activity
Adia et al revealed that the ethyl acetate, methanol and aqueous extracts from the leaves of Clerodendrum rotundifolium exhibit significantly in vitro antiplasmodial activity against the chloroquine-sensitive and chloroquine resistant Plasmodium falciparum strains with the IC50 < 5 μg/ml for the first time.124
3.4.4. Insecticidal activity
Lots of pharmacological tests and clinical observations have shown that different extract and/or compound prescriptions derived from C. chinense have significant insecticidal effects against diseases and organisms including schistosomiasis and trichomoniasis. Govindarajan et al reported that C. chinense-fabricated silver nanoparticles (Ag NPs) display higher toxicity against Anisops subpictus, Anisops albopictus, and Clerodendrum tritaeniorhynchus with the LC50 values of 10.23, 11.10, and 12.38 μg/ml, respectively. Also, C. chinense-fabricated Ag NPs were found safer to non-target organisms Diplonychus indicus, Anisops bouvieri and Gambusia affinis, with respectively LC50 values ranging from 647.05 to 6877.28 μg/ml.125 These results indicated that C. chinense-fabricated Ag NPs are a promising and eco-friendly tool against larval populations of mosquito vectors of medical and veterinary importance, with negligible toxicity against non-target aquatic organisms.
3.5. Antihypertensive activity
Lokesh et al evaluated the anti-hypertensive potential of the aqueous extract, and its aqueous, n-butanol, ethyl-acetate and chloroform fractions of C. colebrookianum leaves using fructose-induced hypertension model in rats and isolated frog heart. The results showed that the each fraction display negative inotropic and chronotropic effect on isolated frog heart and significant reduction in systolic blood pressure and heart rate in hypertensive rats. Moreover, each fraction at 100 mg/ml showed calcium antagonism in rat ileum and at 50 mg/ml and 75 mg/ml doses exhibited Rho-kinase (ROCK-II) and phosphodiesterase-5 (PDE-5) inhibition, respectively.126 The antihypertensive activity of C. colebrookianum may mediate mainly by cholinergic action and following ROCK-II and PDE-5 inhibition. Liu et al demonstrated that four compounds 15-dehydrocyrtophyllone A (64), verbascoside (166), leucosceptoside A (178), and isoacteoside (196), isolated from dried roots of C. bungei showed inhibitory effects against angiotensin converting enzyme (ACE) and a-glucosidase. Among of them, 5-dehydrocyrtophyllone A exhibited an inhibitory effect against ACE with an IC50 value of 42.7 μM, while the three phenylethanoid glycosides, verbascoside, leucosceptoside A, and isoacteoside, exhibited stronger inhibitory effects against a-glucosidase, with IC50 values of 0.5 mM, 0.7 mM, and 0.1 mM, respectively.40
3.6. Anti-diabetic activity
Bachhawat et al reported that the methanolic extract (100 mg/ml) of C. serratum roots was evaluated for alpha-glucosidase inhibitory activity using enzyme assay. The extract was not found significantly effective (32.3% inhibition rate with IC50 value 265 μg/ml) and may require higher dose to produce the effect.127
3.7. Anti-obesity activity
Obesity, initially thought as a problem of the developed world, has now become a worldwide malady because of increasing prevalence in the developing countries as well as developed countries.128 The impact of methanolic extract of C. phlomidis on weight reduction in feeding high fat diet induced obesity in female mice had been investigated. The studies showed that the methanolic extract of C. phlomidis at 200 and 400 mg/kg significantly decrease food consumption, body weight, adiposity index, pancreatic lipase activity, adiposity diameter, glucose, insulin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), triglycerides (TG), total cholesterol (TC) and low-density lipoprotein (LDL-c) levels induced by feeding high fat diet induced obesity in female mice, and the LD50 value was found to be more than 2000 mg/kg.129 Jadeja et al reported that the aqueous extract from the leaves of Clerodendron glandulosum exhibited significant anti-adipogenic effect by decreasing adipogenesis, TG accumulation, leptin release and glyceraldehyde 3-phosphate dehydrogenase (G3PDH) activity along with higher glycerol release without significantly altering viability of 3T3L1 pre-adipocytes in vitro.130 This study was a profound scrutiny of C. glandulosum extract and its role in preventing adipocyte differentiation and visceral adiposity by down regulation of PPARγ-2 related genes and leptin expression. This study validates the traditional therapeutic claim of use of CG extract in controlling obesity.
3.8. Anti-diarrheal activity
Pal. et al reported that the methanolic extract and chloroform fraction from the C. indicum at a dose of 400 mg/kg produced 21.74% and 26.96% inhibition of defecation in castor oil-induced diarrhea testing, respectively, which were found to be comparable to that of standard drug loperamide (37.39% inhibition at 50 mg/kg) with regard to the severity of diarrhea.104 The methanolic extract (at doses of 600 and 800 mg/kg, p.o.) from the leaves of the C. phlomidis showed significant inhibitory activity against castor oil induced diarrhea and PGE2 induced enteropooling in rats. Also, the extract also showed a significant reduction in gastrointestinal motility in charcoal meal test in rats.131 Anti-diarrheal activity of the plant supported its traditional use in diarrhea by the people of Australia and India.
3.9. Hepatoprotective activity
Gopal et al reported that the ethanolic extract of C. inerme leaves exhibit hepatoprotective activity on CCl4-induced (0.5 ml/kg, i.p.) liver damage in rats at a dose of 200 mg/kg. The extract significantly decreases the serum enzyme alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), triglycerides (TGL), total cholesterol (TC), and significantly increased the glutathione level.132 Vidya et al reported that administration of an alcoholic extract from the roots of C. serratum (20 mg/kg) for two weeks significantly reduced the level of serum bilirubin and liver function marker enzymes in carbon tetrachloride (CCl4) induced wistar rats indicating its potential as a hepatoprotective agent possibly due to the radical scavenging activity of the flavonoids present in the drug.133
Also, Agrawal et al found that the alcoholic (200 mg/kg. p.o.) and aqueous extract (200 mg/kg, p.o.) from the leaves of C. serratum possess significant hepatoprotective effects by restoring the normal level of AST, ALT, and ALP with significant reduction in liver weight.134 Reports on the biomarker ursolic acid, isolated from alcoholic root extract suggested restorative effects on the levels of AST, ALT and ALP towards respective normal value, to stabilize the plasma membranes as well as to repair hepatic tissue damage caused by CCl4. Ursolic acid was found to normalize the disturbed antioxidant status by maintaining the levels of glutathione and by inhibiting the production of malondialdehyde or may be due to the inhibition of toxicant activation and the enhancement of body defense system.99
The ethanol extract of the polyherbal composition from the roots of C. serratum showed significant protection against acetaminophen-induced hepatotoxicity in rats, and the function may be through DPPH free radical scavenging activity.135 The methanolic extract (at doses of 100, 200 and 400 mg/kg, i.g.) of C. umbellatum significantly reduced ALT activity and increase total protein level.113 These findings provided scientific evidence to the ethnomedicinal reports of C. serratum in treating acute jaundice; however investigations are still required to fully explicate the exact mechanisms behind the protection.133
3.10. Hypoglycemic and hypolipidemic activities
Adeneye et al reported that the fresh leaves aqueous extract of Clerodendrum capitatum possess obvious hypoglycemic and hypolipidemic activities, the extracts (at doses of 100, 400 and 800 mg/kg, i.g.) could significantly reduce the mean fasting plasma glucose concentration in a dose-dependent lowering effects. Furthermore, the extracts also could notably decrease the total cholesterol, VLDL-c and LDL-c with a dose-related, but significant elevate the triglycerides and HDL-c with a dose-related in plasma.136 Jadeja et al reported that the aqueous extract (200, 400 and 800 mg/kg, i.g.) of C. glandulosum leaves significantly prevented increment in plasma and tissue lipid profiles in high fat diet (HFD) fed rats, suppressed activity levels of HMG CoA reductase (Hepatic) and cholesterol ester synthase (Hepatic and intestinal), and increased the activity levels of plasma lecithin cholesterol acyl transferase and lipoprotein lipase (plasma, hepatic and adipose), and increased excretion of triglycerides, cholesterol and bile acids through faeces.137
3.11. Memory enhancing effects
Gupta et al reported that the methanolic extract of C. infortunatum leaves exhibited promising memory enhancing effects at dose of 200 mg/kg (i.g.), and the effects was closely approximated the results for the standard drug Brahmi, the higher dose evoking pronounced alteration behavior and better learning assessments.138 The presence of steroids, terpenoids, fats and flavonoids were confirmed in this extract by TLC. The extract is likely to develop a promising nootropic to prevent dementia senilis disease.
3.12. Neuroprotective effects
One flavonoid acacetin (146) isolated from the C. inerme was investigated for neuroprotective activity. It was observed that acacetin inhibited depolarization-evoked glutamate release and cytosolic free Ca2+ concentration in the hippocampal nerve terminals. Moreover, acacetin (at doses of 10 and 50 mg/kg, i.p.) inhibited glutamate release from hippocampal synaptosomes by attenuating voltage-dependent Ca2+ entry and effectively prevents kainic acid (KA)-induced in vivo excitotoxicity.69
3.13. Other activities
Hazekamp et al found that the ethanolic extract of C. petasites leaves exhibited a dose-dependently tracheal smooth muscle relaxant activity on isolated guinea-pig at concentrations from 2.25 to 9 mg/ml, and the active principle was isolated and identified as the flavonoid hispidulin.9 The results indicated that hispidulin may be beneficial in the treatment of asthma related diseases. In additional, the methanolic extract (at doses of 200,400 and 600 mg/kg, i.g.) of C. phlomidis leaves was found to cause significant reduction in spontaneous activity, and decreases in exploratory behavioral profiles by the Y-maze and head dip test. Also, the extract exhibit significantly reduction in muscle relaxant activity by rotarod, 30° inclined screen and traction tests, as well as significantly potentiated the phenobarbitone sodium-induced sleeping time.139 Huang et al demonstrated for the very first time that hispidulin isolated from the dichloromethane and the n-hexane fractions of ethanol extract of C. inerme significantly reduced methamphetamine-induced hyperlocomotion (MIH) in mice at dose of 100 mg/kg (i.p.) that did not affect their spontaneous locomotor activity or performance in the rotarod test, a measure for motor coordination.62 This study suggested that hispidulin may be a good therapeutic potential in hyper-dopaminergic disorders.
4. Conclusions
In present review, more than 300 chemical constituents have been isolated and identified from the genus of Clerodendrum, and pharmacological studies indicated that the crude extracts and some special monomer compounds of the genus Clerodendrum exert various biological activities, such as anti-inflammatory and anti-nociceptive, antioxidant, anticancer, antimicrobial, anti-hypertensive, anti-obesity, anti-diarrheal, hepatoprotective, memory enhancing, and neuroprotective activities. Terpenes, including monoterpene and its derivatives, sesquiterpene, diterpenoids, triterpenoids, as the major characteristic constituents with significant biological activities, have great potential to be developed into new drugs, especially for anti-inflammatory, antioxidant, anticancer, and antimicrobial agents. In addition, important activities, such as anti-hypertensive, anti-obesity, and hepatoprotective activities indicated that Clerodendrum genus can be a promising source of biologically active compounds for these diseases.
The genus Clerodendrum has gained a wide acceptance for its pharmacological activities against various ailments. Although above 400 species of the genus Clerodendrum were distributed all over the world, only a few of them have been investigated and studied so far. From this review, it can be concluded that phytochemical and pharmacology investigations were mainly focused on C. serratum, C. bungei, C. inerme, C. trichotomum, Clerodendrum chinense, C. colebrookianum, C. phlomidis, C. petasites, C. grayi, and C. indicum. For some species, such as C. grayi was only studied phytochemically, no biological activity was reported up till now. Many other species are totally unknown phytochemically and biologically. Following these species may be of a great importance in discovering new bio-active compounds. On the other hand, few reports have been published concerning the toxic effects of isolated components, and quantitative informations of the genus Clerodendrum were also relatively sparse.
All in all, the omnibearing study on this genus Clerodendrum should be performed as soon as possible, which will provide reliable theory evidence for better exploit and utilize the resources of the species in this genus.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81374019), the Special Project of the “Twelfth Five-year Plan” for Medical Science Development of PLA (BWS12J012), Project of Traditional Chinese Medicine Administration, Gansu Province (GZK-2015-59), Project of Military Medical and Health Research, PLA (CLZ15JA05), and Project of Military Medical and Health Research, PLA (15ZD021). The authors would also like to express their gratitude to Lanzhou University PhD English writing foreign teacher Mike Carter who thoroughly corrected the English in the paper.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Harley R.M., Atkins S., Budantsev A.L. vol. VII. Springer-Verlag; Berlin; Heidelberg, Germany: 2004. (The Family and Genera of Vascular Plants). ISBN: 978-3-540-40593-1. “Labiatae” pages: 167–275. [Google Scholar]
- 2.Yuan Y.W., Mabberley D.J., Steane D.A., Olmstead R.G. Further disintegration and redefinition of Clerodendrum (Lamiaceae): implications for the understanding of the evolution of an intriguing breeding strategy. Taxon. 2010;59:125–133. [Google Scholar]
- 3.Mabberley D.J. 3rd ed. Cambridge University Press; UK: 2008. Mabberley's Plant-book. ISBN: 978-0-521-82071-4. [Google Scholar]
- 4.Staples G.W., Herbst D.R. Bishop Museum Press; Honolulu: 2005. A Tropical Garden Flora. [Google Scholar]
- 5.Muthu C., Baskar K., Ignacimuthu S., Ai-Khaliel Ovicidal and oviposition deterrent activities of the flavonoid pectolinaringenin from Clerodendrum phlomidis against Earias vittella. Phytoparasitica. 2013;41:365–372. [Google Scholar]
- 6.Shrivastava N., Patel T. Clerodendrum and healthcare: an overview. Med Aromat Plant Sci Biot. 2007;1:209–223. [Google Scholar]
- 7.Chethana G.S., Hari V.K.R., Gopinath S.M. Review on Clerodendrum inerme. J Pharm Sci Innov. 2013;2:38–40. [Google Scholar]
- 8.Baker J.T., Borris R.P., Carte B. Natural product drug discovery and development; new perspective on international collaboration. J Nat Prod. 1995;58:1325–1357. doi: 10.1021/np50123a003. [DOI] [PubMed] [Google Scholar]
- 9.Hazekamp A., Verpoorte R., Panthong A., Hazekamp A., Verpoorte R., Panthong A. Isolation of a bronchodilator flavonoid from the Thai medicinal plant Clerodendrum petasites. J Ethnopharmacol. 2001;78:45–49. doi: 10.1016/s0378-8741(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 10.Calis I., Hosny M., Yuruker A. Inerminosides A1, C and D, three novel iridoid glycosides from Clerodendrum inerme. Phytochemistry. 1994;37:1083–1085. doi: 10.1016/s0031-9422(00)89533-3. [DOI] [PubMed] [Google Scholar]
- 11.Harbone J.B. 2nd ed. Chapman and Hall; London, UK: 1984. Phytochemical Methods, Guide to Modern Techniques of Plants Analysis; pp. 37–76. [Google Scholar]
- 12.Nishida R., Kawai K., Amano T., Kuwahara Y. Pharnacophagous feeding stimulate activity of neoclerodane diterpenoids for the turnip sawfly, Athalia rosae ruficornis. Biochem Syst Ecol. 2004;32:15–25. [Google Scholar]
- 13.Achari B., Chaudhuri C., Saha C.R., Dutta P.K., Pakrashi S.C. A clerodane diterpene and other constituents of Clerodendron inerme. Phytochemistry. 1990;29:3671–3673. [Google Scholar]
- 14.Subramanian S.S., Nair A.G.R., Vedantham T.N.C. (24, S)-ethylcholesta-5, 22, 25-triene-3β-ol from four Clerodendrum species. Phytochemistry. 1973;12:2078–2079. [Google Scholar]
- 15.Ganapaty S., Rao D.V. Triterpenoids of the stem bark of Cleodendrum nerifolium. Indian J Pharm Sci. 1985;47:167–168. [Google Scholar]
- 16.Sinha N.K., Seth K.K., Pandey V.B., Dasgupta B., Shah A.H. Flavonoids from the flowers of Clerodendron infortunatum. Planta Med. 1981;42:296–298. doi: 10.1055/s-2007-971645. [DOI] [PubMed] [Google Scholar]
- 17.Chae S., Kang K.A., Ju S.K., Jin W.H., Kang S.S. Trichotomoside: a new antioxidative phenylpropanoid glycoside from Clerodendron trichotomum. Chem Biodivers. 2006;3:41–48. doi: 10.1002/cbdv.200690005. [DOI] [PubMed] [Google Scholar]
- 18.Kim K.H., Kim S., Min Y.J., Ham I.H., Wan K.W. Anti-inflammatory phenylpropanoid glycosides from Clerodendron trichotomum leaves. Arch Pharm Res. 2009;32:7–13. doi: 10.1007/s12272-009-1112-6. [DOI] [PubMed] [Google Scholar]
- 19.Akihisa T., Ghosh P., Thakur S., Nagata H., Tamura T., Matsumoto T. 24, 24-dimethyl-25-dehydrolophenol, a 4α-methylsterol from Clerodendrum inerme. Phytochemistry. 1990;29:1639–1641. [Google Scholar]
- 20.Xu R.L., Wang R., Wei H., Shi Y.P. New cyclohexylethanoids from the leaves of Clerodendrum trichotomum. Phytochem Lett. 2014;7:111–113. [Google Scholar]
- 21.Jadeja R., Thounaojam M., Ansarullah, Ramchandran A.V., Devkar R. Phytochemical constituents and free radical scavenging activity of Clerodendron glandulosum Coleb methanolic extract. J Compl Integr Med. 2009;6:1–23. [Google Scholar]
- 22.Adsersen A., Adsersen H., Brimer L. Cyanogenic constituents in plants from the Galapagos Islands. Biochem Syst Ecol. 1988;16:65–77. [Google Scholar]
- 23.Yang H., Hou A.J., Jiang B., Lin Z.W., Sun H.D., Serratumin A. A novel compound from Clerodendrum serratum. Acta Bot Yun. 2000;22:75–80. [Google Scholar]
- 24.Yang H., Jiang B., Zhi N.A.Z., Guo Y.P., Sun H.D. Two new iridoid glucosides from Clerodendrum serratum. Chin Chem Lett. 2000;11:231–234. [Google Scholar]
- 25.Wu M.M., Wang L.Q., Hua Y. The study progress of chemical constituents and biological activity of the Clerodendrum. Chem Eng Eq. 2011;4:112–116. [Google Scholar]
- 26.Nan H., Wu J., Zhang S. A new phenylethanoid glycoside from Clerodendrum inerme. Pharmazie. 2005;60:798–799. [PubMed] [Google Scholar]
- 27.Jacke G., Rimpler H. Distribution of iridoid glycosides in Clerodendrum species. Phytochemistry. 1983;22:1729–1734. [Google Scholar]
- 28.Kanchanapoom T., Kasai R., Chumsric P., Hiraga Y., Yamasaki K. Megastigmane and iridoid glucosides from Clerodendrum inerme. Phytochemistry. 2001;58:333–336. doi: 10.1016/s0031-9422(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 29.Calis I., Hosny M., Yuruker A., Wright A.D., Sticher O. Inerminosides A and B, two novel complex iridoid glycosides from Clerodendrum inerme. J Nat Prod. 1994;57:494–500. doi: 10.1021/np50106a008. [DOI] [PubMed] [Google Scholar]
- 30.Stenzel E., Rimpler H., Hunler D. Iridoid glucosides from Clerodendrum incisum. Phytochemistry. 1986;25:2557–2561. [Google Scholar]
- 31.Kanchanapoom T., Chumsri P., Kasai R., Otsuka H., Yamasaki K. A new iridoid diglycoside from Clerodendrum chinense. J Asian Nat Prod Res. 2005;7:269–272. doi: 10.1080/10286020410001690145. [DOI] [PubMed] [Google Scholar]
- 32.Liu S.S., Zhou T., Zhang S.W., Xuan L.J. Chemical constituents from Clerodendrum bungei and their cytotoxic activities. Helv Chim Acta. 2009;92:1070–1079. [Google Scholar]
- 33.Fan T., Min Z., Song G., Iinuma M., Tanaka T. Abietane diterpenoids from Clerodendrum mandarinorum. Phytochemistry. 1999;51:1005–1008. [Google Scholar]
- 34.Fan T.P., Min Z.D., Iinuma M. Two novel diterpenoids from Clerodendrum bungei. Chem Pharm Bull. 1999;47:1797–1798. [Google Scholar]
- 35.Pandey R., Verma R.K., Gupta M.M. Neo-clerodane diterpenoids from Clerodendrum inerme. Phytochemistry. 2005;66:643–648. doi: 10.1016/j.phytochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Pandey R., Verma R.K., Singh S.C., Gupta M.M. 4α-methyl-24β-ethyl-5α-cholesta-14, 25-dien-3β-ol and 24β-ethylcholesta-5, 9(11), 22E-trien-3β-ol, sterols from Clerodendrum inerme. Phytochemistry. 2003;63:415–420. doi: 10.1016/s0031-9422(03)00146-8. [DOI] [PubMed] [Google Scholar]
- 37.Raha P., Das A.K., Adityachaudhri N., Majumder P.L. Cleroinermin, a neo-clerodane diterpenoid from Clerodendrum inerme. Phytochemistry. 1991;30:3812–3814. [Google Scholar]
- 38.Krishna G.N.K., Balachandran J., Aravind S., Ganesh M.R. Antifeedant and growth inhibitory effects of some neo-clerodane diterpenoids isolated from Clerodendrum species (Verbenaceae) on Earias vitella and Spodoptera litura. J Agric Food Chem. 2003;51:1555–1559. doi: 10.1021/jf025920a. [DOI] [PubMed] [Google Scholar]
- 39.Li L.Z., Wang M.H., Sun J.B., Liang J.Y. Abietane diterpenoids and other constituents from Clerodendrum trichotomum. Biochem Syst Ecol. 2014;56:218–220. [Google Scholar]
- 40.Liu Q., Hu H.J., Li P.F. Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and a-glucosidase. Phytochemistry. 2014;103:196–202. doi: 10.1016/j.phytochem.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Wang W.X., Xiong J., Tang Y. Rearranged abietane diterpenoids from the roots of Clerodendrum trichotomum and their cytotoxicities against human tumor cells. Phytochemistry. 2013;89:89–95. doi: 10.1016/j.phytochem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Nan H.H., Wu J., Yin H., Zhang S. Terpenoid compounds from Clerodendrum inerme. Chin Tradit Herb Drugs. 2006;37:508–509. [Google Scholar]
- 43.Wang W.X., Zhu J.J., Zou Y. Trichotomone, a new cytotoxic dimericabietane-derived diterpene from Clerodendrum trichotomum. Tetrahedron Lett. 2013;54:2549–2552. [Google Scholar]
- 44.Sun L., Wang Z.Z., Ding G., Bi Y., Meng Z.Q., Xiao W. Isolation and structure characterization of two new diterpenoids from Clerodendrum bungei. Phytochem Lett. 2014;7:221–224. [Google Scholar]
- 45.Xu M.F., Jia O.Y., Wang S.J., Zhu Q. A new bioactive diterpenoid from Pestalotiopsis adusta, an endophytic fungus from Clerodendrum canescens. Nat Prod Res. 2016;2:1–6. doi: 10.1080/14786419.2016.1138297. [DOI] [PubMed] [Google Scholar]
- 46.Yang H., Wang J., Hou A., Lin Z.W., Sun H.D. Two new diterpenoid glucosides from Clerodendrum serratum. Chin Chem Lett. 1999;10:1023–1026. [Google Scholar]
- 47.Hu H.J., Liu Q., Yang Y.B., Yang L., Wang Z.T. Chemical constituents of Clerodendrum trichotomum leaves. J Chin Med Mater. 2014;37:1590–1593. [PubMed] [Google Scholar]
- 48.Nan H.H., Zhang S., Wu J. Chemical constituents from Clerodendrum inerme. Chin Tradit Herb Drugs. 2005;36:492–494. [Google Scholar]
- 49.Somwong P., Moriyasu M., Suttisri R. Chemical constituents from the roots of Clerodendrum indicum and Clerodendrum villosum. Biochem Syst Ecol. 2015;63:153–156. [Google Scholar]
- 50.Gao L.M., Wei X.M., He Y.Q. Studies on chemical constituents of Clerodendrum bungei. China J Chin Mater Med. 2011;28:1042–1044. [PubMed] [Google Scholar]
- 51.Dong X.P., Qiao R.X., Guo L., Xie J., Liu B. Study on the chemical constituents of Clerodendrum bungei Stedu. Nat Prod Res Dev. 1999;11:8–10. [Google Scholar]
- 52.Jia L., Min Z.D. Chemical constituents from Clerodendrum canescens. Chin Tradit Herb Drugs. 2007;38:161–163. [Google Scholar]
- 53.Ibrahim S.R.M., Alshali K.Z., Fouad M.A., Elkhayat E.S., Haidari R.A.A., Mohamed G.A. Chemical constituents and biological investigations of the aerial parts of Egyptian Clerodendrum inerme. Bull Fac Pharm Cairo Univ. 2014;52:165–170. [Google Scholar]
- 54.Toyota M., Msonthi J.D., Hostettmann K. A molluscicidal and antifungal triterpenoids saponin from the roots of Clerodendrum wildii. Phytochemistry. 1990;29(9):2849–2851. [Google Scholar]
- 55.Tian J., Sun H.D. Chemical constituents from Clerodendrum japonicum. Acta Bot Yun. 1995;17:103–108. [Google Scholar]
- 56.Fan J.D., Long Q.D., Yang J., Luo X.C. Studies on the chemical constituents of Clerodendrum serratum (L.) Moon. Lishizhen Med Mater Med Res. 2008;19:1894–1895. [Google Scholar]
- 57.Yue J.R., Feng D.Q., Xu Y.K. A new triterpenoid bearing octacosanoate from the stems and roots of Clerodendrum philippinum var. simplex (Verbenaceae) Nat Prod Res. 2015;29:1228–1234. doi: 10.1080/14786419.2015.1023725. [DOI] [PubMed] [Google Scholar]
- 58.Yang H., Mu Q., He Y.N., Sun H.D. A new triterpenoid saponins: Se-saponin A. Chin Chem Lett. 2000;11:333–336. [Google Scholar]
- 59.Parveen M., Khanam Z., Ali M., Rahman S.Z. A novel lupene-type triterpenic glucoside from the leaves of Clerodendrum inerme. Nat Prod Res. 2010;24:167–176. doi: 10.1080/14786410902975566. [DOI] [PubMed] [Google Scholar]
- 60.Masevhe N.A., Awouafack M.D., Ahmed A.S., McGaw L.J., Eloff J.N. Clerodendrumic acid, a new triterpenoid from Clerodendrum glabrum (Verbenaceae), and antimicrobial activities of fractions and constituents. Cheminform. 2013;96:1693–1703. [Google Scholar]
- 61.Van O.H., Sinh P.X., An N.T. A new rearranged abietane diterpene and other constituents from Clerodendrum philippinum. Nat Prod Commun. 2009;4:323–325. [PubMed] [Google Scholar]
- 62.Huang W.J., Lee H.J., Chen H.L., Fan P.C., Ku Y.L., Chiou L.C. Hispidulin, a constituent of Clerodendrum inerme that remitted motor tics, alleviated methamphetamine-induced hyperlocomotion without motor impairment in mice. J Ethnopharmacol. 2015;26:18–22. doi: 10.1016/j.jep.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian S.S., Nair A.G.R. Scutellarin and hispidulin-7-O-glucuronide from the leaves of Clerodendrum indicum and Clerodendrum infortunatum. Phytochemistry. 1973;12:1195. [Google Scholar]
- 64.Nair A.G.R., Vedantham T.N.C., Kannabiram B. Crystalline components of Clerodendrum serratum. Curr Sci. 1976;45:391. [Google Scholar]
- 65.Vendantham T.N., Subramanian S.S., Harborne J.B. 4′-methyl-scutellarein and pectolinarigenin from Clerodendron inerme. Phytochemistry. 1977;16:294–295. [Google Scholar]
- 66.Anam E.M. Novel flavone and chalcone glycosides from the Clerodendrum phlomidis (Verbenaceae) India J Chem. 1997;36:897–900. [Google Scholar]
- 67.Gao L.M., Wei X.M., He Y.Q. Studies on chemical constituents in leaf of Clerodendrum fragrans. China J Chin Mater Med. 2003;28:948–951. [PubMed] [Google Scholar]
- 68.Bhujbal S.S., Nanda R.K., Deoda R.S. Structure elucidation of a flavonoid glycoside from the roots of Clerodendrum serratum (L.) Moon, Lamiaceae. J Pharmacogn. 2010;20:1001–1002. [Google Scholar]
- 69.Lin T.Y., Huang W.J., Wu C.C., Lu C.W., Wang S.J. Acacetin inhibits glutamate release and prevents kainic acid-induced neurotoxicity in rats. PLoS One. 2014;9:e88644. doi: 10.1371/journal.pone.0088644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thitilertdecha P., Guy R.H., Rowan M.G. Characterisation of polyphenolic compounds in Clerodendrum petasites S. Moore and their potential for topical delivery through the skin. J Ethnopharmacol. 2014;154:400–407. doi: 10.1016/j.jep.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 71.Zhu M., Phillipson J.D., Greengrass P.M., Bowery N.G. Chemical and biological investigations of the root bark of Clerodendrum mandarinorum. Planta Med. 1996;62:393–396. doi: 10.1055/s-2006-957923. [DOI] [PubMed] [Google Scholar]
- 72.Yao Z.Q., Guo Q. Chemical constituents of Clerodendron trichotomum Thunb leaves. Chin J Exp Tradit Med Formulae. 2010;16:103–104. [Google Scholar]
- 73.Fan J.D., Long Q.D., Luo X.R., Yang J. Studies on the chemical constituents of Clerodendrum serratum (L.) Moon. Med J Chin People's Health. 2007;19:611–612. [Google Scholar]
- 74.Rammohan A., Munikishore R., Gunasekar D., Blond A., Bodo B. Two new chalcones from Clerodendrum phlomidis. J Asian Nat Prod Res. 2015;17:343–347. doi: 10.1080/10286020.2014.968561. [DOI] [PubMed] [Google Scholar]
- 75.Nan H.H., Yin H., Zhang S. Phenylethanoid glycosides from Clerodendrum inerme. Nat Prod Res Dev. 2008;20:1008–1011. [Google Scholar]
- 76.Li Y.B., Li J., Li P., Tu P.F. Isolation and characterization of phenylethanoid glycosides from Clerodendron bungei. Acta Pharm Sin. 2005;40:722–727. [PubMed] [Google Scholar]
- 77.Chae S., Kim J.S., Kang K.A. Anti-oxidant activity of jionoside D from the Clerodendrum trichotomum. Biol Pharm Bull. 2004;27:1504–1508. doi: 10.1248/bpb.27.1504. [DOI] [PubMed] [Google Scholar]
- 78.Yang H., Hou A.J., Mei S.X., Sun H.D., Che C.T. Constituents of Clerodendrum bungei. J Asian Nat Prod Res. 2002;4:165–169. doi: 10.1080/1028602021000000053. [DOI] [PubMed] [Google Scholar]
- 79.Yang H., Hou A.J., Mei S.X., Peng L.Y., Sun H.D. A new phenylpropanoid glycoside: serratumoside A from Clerodendrum serratum. Chin Chem Lett. 2000;11:323–326. [Google Scholar]
- 80.Raju R., Akoni J.L., Subban R. Chemical constituents from Clerodendrum serratum. J Asian Nat Prod Res. 2008;10:652–655. [Google Scholar]
- 81.Macabeo A.P., Villafranca M.C., Aguinaldo A.M., Hussain H., Krohn K. Clerosterols from Clerodendrum quadriloculare. Biochem Syst Ecol. 2008;36:659–660. [Google Scholar]
- 82.Xu R.L., Wang R., Ding L., Shi Y.P. New cytotoxic steroids from the leaves of Clerodendrum trichotomum. Steroids. 2013;78:711–716. doi: 10.1016/j.steroids.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Akihisa T., Ghosh P., Thakur S., Oshikiri S., Tamura T., Massumoto T. 24β-methylcholesta-5, 22E, 25-trien-3β-ol and 24α-ethyl-5α-cholest-22E-en-3β-ol from Clerodendrum fragrans. Phytochemistry. 1988;27:241–244. [Google Scholar]
- 84.Yang H., Wang J., Hou A.J., Guo Y.P., Lin Z.W., Sun H.D. New steroids from Clerodendrum colebrookianum. Fitoterapia. 2000;71:641–648. doi: 10.1016/s0367-326x(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 85.Akihisa T., Matsubara Y., Ghosh P., Thakur S., Tamura T., Matsumoto T. Sterols of some Clerodendrum species (Verbenaceae): occurrence of the 24 alpha- and 24 beta-epimers of 24-ethylsterols lacking a delta 25-bond. Sterols. 1989;53:625–638. doi: 10.1016/0039-128x(89)90036-6. [DOI] [PubMed] [Google Scholar]
- 86.Zhu H.C., Huan L.J., Chen C.M. A pair of unprecedented cyclohexyl ethanoid enantiomers containing unusual trioxabicyclo [4.2.1]nonane ring from Clerodendrum bungei. Tetrahedron Lett. 2014;55:2277–2279. [Google Scholar]
- 87.Miller R.E., McConville M.J., Woodrow I.E. Cyanogenic glycosides from the rare Australian endemic rainforest tree Clerodendrum grayi (Lamiaceae) Phytochemistry. 2006;67:43–51. doi: 10.1016/j.phytochem.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 88.Shanker K., Singh S.C., Pant S. Quantitative TLC analysis of sterol (24β-ethylcholesta-5, 22E, 25-triene-3β-ol) in Agnimantha (Clerodendrum phlomidis Linn) Chromatographia. 2008;67:269–274. [Google Scholar]
- 89.Xu M.F., Wang S.J., Jia O.Y., Zhu Q., Shi L.E. Bioactive diterpenoids from Clerodendrum kiangsiense. Molecules. 2016;21:86–93. doi: 10.3390/molecules21010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prakash B.N., Saravanan S., Pandikumar P., Krishna K.B., Raj M.K., Ignacimuthu S. Anti-inflammatory and anti-arthritic effects of 3-hydroxy, 2-methoxy sodium butanoate from the leaves of Clerodendrum phlomidis L.f. Inflamm Res. 2014;63:127–138. doi: 10.1007/s00011-013-0681-5. [DOI] [PubMed] [Google Scholar]
- 91.Panthong A., Kanjanapothi D., Taesotikul T., Wongcome T., Reutrakul V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S. Moore. J Ethnopharmacol. 2003;85:151–156. doi: 10.1016/s0378-8741(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 92.Phosrithong N., Nuchtavorn N. Antioxidant and anti-inflammatory activities of Clerodendrum leaf extracts collected in Thailand. Eur J Integr Med. 2015;8:281–285. [Google Scholar]
- 93.Joseph J., Bindhu A.R., Aleykutty N.A. In vitro and in vivo antiinflammatory activity of Clerodendrum paniculatum Linn leaves. Indian J Pharm Sci. 2013;75:376–379. doi: 10.4103/0250-474X.117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Srisook K., Srisook E., Nachaiyo W. Bioassay-guided isolation and mechanistic action of anti-inflammatory agents from Clerodendrum inerme leaves. J Ethnopharmacol. 2015;165:94–102. doi: 10.1016/j.jep.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 95.Bhangare N.K., Pansare T.A., Ghongane B.B., Nesari T.M. Screening for anti-inflammatory and antiallergic activity of Bharangi (Clerodendrum serratum Moon.) in animals. Int J Pharm Bio Sci. 2012;3:245–254. [Google Scholar]
- 96.Bhangare N.K., Ghongane B.B. Screening for anti-inflammatory and antiallergic activity of Bharangi (Clerodendrum serratum Moon.) Indian J Pharmacol. 2011;43:197. [Google Scholar]
- 97.Phacharoen A. Graduate School, Chiang Mai University; Chiang Mai: 2007. Analgesic, Anti-inflammatory and Vascular Effects of Clerodendrum Serratum Linn Extract. [Google Scholar]
- 98.Narayanan N., Thirugnanasambantham P., Viswanathan S., Vijayasekaran V., Sukumar E. Antinociceptive, anti-inflammatory and antipyretic effect of ethanol extract of Clerodendrum serratum roots in experimental animals. J Ethnopharmacol. 1999;65:237–241. doi: 10.1016/s0378-8741(98)00176-7. [DOI] [PubMed] [Google Scholar]
- 99.Patel J.J., Acharya S.R., Acharya N.S. Clerodendrum serratum (L.) Moon. – a review on traditional uses, phytochemistry and pharmacological activities. J Ethnopharmacol. 2014;154:268–285. doi: 10.1016/j.jep.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 100.Kajaria D., Tripathi J.S., Tiwari S.K., Pandey B.L. Antimicrobial and antiinflammatory effect of an indigenous Ayurvedic drug – Bharangyadi. Nov Sci Int J Pharm Sci. 2012;1:479–483. [Google Scholar]
- 101.Saha D., Talukdar A., Das T., Ghosh S.K., Rahman H. Evaluation of analgesic activity of ethanolic extract of Cleodendrum serratum Linn. leaves in rats. Int Res J Pharm Appl Sci. 2012;2:33–37. [Google Scholar]
- 102.Liu J.X., Zhou L., Zhou Q., Lian Q.S. The study on antinociceptive effect of Clerodendrum bungei Steud roots n-butyl alcohol extract in mice. Chin J Pain Med. 2007;13(6):349–352. [Google Scholar]
- 103.Zhou H.L., Liu J.X., Zhou L., Zhou Q., Lian Q.S. Anti-inflammatory, analgesic and anti-hypersensitivity actions of extracts from Clerodendrum bungei. Chin J New drugs. 2006;15:2027–2029. [Google Scholar]
- 104.Pal A., Mahmud Z.A., Akter N., Islam S., Bachar S.C. Evaluation of antinociceptive, antidiarrheal and antimicrobial activities of leaf extracts of Clerodendrum indicum. Pharmacogn J. 2012;4:41–46. [Google Scholar]
- 105.Thirumal M., Srimanthula S., Kishore G., Vadivelan R., Anand Kumar A.V.S. Analgesic and antipyretic effects of aqueous extract from Clerodendrum inerme (L.) Gaertn leaves in animal models. Der Pharm Lett. 2013;5:315–323. [Google Scholar]
- 106.Gouthamchandra K., Mahmood R., Manjunatha H. Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L. Environ Toxicol Pharmacol. 2010;30:11–18. doi: 10.1016/j.etap.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 107.Adefegha S.A., Oboh G. Antioxidant and inhibitory properties of Clerodendrum volubile leaf extracts on key enzymes relevant to non-insulin dependent diabetes mellitus and hypertension. J Taibah Univ Sci. 2016;10:521–533. [Google Scholar]
- 108.Min H., Zhao Z.M., Guo W.Y., Yang D.P., Cheng J.L. Chemical constituents of Clerodendrum lindleyi and their free radical scavenging activities. Chin Tradit Herb Drugs. 2012;43:1050–1056. [Google Scholar]
- 109.Bhujbal S.S., Kewatkar S.M.K., More L.S., Patil M.J. Antioxidant effects of roots of Clerodendrum serratum Linn. Pharm Res. 2009;1:294–298. [Google Scholar]
- 110.Mohamed A.J., Mohamed E.A.H., Abdalrahim A.F.A. Antioxidant, antiangiogenic and vasorelaxant activities of methanolic extract of Clerodendrum serratum (Spreng.) leaves. J Med Plants Res. 2012;6:348–360. [Google Scholar]
- 111.Prasad M.P., Sushant S., Chikkaswamy B.K. Phytochemical analysis, antioxidant potential, antibacterial activity and molecular characterization of Clerodendrum species. Int J Mol Biol. 2012;3:71–76. [Google Scholar]
- 112.Feng J.N., Huang H.Y., Yu R.J., Deng B. Extraction of flavonoid compound from Clerodendrum bungei and it's antioxidant property. Chin J Spectrosc Lab. 2013;30:3215–3220. [Google Scholar]
- 113.Jatsa H.B., Kenfack C.M., Simo D.N. Schistosomicidal, hepatoprotective and antioxidant activities of the methanolic fraction from Clerodendrum umbellatum Poir leaves aqueous extract in Schistosoma mansoni infection in mice. BMC Complement Altern Med. 2015;15:1–9. doi: 10.1186/s12906-015-0788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arokiyaraj S., Sripriya N., Bhagya R., Radhika B., Prameela L., Udayaprakash N.K. Phytochemical screening, antibacterial and free radical scavenging effects of Artemisia nilagirica, Mimosa pudica and Clerodendrum siphonanthus – an in-vitro study. Asian Pac J Trop Biomed. 2012;2:S601–S604. [Google Scholar]
- 115.Chinchali J.F., Sanakal R.D., Kaliwal B.B. Evaluation of anticarcinogenic activity of Clerodendrum serratum leaf extract on liver and kidney of 7, 12-dimethylbenz[a]anthracene (DMBA) induced skin carcinogenesis in mice. Eur J Exp Biol. 2011;1:130–141. [Google Scholar]
- 116.Chinchali J.F., Sanakal R.D., Kaliwal B.B. Effect of Clerodendrum serratum leaf extract on biochemical and oxidative stress parameters of testis in 7, 12-dimethylbenz[a]anthracene induced skin carcinogenesis in Swiss albino mice. Recent Res Sci Technol. 2012;4:8–15. [Google Scholar]
- 117.Zalke A.S., Kulkarni A.V., Shirode D.S., Duraiswamy B. In-vivo anticancer activity of Clerodendrum serratum (L) Moon. Res J Pharm Biol Chem Sci. 2010;1:89–98. [Google Scholar]
- 118.Nagdeva, Katiyar P.K., Singh R. Anticancer activity of leaves of Clerodendron serratum Spreng. Am J PharmTech Res. 2012;2:452–461. [Google Scholar]
- 119.Hu Q., Zhou K.J., Tan X.N., Li Y.M. Experimental study of total flavonoids isolated from Clerodendrum bungei on human hepatoma HepG2 cells proliferative effect. Hunan J Tradit Chin Med. 2015;31:166–168. [Google Scholar]
- 120.Liu J.X., Li Y., Lian L.F., Zhang W.P. Antibacterial activity of the n-butyl alcohol extract of Clerodendrum bungei Steud in vitro study. Lishizhen Med Mater Med Res. 2015;26:1849–1850. [Google Scholar]
- 121.Lin N., Yin L.G., Chen C.Z., Wei Q., Li H.L. Study on the antimicrobial effect of the extracts of Clerodendrum bungei roots. Agric Sci Technol. 2009;10:130–133. [Google Scholar]
- 122.Gong B., Yao X.H., Zhang Y.Q., Fang H.Y., Pang T.C., Dong Q.L. A cultured endophyte community is associated with the plant Clerodendrum inerme and antifungal activity. Genet Mol Res. 2015;14:6084–6093. doi: 10.4238/2015.June.8.6. [DOI] [PubMed] [Google Scholar]
- 123.Waliullah T.M., Yeasmin A.M., Alam A., Islam W., Hassan P. In vitro antimicrobial study for biological evaluation of Clerodendrum infortunatum Linn. Recent Pats Antiinfect Drug Discov. 2015;10:98–104. doi: 10.2174/1574891x10666150512104405. [DOI] [PubMed] [Google Scholar]
- 124.Adia M.M., Emami S.N., Byamukama R., Faye I., Borq-Karlson A.K. Antiplasmodial activity and phytochemical analysis of extracts from selected Ugandan medicinal plants. J Ethnopharmacol. 2016;186:14–19. doi: 10.1016/j.jep.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 125.Govindarajan M., Rajeswary M., Hoti S.L. Clerodendrum chinense-mediated biofabrication of silver nanoparticles: mosquitocidal potential and acute toxicity against non-target aquatic organisms. J Asia-Pacific Entomol. 2016;19:51–58. [Google Scholar]
- 126.Lokesh D., Amitsankar D. Evaluation of mechanism for antihypertensive action of Clerodendrum colebrookianum walp, used by folklore healers in north-east India. J Ethnopharmacol. 2012;143:207–212. doi: 10.1016/j.jep.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 127.Bachhawat A.J., Sham M.S., Thirumurugan K. Screening of fifteen Indian ayurvedic plants for alpha-glucosidase inhibitory activity and enzyme kinetics. Int J Pharm Pharm Sci. 2011;3:267–274. [Google Scholar]
- 128.Hoffman D.J. Obesity in developing countries: causes and implications. Food Nutr Agric. 2001;28:35–42. [Google Scholar]
- 129.Chidrawar V.R., Patel K.N., Chitme H.R., Shiromwar S.S. Pre-clinical evolutionary study of Clerodendrum phlomidis as an antiobesity agent against high fat diet induced C57BL/6J mice. Asian Pac J Trop Biomed. 2012;2:1509–1519. [Google Scholar]
- 130.Jadeja R.N., Thounaojam M.C., Ramani U.V., Devkar R.V., Ramachandran A.V. Anti-obesity potential of Clerodendron glandulosum Coleb leaf aqueous extract. J Ethnopharmacol. 2011;135:338–343. doi: 10.1016/j.jep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 131.Rani S., Ahamed N., Rajaram S., Saluja R., Thenmozhi S., Murugesan T. Anti-diarrhoeal evaluation of Clerodendrum phlomidis Linn leaf extract in rats. J Ethnopharmacol. 1999;68:315–319. doi: 10.1016/s0378-8741(99)00103-8. [DOI] [PubMed] [Google Scholar]
- 132.Gopal N., Sengottuvelu S. Hepatoprotective activity of Clerodendrum inerme against CCL4 induced hepatic injury in rats. Fitoterapia. 2008;79:24–26. doi: 10.1016/j.fitote.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 133.Vidya S.M., Krishna V., Manjunatha B.K., Mankani K.L., Ahmed M., Singh S.D.J. Evaluation of hepatoprotective activity of Clerodendrum serratum L. Indian J Exp Biol. 2007;45:538–542. [PubMed] [Google Scholar]
- 134.Agrawal S.K., Jat R.K., Chhipa R.C. Pharmacological evaluation of hepatoprotective activity of Clerodendrum serratum. Int J Pharmacol Toxicol. 2013;3:67–70. [Google Scholar]
- 135.Tulsiani P., Deshmukh P., Silawat N., Akhbar Z. Protective effect of polyherbal preparation against acetaminophen-induced hepatotoxicity in rats. Drug Invent Today. 2009;1:119–120. [Google Scholar]
- 136.Adeneye A.A., Adeleke T.I., Adeneye A.K. Hypoglycemic and hypolipidemic effects of the aqueous fresh leaves extract of Clerodendrum capitatum in wistar rats. J Ethnopharmacol. 2008;116:7–10. doi: 10.1016/j.jep.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 137.Jadeja R.N., Thounaojam M.C., Ansarullah, Devkar R.V., Ramchandran A.V. Clerodendron glandulosum Coleb, Verbenaceae, ameliorates high fat diet-induced alteration in lipid and cholesterol metabolism in rats. Rev Bras Farmacogn. 2010;20:117–123. [Google Scholar]
- 138.Gupta R., Singh H.K. Nootropic potential of Alternanthera sessilis and Clerodendrum infortunatum leaves on mice. Asian Pac J Trop Dis. 2012;2:465–470. [Google Scholar]
- 139.Murugesan T., Saravanan K.S., Lakshmi S., Ramya G., Thenmozhi K. Evaluation of psychopharmacological effects of Clerodendrum phlomidis Linn extract. Phytomedicine. 2001;8:472–476. doi: 10.1078/S0944-7113(04)70068-9. [DOI] [PubMed] [Google Scholar]