Abstract
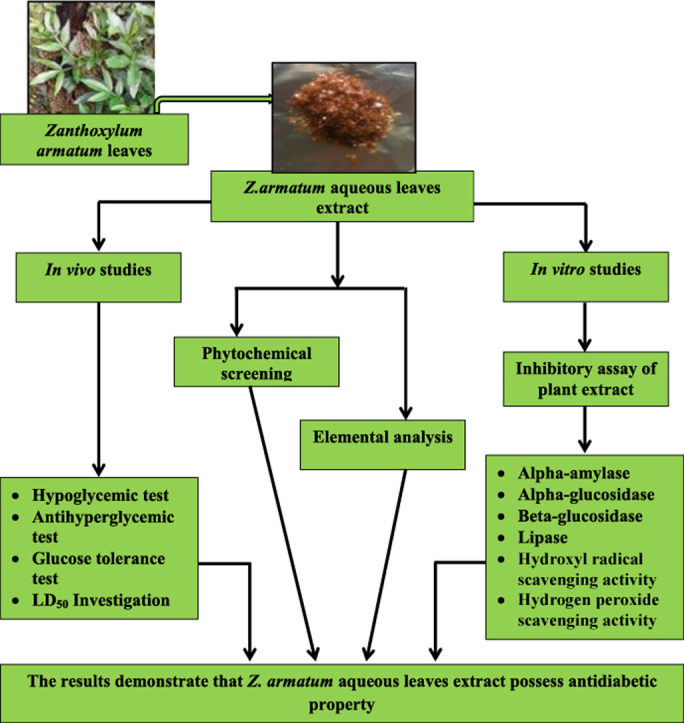
The present study was designed to evaluate the antidiabetic potential of the aqueous leaves extract of Zanthoxylum armatum DC. leaves using in vivo and in vitro approaches. For in vivo studies, blood glucose level was monitored at different intervals after administration of varying doses of the extract for its hypoglycemic (100–6000 mg/kg b.w.) and antihyperglycemic (250 mg/kg b.w.) effect in normoglycemic and diabetic mice. In vitro enzymatic inhibition activity was tested against α-amylase, α- and β-glucosidase and lipase. Additionally hydroxyl radical, hydrogen peroxide scavenging assay and phytochemical screening were also performed. Element analysis of the plant was studied by Atomic Absorption Spectrometry (AAS) and Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES). The plant extract showed significant hypoglycemic and antihyperglycemic effect in normoglycemic and diabetic mice. The IC50 values of extract for α-amylase, β-glucosidase, lipase, hydroxyl radical scavenging activity, hydrogen peroxide scavenging activity were 7.40 mg/ml, 0.30 mg/ml, 8.35 mg/ml, 3.25 mg/ml, 9.62 mg/ml respectively and the percentage of inhibition for α-glucosidase was 79.82% at 0.8 mg/ml. In vitro studies were compared with their respective standards. Elemental analysis revealed the presence of essential elements such as Mg, V, Fe, Cr, Zn, Cu, Mo, Mn, K, Ca, P and Sr which are all known to play a role in regulating blood glucose. The results demonstrate that Z. armatum aqueous leaves extract possess antidiabetic property in both in vivo and in vitro condition.
Keywords: α-Amylase, α-Glucosidase, Antihyperglycemic, β-Glucosidase, Lipase, Zanthoxylum armatum
Graphical abstract
1. Introduction
Diabetes mellitus (DM) is a group of commonly known metabolic diseases characterized by chronic hyperglycemia due to defects in insulin secretion, insulin action or both.1 Many different therapeutic approaches are available for treating diabetes and one of the treatment includes retarding absorption of glucose by inhibiting the carbohydrate hydrolysing enzymes like amylase and glucosidases.2, 3, 4 The human α-amylase (EC 3.2.1.1), is commonly found in the pancreatic juice and saliva which breaks down large insoluble starch molecules into simple and absorbable sugars.2 On the other hand, α-glucosidase (EC 3.2.1.20) and β-glucosidase (EC 3.2.1.21) are key enzymes that are located at the border brush of the small intestine which catalyses the cleavage of glycosidic bonds releasing glucose from the non-reducing end of an oligo- or polysaccharide chains.4 Hence, inhibition of α-amylase and glucosidase enzymes will result in delaying the breakdown of carbohydrates in the small intestine thus diminishing the postprandial blood glucose level. Further, hyperlipidaemia which is known to be associated with disturbances in the lipid metabolism have been linked to the development of obesity and diseases including diabetes. The important strategy in the prevention and treatment of hyperlipidaemia includes delaying fat digestion and absorption through gastrointestinal mechanisms such as the inhibition of pancreatic lipase (EC 3.1.1.3).5 Therefore, the use of lipase inhibitors will result in reduced absorption of glycerides and fatty acids from the gut and consequently, less fat will be synthesised in the body.6 Further, it has also been shown in a number of studies that DM is associated with oxidative stress, leading to an increased production of reactive oxygen species (ROS), including superoxide radical (O2•), hydrogen peroxide (H2O2), and hydroxyl radical (OH•) or reduction of antioxidant defence system.7 Antioxidants play an important role in scavenging the free radicals and protect the human body from oxidative stress.8 Therefore, a drug with both antioxidant and antidiabetic property would be useful for the treatment of DM. Although various types of antidiabetic drugs are easily available for controlling blood glucose level however, these medications are known to be associated with undesirable effects. Thus, managing diabetes using currently available drugs devoid from side effects is still a challenge.9 Hence, the search for more effective and safer therapeutic agents of natural origin is continuing as it is considered valuable.9 WHO has suggested the evaluation of traditional plant therapies for DM as they are effective, nontoxic, with less or no side effects and are considered to be excellent candidates for oral therapy.10 There are various mechanisms by which plants show antihyperglycemic activity by acting either insulinomimetic or secretagogues properties, some resemble the effect of metformin, others inhibit enzymes such as α-amylase, α-/β-glucosidase, lipase and some are due to their antioxidant potential.11, 12, 13, 14, 15 Therefore, documenting and validating the efficacy of medicinal plants having antidiabetic property has increased and characterization of chemical constituents is focused in drug discovery programmes to bring a better lead molecule for diabetes treatment.16
Zanthoxylum armatum DC. (Rutaceae) is a small tree or large spiny shrub. It is widely distributed in India from Kashmir to Bhutan at altitudes up to 2500 m and occurs through North East India.17 The parts of the plant such as the leaves, bark, stem, fruits and seeds are extensively used in indigenous system of medicine as a tonic, carminative, stomachic and anthelmintic.18, 19 Z. armatum is also commonly used in traditional practices by the Khasi tribe of Meghalaya in North-Eastern India and in neighbouring regions including South-East Asia.20 People of Meghalaya used the aromatic fruits (local name: Jaiur) and leaves as spices for preparing traditional foods.21 Z. armatum extract has shown to possessed antifungal activity, hepatoprotective activity, anti-inflammatory activity, antioxidant and antimicrobial activities.22, 23, 24, 25 Other genus of Zanthoxylum are also known to possessed potent antidiabetic property.26, 27 Previous studies has shown that the hydromethanolic bark extract of Z. armatum is also known to possessed antidiabetic property.28 Currently, there is no scientific validation displaying the antidiabetic potential of Z. armatum aqueous leaves extract using in vivo and in vitro approaches. Hence, the present study was aimed to investigate the antidiabetic activity of aqueous leaves extract of Z. armatum in diabetic mice using the above mentioned approaches.
2. Material and methods
2.1. Chemicals
Alloxan was procured from Sigma Co., USA; Insulin from Knoll Pharmaceutical Ltd., India; Metformin from USV Ltd., India; Acarbose from Bayer Zydus Pharma, India; Orlistat from Meyer Organics Pvt. Ltd., India. Other chemicals used were of analytical grade obtained from Sisco Research Laboratories (SRL), India and Himedia, India.
2.2. Collection of plant material
Leaves of Z. armatum (ZA) were collected from Diengpasoh, East Khasi Hills, Shillong, Meghalaya, India. The plant was authenticated by a Herbarium curator, Dr. P. Gurung, Department of Botany, NEHU, Shillong, Meghalaya, India, with a voucher No. 11963.
2.3. Preparation of the plant extract
The leaves were properly washed, dried in oven at 40 °C and grounded. 40 g of powdered leaves dissolved in 200 ml of distilled water was filtered using Whatmann filter paper No.1. The filtrate was then evaporated using rotary evaporator and then lyophilized to dryness.29 The lyophilized powder was stored at 4 °C for further use. The yield percentage of Z. armatum extract (ZAE) was 6.31%.
2.4. In vivo studies
2.4.1. Experimental animals
Female swiss albino mice, weighing about 25–30 g were obtained from Pasteur Institute, Shillong, India, and used for the study. Mice were housed in a room kept under control conditions with temperature maintained at 22 °C on a 12-h dark cycle and fed with standard mice feed. Mice were fasted overnight before performing the following experiment but given water ad libitum. Food was again fed after 6 h to mice during the hypoglycemic and antihyperglycemic study and after 120 min during glucose tolerance test. The experiments were conducted after the approval by the Institutional Ethics Committee (IEC) (Dated: 01.09.2014) of North-Eastern Hill University, Shillong, Meghalaya, India.
2.4.2. Lethal dose investigation
Single dose of ZAE (100–6000 mg/kg b.w.) was administered intraperitoneally (i.p.). The mice were kept under observation for 24 h. The LD50 was calculated using Karber30 method with modification by Aliu, Nwude.31 The calculation is given below:
| (1) |
n = total number of animal in a group.
a = the difference between two successive doses of administered extract/substance.
b = the average number of dead animals in two successive doses.
c = maximum dose.
2.4.3. Hypoglycemic activity in normoglycemic mice
The extract in varying doses ranging from 100 to 6000 mg/kg b.w. were administered to the normoglycemic mice by i.p. injection and glucose level was monitored at 2, 4, 6 and 24 h. SD check glucometer was used for measuring the blood glucose level. The control mice were given only distilled water.
2.4.4. Induction of diabetes
Alloxan monohydrate dissolved in sodium acetate buffer 0.15 M pH 4.5 at 80 mg/kg b.w. were intravenously administered to overnight fasted mice.32 After 48 h mice showing blood glucose level above 200 mg/dl were used for the study. The control group received only the buffer.
2.4.5. Antihyperglycemic activity in diabetic mice
The extract at the dose of 250 mg/kg b.w. were administered through i.p. injection to the diabetic mice. Metformin at 250 mg/kg b.w. (MET) and insulin at 0.1 IU/kg b.w. (INS) were used as reference drugs. The blood glucose level was determined after extract/standard drugs administration at 2, 4, 6 and 24 h.33
2.4.6. Glucose tolerance test in normoglycemic and diabetic mice
Normoglycemic or diabetic mice were administered with ZAE/standard drugs one and a half hour prior to the glucose load (2000 mg/kg b.w.).33 Glucose concentration was measured at 0, 30, 60, 120 and 1440 min after the glucose load. The control group received only the glucose load.
2.5. In vitro studies
2.5.1. Inhibition assay of α-amylase, lipase, α- and β-glucosidase
The enzymes inhibition procedure of α-amylase, α-glucosidase, β-glucosidase and lipase was according to the method of Hansawasdi et al34; Kim et al35; Sanchez-Medina et al14; Kumar et al36 respectively. Acarbose was used as a standard drug for α-amylase, α- and β-glucosidase while orlistat for lipase inhibition assay. The percentage (%) of inhibition for all the enzymes except for α-glucosidase was also expressed as the half maximal inhibitory concentration (IC50). The formula for % of inhibition is given below:
| (2) |
where Abscontrol is the absorbance read without the extract, Abssample is the absorbance read in the presence of the extract.
2.5.2. Antioxidant assay
-
(i)
Hydroxyl radical scavenging activity of ZAE/ascorbic acid (standard) was performed according to the method of Halliwell et al.37 The formula for % of hydroxyl radical scavenging activity is given below:
| (3) |
where Abscontrol was the absorbance read without the extract, Absextract was the absorbance read in the presence of extract. The % of hydroxyl radical scavenging activity of the extract was also expressed in IC50.
-
(ii)
The titration method using sodium thiosulphate was performed for hydrogen peroxide scavenging activity of ZAE/ascorbic acid (standard) according to Zhao et al.38 The hydrogen peroxide scavenging activity was calculated as:
| (4) |
where Vcontrol: volume of sodium thiosulfate used for titration in the presence of H2O2 (without extract), Vextract: volume of sodium thiosulfate solution used for titration in the presence of H2O2 (presence of extract). The % of hydrogen peroxide scavenging activity of the extract was expressed in IC50.
2.6. Phytochemical screening
Preliminary qualitative phytochemical analysis was carried out to identify the secondary metabolites present in the ZAE.39, 40, 41, 42, 43, 44, 45, 46
2.7. Elemental analysis
The elements such as Magnesium (Mg), Vanadium (V), Iron (Fe), Chromium (Cr), Lead (Pb), Zinc (Zn), Copper (Cu), Molybdenum (Mo), Manganese (Mn) was determined by Graphite furnace-AAS analysis (AAS Perkin Elmer 3110) at Sophisticated Analytical Instrumental Facility Centre (SAIF), North-Eastern Hill University, Shillong, India. Potassium (K), Calcium (Ca), Strontium (Sr), Phosphorus (P), Selenium (Se), Titanium (Ti) and Arsenic (As) were analysed using ICP-AES (Arcos from M/S. Spectro, Germany), at SAIF, Indian Institute of Technology, Mumbai, India.
2.8. Statistical analysis
For in vivo study, data were expressed as mean ± standard error of the mean (SEM). To determine the level of significance, the data were analysed by student's t-tests. The values p < 0.05∗, p < 0.01∗∗, p < 0.001∗∗∗ were considered as statistically significant.
For in vitro enzyme inhibitory activity and antioxidant activity, the data were expressed as means ± SEM of the % of inhibition. The concentration of ZAE required to inhibit 50% (IC50) were determined by linear regression analysis between the % of inhibition versus the extract concentration by using the excel program.
3. Results
3.1. In vivo studies
3.1.1. Lethal dose investigation
Administration of ZAE did not show mortality in mice treated with doses of 100–4000 mg/kg b.w. whereas at the dose of 6000 mg/kg b.w. 100% mortality was observed within 24 h. The calculated LD50 was found to be 5000 mg/kg b.w.
3.1.2. Hypoglycemic activity and glucose tolerance in normoglycemic mice
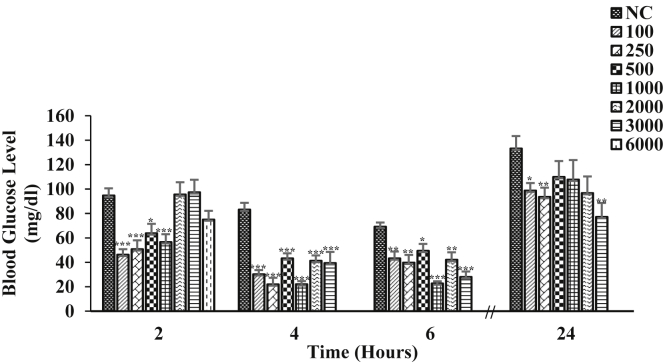
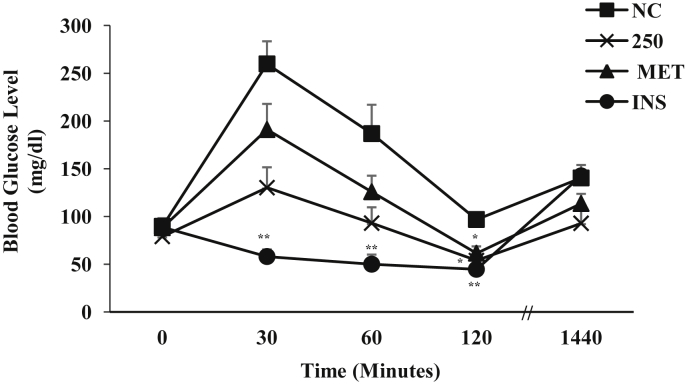
The effect of different doses (100–6000 mg/kg b.w.) of the ZAE on the normoglycemic mice is illustrated in Fig. 1. Among the lower doses, 250 mg/kg b.w. was found to be more effective in reducing the blood glucose level and it showed maximum reduction at 4 h by 73.75% (p < 0.001) when compared to the control. The result of the glucose tolerance test performed in normoglycemic mice is given in Fig. 2. Half an hour after the administration of the glucose load, there was a marked increase in the blood glucose level of the control and treated mice. The mice treated with ZAE suppressed the glucose peak significantly at 120 min by 31.86% (p < 0.05) compared to 0 min. The mice treated with MET and INS was able to significantly suppressed the glucose peak at 120 min by 30.25% (p < 0.05) and 49.72% (p < 0.01) respectively. The effect of ZAE on the pattern of reduction of blood glucose level at 30, 60 and 120 min was more pronounced than MET but showed lower effect than INS.
Fig. 1.
Effect of varying doses (represented as mg/kg b.w.) of ZAE in normoglycemic mice (n = 6) where NC is normoglycemic control. Values are expressed in mean ± SEM (p < 0.05∗, p < 0.01∗∗, p < 0.001∗∗∗).
Fig. 2.
Glucose tolerance test in normoglycemic mice (n = 6) administered with ZAE (250 mg/kg b.w.)/metformin (MET; 250 mg/kg b.w.) and insulin (INS; 0.1 IU/kg b.w.) where NC is normoglycemic control. Values are expressed in mean ± SEM (p < 0.05∗, p < 0.01∗∗, p < 0.001∗∗∗).
3.1.3. Antihyperglycemic activity and glucose tolerance test in diabetic mice
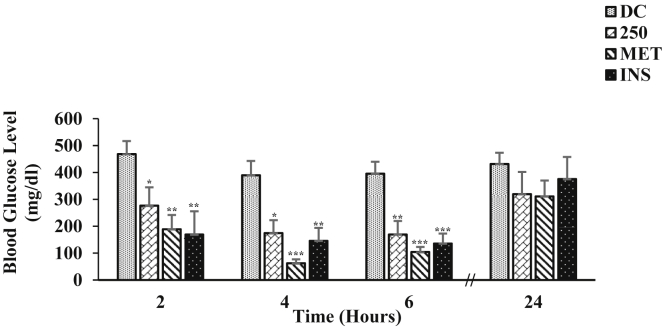
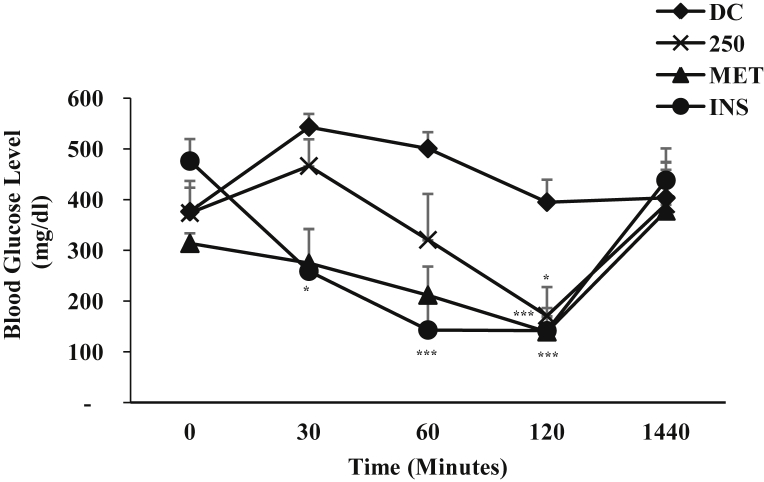
The effect of single i.p. administration of ZAE (250 mg/kg b.w.) on blood glucose level of diabetic mice is represented in Fig. 3. ZAE displayed its antihyperglycemic effect starting from 2 h as seen in the hypoglycemic study and maximum reduction in the blood glucose level was observed at 6 h (57.23%; p < 0.01) when compared to the diabetic control. MET showed antihyperglycemic effect at 4 h by 84.00% (p < 0.001) and at 6 h by 73.66% (p < 0.001) respectively while INS showed antihyperglycemic effect at 2 h by 63.93% (p < 0.01), at 4 h by 62.57% (p < 0.01) and at 6 h by 65.69% (p < 0.001) respectively when compared to the diabetic control. Glucose tolerance test was also performed in diabetic mice and the result is given in Fig. 4. As seen in the graph, at 30 min after the glucose load there was a marked increase on the blood glucose level of the diabetic control and a slight increase in the mice treated with ZAE/standard drug. The blood glucose level of the diabetic mice treated with ZAE (250 mg/kg b.w.) was able to improve the blood glucose pattern by 54.24% (p < 0.05) at 120 min. MET was able to reduce the elevated blood glucose level by 55.61% (p < 0.001) whereas, insulin showed the highest percentage reduction (70.30%, p < 0.001) at 120 min when compared to its respective 0 min. In both antihyperglycemic activity and glucose tolerance test, ZAE improved the blood glucose level in diabetic mice, however, its magnitude of effect on the blood glucose level was lower than MET including INS at all-time point.
Fig. 3.
Effect of ZAE (250 mg/kg b.w.)/metformin (MET; 250 mg/kg b.w.) and insulin (INS; 0.1 IU/kg b.w.) on blood glucose level of diabetic mice (n = 6) where DC is diabetic control. Values are expressed in mean ± SEM (p < 0.05∗, p < 0.01∗∗, p < 0.001∗∗∗).
Fig. 4.
Glucose tolerance test in diabetic mice (n = 6) administered with ZAE (250 mg/kg b.w.)/metformin (MET; 250 mg/kg b.w.) and insulin (INS; 0.1 IU/kg b.w.) where DC is diabetic control. Values are expressed in mean ± SEM (p < 0.05∗, p < 0.01∗∗, p < 0.001∗∗∗).
3.2. In vitro studies
3.2.1. Inhibition assay of α-amylase, lipase, α- and β-glucosidase
The IC50 of extract against α-amylase was 7.40 mg/ml while acarbose was 4.42 mg/ml (Table 1), β-glucosidase was 0.30 mg/ml for extract while acarbose showed IC50 of 0.07 mg/ml (Table 1) and lipase showed IC50 value of 8.33 mg/ml for extract while orlistat was 3.97 mg/ml (Table 1). The ZAE extract was found to inhibit α-glucosidase by 79.82% (0.8 mg/ml) whereas acarbose was able to inhibit 23.83% (0.8 mg/ml) (Table 2).
Table 1.
In vitro inhibitory effects of ZAE on β-glucosidase, α-amylase, lipase and hydroxyl radical and hydrogen peroxide scavenging activity.
| β-glucosidase (% of inhibition) |
α-amylase (% of inhibition) |
Lipase (% of inhibition) |
% of hydroxyl radical activity |
% of hydrogen peroxide activity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/ml | ZAE | STD | mg/ml | ZAE | STD | ZAE | STD | ZAE | STD | ZAE | STD |
| 0.02 | 05.40 ± 00.57 | 34.91 ± 2.88 | 0.2 | 15.92 ± 01.66 | 18.20 ± 0.95 | 13.58 ± 1.62 | 19.58 ± 4.87 | 31.86 ± 1.74 | 15.05 ± 1.38 | 18.92 ± 1.74 | 12.58 ± 0.71 |
| 0.04 | 08.36 ± 01.35 | 39.27 ± 1.75 | 0.4 | 20.17 ± 01.25 | 22.75 ± 1.51 | 18.15 ± 1.78 | 24.07 ± 4.33 | 34.09 ± 4.31 | 18.16 ± 1.33 | 25.18 ± 0.51 | 15.88 ± 0.80 |
| 0.06 | 18.04 ± 04.49 | 47.64 ± 2.92 | 0.6 | 24.82 ± 01.75 | 30.69 ± 1.76 | 25.03 ± 4.25 | 39.66 ± 3.99 | 39.05 ± 3.05 | 25.30 ± 2.46 | 30.89 ± 1.23 | 26.65 ± 2.44 |
| 0.08 | 11.01 ± 03.34 | 59.76 ± 2.28 | 0.8 | 30.77 ± 01.77 | 38.97 ± 1.68 | 23.48 ± 4.08 | 41.64 ± 3.83 | 40.01 ± 1.96 | 29.20 ± 1.95 | 37.82 ± 1.74 | 34.99 ± 1.81 |
| 0.1 | 21.14 ± 04.39 | 68.09 ± 0.89 | 1.0 | 29.46 ± 0.84 | 38.72 ± 1.65 | 29.97 ± 2.66 | 44.95 ± 5.77 | 46.07 ± 2.19 | 32.05 ± 2.65 | 47.54 ± 3.30 | 39.82 ± 1.96 |
| 0.2 | 47.17 ± 02.26 | 72.78 ± 1.20 | 2.0 | 36.11 ± 4.43 | 45.42 ± 1.57 | 31.45 ± 2.55 | 51.34 ± 3.74 | 48.89 ± 1.96 | 35.22 ± 1.28 | 54.30 ± 4.13 | 40.39 ± 2.65 |
| 0.4 | 53.85 ± 05.59 | 75.19 ± 0.96 | 4.0 | 46.75 ± 2.28 | 51.87 ± 1.51 | 39.82 ± 4.88 | 57.80 ± 4.54 | 54.47 ± 1.99 | 39.06 ± 2.82 | 60.98 ± 2.44 | 44.91 ± 1.93 |
| 0.6 | 54.59 ± 08.61 | 76.94 ± 1.47 | 6.0 | 49.80 ± 1.02 | 58.88 ± 1.28 | 41.27 ± 2.74 | 61.26 ± 5.78 | 58.04 ± 2.31 | 44.84 ± 1.87 | 66.98 ± 2.48 | 47.65 ± 0.96 |
| 0.8 | 57.18 ± 04.77 | 79.61 ± 2.31 | 8.0 | 51.66 ± 0.70 | 67.88 ± 1.57 | 47.51 ± 2.56 | 63.91 ± 5.08 | 63.50 ± 1.74 | 47.47 ± 1.67 | 70.63 ± 0.96 | 50.60 ± 0.83 |
| 1.0 | 67.79 ± 10.10 | 83.59 ± 1.97 | 10 | 52.21 ± 1.16 | 72.31 ± 2.74 | 54.20 ± 1.27 | 68.67 ± 5.23 | 68.54 ± 1.63 | 50.30 ± 1.13 | 74.99 ± 1.01 | 53.16 ± 1.25 |
Values are represented as mean ± SEM of % of inhibition (n = 5). Results are also expressed in IC50.The IC50 values are as follows: For β-glucosidase: 0.30 mg/ml ZAE and 0.07mg/ml acarbose as standard (STD); α-amylase: 7.40 mg/ml ZAE and 4.42 mg/ml acarbose (STD): lipase: 8.33 mg/ml ZAE, 3.97 mg/ml orlistat (STD); hydroxyl radical activity: ZAE 8.46mg/ml and 1.10 mg/ml ascorbic acid (STD); hydrogen peroxide activity 9.62 mg/ml ZAE and 3.25 mg/ml ascorbic acid (STD).
Table 2.
In vitro inhibitory effect of ZAE on α-glucosidase.
| Concentration |
% of inhibition |
|
|---|---|---|
| mg/ml | ZAE | Acarbose |
| 0.2 | 74.46 ± 2.40 | 25.85 ± 4.37 |
| 0.4 | 69.48 ± 0.66 | 24.61 ± 1.29 |
| 0.6 | 79.21 ± 1.81 | 24.19 ± 0.16 |
| 0.8 | 79.82 ± 0.96 | 23.83 ± 0.45 |
Values are represented as mean ± SEM (n = 3). Results are expressed in % of inhibition.
3.2.2. Antioxidant assay
The IC50 of hydroxyl radical assay was found to be 8.46 mg/ml and 1.10 mg/ml for ZAE and ascorbic acid respectively (Table 1). The IC50 of hydrogen scavenging assay was found to be 9.62 mg/ml and 3.25 mg/ml for ZAE and ascorbic acid respectively (Table 1).
3.3. Phytochemical screening
The preliminary phytochemical screening tests for ZAE revealed the presence of carbohydrates, steroids, saponins, alkaloids, terpenoids, phenols, tannins and proteins (Table 3).
Table 3.
Phytochemical test for the presence of different constituents in ZAE.
| Plant constituents | Test | Present (+)/Absent (−) |
|---|---|---|
| Carbohydrates | Molisch's tests | + |
| Fehling's tests | + | |
| Proteins | + | |
| Fatty acids | − | |
| Steroids | + | |
| Saponins | + | |
| Alkaloids | Wagner's test | − |
| Dragendorff's test | + | |
| Terpenoids | + | |
| Phenols | + | |
| Tannins | + | |
| Flavonoids | Pew's test | − |
| Alkaline reagent test | − |
3.4. Elemental analysis
The presence of the essential elements such as Mg, V, Fe, Cr, Zn, Cu, Mo, Mn, K, Ca, P and Sr in the plant sample is depicted in Table 4, Table 5.
Table 4.
Elemental analysis of Z. armatum leaves using AAS.
| Elements | Mg | V | Fe | Cr | Pb | Zn | Cu | Mo | Mn |
|---|---|---|---|---|---|---|---|---|---|
| Values ppm | 0.41 ± 0.003 | 0.39 ± 0.001 | 0.30 ± 0.001 | 0.13 ± 0.003 | 0.13 ± 0.001 | 0.13 ± 0.002 | 0.11 ± 0.003 | 0.06 ± 0.003 | 0.053 ± 0.002 |
Result are expressed in parts per million (ppm).
Table 5.
Elemental analysis of Z. armatum leaves using ICP-AES.
| Elements | K | Ca | P | Sr | Se | Ti | As |
|---|---|---|---|---|---|---|---|
| Values % | 2.089 | 1.424 | 0.274 | 0.0039 | ND | ND | ND |
Results are expressed in percentage (%). ND (Not detectable).
4. Discussion
The ZAE was found to have a good safety profile with calculated LD50 of 5000 mg/kg b.w. The effects of the higher doses (1000–3000 mg/kg b.w.) were found to be similar as in lower doses (100–500 mg/kg b.w.). The lower doses of extract would be more preferred over higher doses since lower doses are considered to be less toxic and safe.47 The reduction in blood glucose level was observed from 2 h in all the doses that was used in the study. The hypoglycemic result indicates that ZAE has compound(s) that can attenuate the blood glucose level of the normoglycemic mice when compared to the control. Alloxan, a beta cytotoxin chemical, used for inducing diabetes, works by destroying the pancreatic beta-cells resulting in decreased level of insulin production and ultimately leading to increase in the blood glucose level.48 In the present study, the mice administered with alloxan were seen to have elevated blood glucose level whereas administration of ZAE caused a decline in the rise of the blood glucose level in the diabetic mice. During glucose tolerance test, ZAE was observed to be more effective than MET in reducing the blood glucose level in normoglycemic mice while in diabetic mice, MET was more effective than ZAE. The pattern of blood glucose reduction at all-time point shown by the plant extract in normoglycemic and diabetic mice suggested that the extract contain principle(s) that work differently from MET. MET, a biguanide, an antihyperglycemic drug do not cause hypoglycemia in normal subjects even when taken in excessive dose49, 50 and this is because it lowers blood glucose without increasing insulin secretion.51 Therefore in our study, MET administration showed more pronounced blood glucose lowering effect in diabetic mice than in normoglycemic mice. Plant extract producing hypoglycemic effects can act by various mode and mechanisms. Some of the plant extract have been shown to have glibenclamide like activity.52 Glibenclamide is a sulfonylurea derivative, causes hypoglycemia by stimulating pancreatic beta-cells to release more insulin and inhibiting glucagon secretion.53 Other plant extracts have either insulin-like effect, increase insulin secretion from beta-cells of pancreas, regenerate gamma-cell from islets of langerhans, reduce absorption of glucose from gastrointestinal tract, increase glucose utilization, etc.54 Therefore, ZAE may possibly exert multiple actions involving different mechanisms in exerting hypoglycemic and, antihyperglycemic effects.
Inhibition of α-amylase, α- and β-glucosidase activities by the extract could have contributed to its antidiabetic effect, thereby slowing down the degradation of starch to disaccharide and ultimately reducing the elevated blood glucose level which has been noted in diabetic mice treated with the extract. Pancreatic lipase inhibition is also a valuable target for the treatment of diet-induced hyperglycemia. Hence, ZAE simultaneously inhibited both the carbohydrates and lipid hydrolyzing enzymes. Previous report has already discussed the beneficial effect of other plants having enzymes inhibitory effects against amylase, glucosidases and lipase.12, 13, 14, 15 During hyperglycemia, scavenging ROS such as hydrogen peroxide (H2O2) and hydroxyl radical (OH) are considered to be crucial as they can lead to oxidative stress.16 The ability of the extract to scavenge the H2O2 and OH radicals can be beneficial for reducing the risk for developing diabetes and its complication.
The presence of one or more compounds in the plant extract may involve in decreasing the blood glucose suggesting that the natural constituents could have act separately or synergistically to induce the hypoglycemic effect.55 The antidiabetic property of some medicinal plants have also assumed to be due to the presence of the phytochemical compounds.56, 57 Therefore, the antidiabetic property display by ZAE might have been attributed by the presence of various chemical constituent(s) such as alkaloids, flavonoids, saponins, and tannins.
A number of studies have also link diabetes with deficiency of some important elements viz. Mg V, Fe, Cr, Zn, Cu, Mo, Mn, K, Ca and P. Plants with antidiabetic property are widely known to contain these essential elements through which they can be a good alternative as a supplement. Mg is known to be present in various enzymes involved in glucose oxidation pathways and also play a role in glucose transport mechanism.58 V affects carbohydrate metabolism including glucose transport, glycolysis, glucose oxidation and glycogen synthesis.59 Insulinomimetic property is a possible mechanism of action of V in glycemic control by up regulation of insulin receptors.60 Glucose metabolism and insulin action can be influenced by the presence of Fe.61 Cr is a crucial trace element which is important in glucose homeostasis.62 Zn play an important role in maintaining the structural integrity of insulin. In which, deficiency of Zn causes a large number of metabolic disturbances such as impaired glucose tolerance, insulin degradation and reduced pancreatic insulin content.63 Cu is known to possess an insulin-like activity and promotes lipogenesis.64 Studies has shown that Mn has the ability to decrease glucose intolerance.65 Mo can increase receptor autophosphorylation and phosphorylation of its substrate and augment glucose transport.66 K is a well proven insulin secretagogue in the intact organism and the isolated pancreas.67 Increased in cytosolic Ca ions is important for mediating the effect of glucose by stimulating the insulin from beta cells in which alteration in Ca flux can leads to adverse effect of the insulin producing cells.68 P has a property of maintaining normal blood glucose.69 The biological role of Ti and Sr is not much known. Therefore, the aforementioned elements that were found in the leaves might have played a direct or indirect role in maintaining the glucose level. The level of the element is in accordance with other plants and is within the permissible limit.70
5. Conclusion
In conclusion, both the in vivo and in vitro approaches give insight understanding about the antidiabetic potential of Z. armatum aqueous leaves extract. Inhibition of α-amylase, glucosidases, lipase and its antioxidant activities studied under in vitro condition including the presence of phytochemical compounds in ZAE could be associated with its hypoglycemic and antihyperglycemic activity. This study has also established that ZAE have appreciable quantities of some of the trace elements associated with glucose lowering effects and the antidiabetic property of Z. armatum aqueous leaves extract reported in this study can be attributed to, among others, some of these trace elements present in ZAE.
Further studies can be undertaken at the cellular and molecular levels, which may further elucidate its mechanism in detail. Additionally, toxicity tests involving long duration administration of the extract to animal model are needed to comprehensively evaluate the safety of ZAE. Therefore, the present investigation has opened avenue for further research especially with reference to the development of method for isolating, identifying and characterizing the active constituent (s) that are responsible for the antidiabetic property of Z. armatum aqueous leaves extract.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgements
Research was partly supported by Science & Engineering Research Board (SERB), Department of Science & Technology (DST), Government of India, New Delhi (Sanction No: SERB/F/5362/2013-14 dated 19.11.2013). Additional support was provided by the Department of Biotechnology & Bioinformatics, North-Eastern Hill University, Shillong, Meghalaya. Additional support was provided by the Department of Biotechnology & Bioinformatics, North-Eastern Hill University, Shillong, Meghalaya through grants received from funding agency i.e., Department of Biotechnology (DBT-M.Sc. Biotechnology programme; BT/HRD/01/06/2006/vol II dated 27.10.2014), Government of India (GoI), New Delhi and Department of Science & Technology (DST-FIST programme, 100/IFD/12861/2012-2011 dated 09.03.2011), GoI, New Delhi.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.World Health Organisation . Diagnosis and Classification of Diabetes Mellitus; Geneva: 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications, Report of a WHO Consultation Part 1; p. 2. [Google Scholar]
- 2.Afifi A.F., Kamel E.M., Khalil A.A. Purification and characterization of α-amylase from Penicillium olsonii under the effect of some antioxidant vitamins. Glob J Biotech Biochem. 2008;3:14–21. [Google Scholar]
- 3.De Melo E.B., Da Silveira Gomes A., Carvalho I. α and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron. 2006;62:10277–10302. [Google Scholar]
- 4.Raptis S.A., Dimitriadis G.D. Oral hypoglycemic agents: insulin secretagogues, α-glucosidase inhibitors and insulin sensitizers. Exp Clin Endocrinol Diabetes. 2001;109(2):S265–S287. doi: 10.1055/s-2001-18588. [DOI] [PubMed] [Google Scholar]
- 5.Ros E. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis. 2000;151:357–379. doi: 10.1016/s0021-9150(00)00456-1. [DOI] [PubMed] [Google Scholar]
- 6.Patrick G.L. 5th ed. Oxford University Press; United Kingdom: 2013. An Introduction to Medicinal Chemistry; p. 90. [Google Scholar]
- 7.Matough F.A., Budin S.B., Hamid Z.A. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eurich D.T., Mcalister F.A., Blackburn D.F. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. Br Med J. 2007;335:497–501. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antu K.A., Riya M.P., Mishra A. Antidiabetic property of Symplocos cochinchinensis mediated by inhibition of alpha-glucosidase and enhanced insulin sensitivity. Plos One. 2014;9:1–13. doi: 10.1371/journal.pone.0105829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day C. Traditional plant treatments for diabetes mellitus: pharmaceutical foods. Br J Nutr. 1998;80:203–208. doi: 10.1017/s0007114598001718. [DOI] [PubMed] [Google Scholar]
- 11.Frankish N., De Sousa Menezes F., Mills C. Enhancement of insulin release from the beta-cell line INS-1 by an ethanolic extract of Bauhinia variegata and its major constituent roseoside. Planta Med. 2010;76:995–997. doi: 10.1055/s-0029-1240868. [DOI] [PubMed] [Google Scholar]
- 12.Lima C.F., Azevedo M.F., Araujo R. Metformin-like effect of Salvia officinalis (common sage); is it useful in diabetes prevention? Br J Nutr. 2006;96:326–333. doi: 10.1079/bjn20061832. [DOI] [PubMed] [Google Scholar]
- 13.Sancheti S., Sancheti S., Seo S.-Y. Antidiabetic and antiacetylcholinesterase effects of ethyl acetate fraction of Chaenomeles sinensis (Thouin) koehne fruits in streptozotocin-induced diabetic rats. Exp Toxicol Pathol. 2013;65:55–60. doi: 10.1016/j.etp.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Medina A., García-Sosa K., May-Pat F. Evaluation of biological activity of crude extracts for plants used in Yucatecan traditional medicine part I. Antioxidant, antimicrobial and β-glucosidase inhibition activities. Phytomedicine. 2001;8:144–151. doi: 10.1078/0944-7113-00020. [DOI] [PubMed] [Google Scholar]
- 15.Mazumdar U.K., Gupta M., Rajeshwar Y. Antihyperglycemic effect and antioxidant potential of Phyllanthus niruri (Euphorbiaceae) in streptozotocin induced diabetic rats. Eur Bull Drug Res. 2005;13:15–23. [Google Scholar]
- 16.Tiwari A.K., Rao J.M. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci. 2002;83:30–38. [Google Scholar]
- 17.Singh T.P., Singh O.M. Phytochemical and pharmacological profile of Zanthoxylum armatum DC. – an overview. Indian J Nat Prod Resour. 2011;2:275–278. [Google Scholar]
- 18.Verma N., Khosa R.L. Hepatoprotective activity of leaves of Zanthoxylum armatum DC. in CCl4 induce hepatotoxicity in rats. Indian J Biochem Biophys. 2010;47:124–127. [PubMed] [Google Scholar]
- 19.Brijwal L., Pandey A., Tamta S. An overview on phytomedicinal approaches of Zanthoxylum armatum DC.: an important magical medicinal plant. J Med Plant Res. 2013;7:366–370. [Google Scholar]
- 20.Kharshiing V.E. Aqueous extracts of dried fruits of Zanthoxylum armatum DC. (Rutaceae) induce cellular and nuclear damage coupled with inhibition of mitotic activity in vivo. Am J Plant Sci. 2012;3:1646–1653. [Google Scholar]
- 21.Kayang H. Tribal knowledge on wild edible plants of Meghalaya, Northeast India. Indian Tradit Knowl. 2007;6:177–181. [Google Scholar]
- 22.Parajuli R.R., Tiwari R.D., Chaudhary R.P. Fungitoxicity of the essential oils of some aromatic plants of manang against Alternaria brassicicola. Sci World J. 2005;3(3):39–43. [Google Scholar]
- 23.Ranawat L., Bhatt J., Patel J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC. in CCl4 induced hepatic damage in rats. J Ethnopharmacol. 2010;127:777–780. doi: 10.1016/j.jep.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Guo T., Deng Y.X., Xie H. Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice. Fitoterapia. 2011;82:347–351. doi: 10.1016/j.fitote.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Karmakar I., Haldar S., Chakraborty M. Antioxidant and cytotoxic activity of different extracts of Zanthoxylum alatum. Free Rad Antiox. 2015;5:21–28. [Google Scholar]
- 26.Kimani C.N., Mbaria J.M., Suleiman M. Antihyperglycemic activity of Zanthoxylum chalybeum stem bark extract in diabetic rats. J Phytopharm. 2015;4(3):183–189. [Google Scholar]
- 27.Aloke C., Nwachukwu N., Ugwuja E.I. Effects of Zanthoxylum zanthoxyloides leaves on blood glucose, lipid profile and some liver enzymes in alloxan induced diabetic rats. Int J Sci Nat. 2012;3(3):497–501. [Google Scholar]
- 28.Karki H., Upadhayay K., Pal H. Antidiabetic potential of Zanthoxylum armatum bark extract on streptozotocin-induced diabetic rats. Int J Green Pharm. 2014;16:77–83. [Google Scholar]
- 29.Venkateswaran P.S., Millman I., Blumberg B.S. Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: in vitro and in vivo studies. Proc Natl Acad Sci. 1987;84:274–278. doi: 10.1073/pnas.84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karber G. Beitrag zur kollecktiven behandlung pharmakologischer reihenversuche. Arch Exptl Pathol Pharmakol. 1931;162:480–483. Cited by Turner, R. Screening methods in pharmacology. New York and London: Academic Press. 1965:63−64. [Google Scholar]
- 31.Aliu Y.O., Nwude N. 1st ed. Baraka Press and Publishers Ltd; Nigeria: 1982. Veterinary Pharmacology and Toxicology Experiments; pp. 104–110. [Google Scholar]
- 32.Hui Z.-G., Zhou X.-W., Li R.-J. Studies on the extraction process of total flavonoids in Radix puerariae and their hypoglycemic effect in mice. Biomed Res. 2015;26(1):51–54. [Google Scholar]
- 33.Syiem D., Syngai G., Khup P.Z. Hypoglycemic effects of Potentilla fulgens L. in normal and alloxan induced diabetic mice. J Ethnopharmacol. 2002;83:55–61. doi: 10.1016/s0378-8741(02)00190-3. [DOI] [PubMed] [Google Scholar]
- 34.Hansawasdi C., Kawabata J., Kasai T. Alpha-amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci Biotechnol Biochem. 2000;64:1041–1043. doi: 10.1271/bbb.64.1041. [DOI] [PubMed] [Google Scholar]
- 35.Kim J.-S., Yang J., Kim M.-J. Alpha glucosidase inhibitory effect, anti-microbial activity and UPLC analysis of Rhus verniciflua under various extract conditions. J Med Plants Res. 2011;5:778–783. [Google Scholar]
- 36.Kumar A.H.S., Kekuda P.T.R., Vinayaka K.S. Anti-obesity (Pancreatic lipase inhibitory) activity of Everniastrum cirrhatum (Fr.) Hale (Parmeliaceae) Phcog J. 2011;3:65–68. [Google Scholar]
- 37.Halliwell B., Gutteridge J.M., Aruoma O.I. The deoxyribose method: a sample test tube assay for determination of rate constant for reaction of hydroxyl radicals. Anal BioChem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 38.Zhao G.R., Xiang Z.J., Ye T.X. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 2006;99:676–774. [Google Scholar]
- 39.Egwaikhide P.A., Gimba C.E. Analysis of chemical content and anti-microbial activity of Plectranthus glandulosis whole plant. Middle East J Sci Res. 2007;2:135–138. [Google Scholar]
- 40.Savithramma N., Rao M.L., Suhrulatha D. Screening of medicinal plants for secondary metabolites. Middle East J Sci Res. 2011;8:579–584. [Google Scholar]
- 41.Rathore S.K., Bhatt S., Dhyani S. Preliminary phytochemical screening of medicinal plant Ziziphus mauritiana Lam. fruits. Int J Curr Pharm Res. 2011;4:160–162. [Google Scholar]
- 42.Mishra A.P., Saklani S. Satyrium nepalense: a rare medicinal orchid of western Himalaya (India); Phytochemical screening, anti-microbial evaluation and conservation studies. Indones J Pharm. 2012;23:162–170. [Google Scholar]
- 43.Saklani S., Mishra A.P., Parcha V. Phytochemical and anti-bacterial evaluation of Satyrium nepalense and Saussurea simpsoniana, the threatened medicinal herbs of Uttarakhand. J Pharm Res. 2011;4:3866–3870. [Google Scholar]
- 44.Usman H., Abdulrahman F., Usman A. Qualitative phytochemical screening and in vitro anti-microbial effects of methanol stem bark extract of Ficus thonningii (Moraceae) Afr J Tradit Complement Altern Med. 2009;6:289–295. doi: 10.4314/ajtcam.v6i3.57178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yadav R.N., Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3:10–14. [Google Scholar]
- 46.Suganya R.S., Priya K., Roxy S. Phytochemical screening and antibacterial activity from Nerium oleander and evaluate their plant mediated nanoparticle synthesis. Int Res J Pharm. 2012;3:285–288. [Google Scholar]
- 47.Jothy S.L., Zakaria Z., Chen Y. Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules. 2011;16:5268–5282. doi: 10.3390/molecules16065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto H., Uchigata Y., Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981;294(5838):284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- 49.Gilmar G.A., Rall W.T., Nies S.A. vol. II. 1992. Pharmacological Basis of Therapeutics. (McCrow-Hill International Editions Medical Series). English edition. [Google Scholar]
- 50.Abuelgasim A.I., Osman M.K.M., Elmahdi B. Effects of Aloe vera (Elsabar) ethanolic extract on blood glucose level in wistar albino rats. J Appl Sci Res. 2008;4(2):1841–1845. [Google Scholar]
- 51.Hundal R.S., Inzucchi S.E. Metformin: new understandings, new uses. Drugs. 2003;63:1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 52.Asad M., Munir T.A., Farid S. Duration effect of Acacia nilotica leaves extract and glebenclamide as hypolipidaemic and hypoglycaemic activity in alloxan-induced diabetic rats. J Pak Med Assoc. 2015;65(12):1266–1270. [PubMed] [Google Scholar]
- 53.Syiem D., Khup P.Z. Study of the traditionally used medicinal plant Osbeckia chinensis for hypoglycemic and anti-hyperglycemic effects in mice. Pharm Biol. 2006;44(8):613–618. [Google Scholar]
- 54.Singab A.N., Youssef F.S., Ashour M.L. Medicinal plants with potential antidiabetic activity and their assessment. Med Aromat Plants. 2014;3(1):151. [Google Scholar]
- 55.Marles R.J., Farnsworth N.R. Antidiabetic plants and their constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 56.Meenatchi P., Purushothaman A., Maneemegalai S. Antioxidant, antiglycation and insulinotrophic properties of Coccinia grandis (L.) in vitro: possible role in prevention of diabetic complications. J Tradit Complement Med. 2017;7:54–64. doi: 10.1016/j.jtcme.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birru E.M., Abdelwuhab M., Shewamene Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. On blood glucose level of normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2015;15:321. doi: 10.1186/s12906-015-0852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velayutharaj A., Saraswathi R., Shivakumar R. Association of serum magnesium with glycemic control and insulin resistance in patients with type 2 diabetes mellitus. Int J Curr Res Rev. 2016;8(13):17–23. [Google Scholar]
- 59.Orvig C., Thompson K.H., Battell M. Vanadium compounds as insulin mimics. Met Ions Biol Syst. 1995;31:575–594. [PubMed] [Google Scholar]
- 60.O'Connell B. Select vitamins and minerals in the management of diabetes. Diabetes Spectr. 2001;14:133–148. [Google Scholar]
- 61.Khaw K.T., Barrett-Connor E. Dietary potassium and blood pressure in a population. Am J Clin Nutr. 1984;39:963–968. doi: 10.1093/ajcn/39.6.963. [DOI] [PubMed] [Google Scholar]
- 62.Guimarães M.M., Carvalho A.C., Silva M.S. Effect of chromium supplementation on the glucose homeostasis and anthropometry of type 2 diabetic patients: double blind, randomized clinical trial. J Trace Elem Med Biol. 2016;36:65–72. doi: 10.1016/j.jtemb.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Nsonwu A.C., Usoro C.A.O., Etukudo M.H. Glycemic control and serum and urine levels of zinc and magnesium in diabetics in Calabar, Nigeria. Pak J Nutr. 2006;5(1):75–78. [Google Scholar]
- 64.Siddiqui K., Bawazeer N., Joy S.S. Variation in macro and trace elements in progression of type 2 diabetes. Sci World J. 2014:461–591. doi: 10.1155/2014/461591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keen C.L., Zidenberg-Cherr S. Manganese. In: Ziegler E.E., Filer L.J., editors. Present Knowledge in Nutrition. 7th ed. ILSI Press; Washington, DC: 1996. pp. 334–343. [Google Scholar]
- 66.Fillat C., Rodríguez-Gil J.E., Guinovart J.J. Molybdate and tungstate act like vanadate on glucose metabolism in isolated hepatocytes. Comparison of insulin action on glucose versus potassium uptake in humans. Biochem J. 1992;282(Pt 3):659–663. doi: 10.1042/bj2820659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen T.Q., Maalouf N.M., Sakhaee K. Comparison of insulin action on glucose versus potassium uptake in humans. Clin J Am Soc Nephrol. 2011;6(7):1533–1539. doi: 10.2215/CJN.00750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arika W.M., Ogola P.E., Nyamai D.W. Mineral elements content of selected Kenyan antidiabetic medicinal plants. Adv Tech Biol Med. 2016;4(1):1–5. [Google Scholar]
- 69.Linder Manria C. vol. 2. Appleton and Lange; Norwalk: 1991. pp. 191–212. (Nutritional Biochemistry and metabolism with clinical applications). [Google Scholar]
- 70.Saraf A., Samant A. Evaluation of mineral and trace element in Achyranthes aspera (Linn) Int J Pharm Sci. 2013;3(3):229–233. [Google Scholar]