Abstract
Cyperus articulatus (CA) rhizomes have demonstrated different properties on nervous system. However, the leaves still have not studied to treat epilepsy. The aim of this study was to determine the effect of CA ethanolic extract on pentylenetetrazol (PTZ) induced seizures in mice as well as measuring its antioxidant activity in vivo and in vitro. Mice were divided into five groups: (1) control (PTZ 80 mg/kg; i.p.), (2) PTZ-Diazepam (1 mg/kg; i.p.), (3–5) PTZ-CA 50, PTZ-CA 150 and PTZ-CA 300 (50, 150 and 300 mg/kg of CA extract, 30 min prior to each PTZ injection). The PTZ-CA 150 group showed lower seizure scores (P < 0.01), latency (P < 0.01), frequency (P < 0.01) and duration (P < 0.01) than control group. The antioxidant activity of CA extract scavenged DPPH radical showed IC 50 = 16.9 ± 0.1 μg/mL and TEAC = 2.28 ± 0.08, mmol trolox/g of extract, the content of gamma amino butyric acid (GABA) and malondialdehyde (MDA) were significantly high (P < 0.01) at dose of 150 mg/kg (82 ± 1.2 ng/g tissue; 1.0 ± 2.2 mol/g tissue, respectively). The present research demonstrated that CA extract possesses a potential effect to prevent PTZ induced seizures, antioxidant activity in addition to increase GABA levels.
Keywords: Pentylenetetrazol, Anticonvulsant, Cyperus articulatus, Antioxidant
Graphical abstract
1. Introduction
Epilepsy is a chronic disease characterized by recurrent seizures and other complications, which has been demonstrated to cause oxidative stress by reactive oxygen species (ROS) in the brain.1 Approximately, more than 50 million people worldwide have been diagnosed with epilepsy, representing a public health problem of all ages, genera and social groups. Medical reports have shown an incidence rate of epilepsy in developed countries of 50 per 100,000 otherwise in developing countries is 100 per 100,000.2
The modern concept of epilepsy proposed by the International League Against Epilepsy (ILAE) involves the occurrence of at least one or more epileptic seizures.3 The etiology can be genetic or sporadic, by consequence, cerebral damage could be linked to congenital cortical abnormality, traumas, neurocysticercosis, infections or cerebral tumor. The pathophysiology of epilepsy is known by depolarization disorders from the neural membrane, ionic medium and neuronal morphology.4, 5 These alterations produce an imbalance of excitatory and inhibitory neurotransmitters like excitatory aminoacids, (glutamate or aspartate) or inhibitory neurotransmissions (gamma amino butyric acid – GABA).6
One of the experimental models for inducing epileptic seizures is pentylenetetrazol (PTZ), this chemical agent produces primary generalized discharges and acts as a noncompetitive agonist of GABAA receptors, also induces oxidative stress on the brain, altering the normal metabolism of phospholipids and proteins. Oxidative stress is an associated factor that leads to epilepsy.7 Brain is extremely susceptive to an oxidative damage by free radicals, due to a high aerobic metabolism, blood perfusion and a significant concentration of polyunsaturated fatty acids compared with others organs with deficient antioxidant systems.8
Several drugs are used by physicians to manage epilepsy. However, such drugs show serious side effects like ischemia, hepatotoxicity depression, cognitive disorders, and motor disability.9 Additionally, 20–30% of those patients are resistant to treatments with synthetic drugs so, it is necessary to discover new treatments by using the natural medicine to reduce the complications or side effects of antiepileptic drugs.
Cyperus articulatus L. (Cyperaceae) commonly called “piri-piri” in Peru is a rhizome-bearing herb found in Africa, Latin America, Asia and Oceania. In Cameroon, Central Africa Republic, Gabon, and Senegal use the decoction of its rhizome for the treatment of headaches, migraines, etc. In addition, Indians of Amazonia use the plant in a similar way.10 However, the leaves of this plant have not been studied, an experimental study based on previous studies was designed in order to determine the anticonvulsant effect of C. articulatus L leaves extract, using pentylenetetrazol as chemical inductor of seizures in mice and evaluate the gamma amino-butyric acid (GABA) and malondialdehyde (MDA) levels on mice brain.
2. Materials and methods
2.1. Animals
A total of 30 Balb/C albino mice (20–30 g) of male sex obtained from the National Institute of Health (Lima-Peru) were used in this study. Mice were placed in Plexiglas cage with access to pelletized food and water ad libitum, housed in animal room with controlled temperature (22–24 °C) and 12 h light/dark cycle. All mice were divided into five groups of six animals each, acclimatized to the laboratory 15 days previous to the experiments. The experimental protocol was carried out according to the guidelines established by the European Union on Animal Care (CCE Council 86/609), and approved by the Ethics Committee of the National Institute of Health (Reg. 21433-13; ET-060-13).
2.2. Plant collection
1 kg of “Piri-piri” leaves were collected from Pucallpa, Ucayali, Peru and kept in kraft paper to be transported to the laboratory. The botanical identification of the plant material was classified and a voucher specimen (13-2014-USM-MHN) was deposited at the National Herbarium of National University of San Marcos (UNMSM), Lima, Peru.
2.3. Preparation of extract
The extraction of C. articulatus leaves was carried out by using a maceration process with alcohol 96%. The extract was concentrated on a rotavap and lyophilized, finally 75 g of extract was obtained and stored at 4 °C until further studies.
2.4. Phytochemical screening
The extract of C. articulatus was analyzed for the presence of phytochemical constituents, such as alkaloids, terpenoids, quinones, flavonoids, saponins, steroids and phenolic compounds, with the standard qualitative phytochemical methods described.11
2.5. Drugs and chemicals
Pentylenetetrazol (PTZ), 1,1-diphenyl-2-picrylhydrazyl (DPPH), Trolox, 2,2′-Azinobis-3-ethylbenzotiazilone-6-sulphonic acid (ABTS), (Sigma Chemical Co), Diazepam injection (Medifarma Pharmaceuticals). All other chemicals used were of analytical grade.
2.6. Antioxidant activity by DPPH assay
The antioxidant activity of extract and positive control (Trolox) were estimated, based on the method of Brand-Williams et al.12 Aliquots (100 μL) of extract at various concentrations and Trolox were mixed with 900 μL of DPPH solution. The solution was shaken and stored at room temperature for 30 min in the dark. The absorbance of the reactive solution was measured by spectrophotometric method at 517 nm. The percentage of antioxidant activity was calculated according to the equation:
| % antioxidant activity = [(ABS control − ABS sample)/ABS control] × 100. |
2.7. 2,2′-Azinobis-3-ethylbenzotiazilone-6-sulphonic acid (ABTS•+) assay
The assay was performed as previously described by Arnao et al.13 The stock solution included 7.4 mM ABTS solution and 2.6 mM potassium persulphate solution to react for 12 h at room temperature in the dark. The solution was diluted to obtain an absorbance of 0.7 ± 0.02 units at 734 nm. Extract (40 μL) was mixed with 1960 μL of ABTS to react for 7 min. UV/Vis spectrophotometer was blanked with distilled water, thus absorbance was recorded at 734 nm. Each assay was made by triplicated. The trolox equivalent antioxidant capacity (TEAC) was calculated as mmol trolox per gram of sample from a calibration standard curve with Trolox.
2.8. Anticonvulsant effect
Mice were randomly divided to five groups (n = 6) and treated according to the experimental protocol: Group I: Control group (distilled water); Group II: Standard group, reference drug (Diazepam, 1 mg/kg i.p.); Group III, IV, V: Test groups, C. articulatus extract (50, 150, 300 mg/kg p.o. respectively).
All the drugs were administered 30 min prior to the administration of PTZ (80 mg/kg, i.p.). Vehicle (distilled water; 10 mL/kg, p.o.) and diazepam (1 mg/kg, i.p.) were administered 15 min respectively before PTZ injection. The animals were placed in Plexiglas cage (20 × 20 × 20 cm) and observed for 30 min for latency of seizure onset, frequency of convulsions, mortality and duration of a seizure. The animals that survived after that period of time were considered to be protected. Furthermore, each seizure was classified according to a modified Racine scale as follows: 1-Mouth and facial movements; 2-Head nodding; 3-Forelimb clonus; 4-Rearing; 5-Rearing and falling.14, 15
2.9. Biochemical parameters
Two biochemical parameters were determined by spectrophotometry in order to determine the effect of the extract on mice brain.
2.9.1. Estimation of GABA by spectrophotometry
GABA (gamma amino butyric acid) was determined from whole brain and was isolated immediately to be transferred to homogenization tube containing 5 mL of 0.01 M hydrochloric acid. Brain homogenate was transferred to bottle containing 8 mL of ice cold absolute alcohol and kept for 1 h at 0 °C. The content was centrifuged for 10 min at 16,000 rpm, supernatant was collected in petridish. Precipitate was washed with 5 mL of 75% alcohol for three times and washes were combined with supernatant. Next, samples were evaporated to dryness at 70 °C on water bath. To the dry mass 1 mL water and 2 mL chloroform were added and centrifuged at 2000 rpm. Upper phase containing GABA (2.0 mL) was separated and 10 mL of it was applied as spot on Whatman paper (Nº 41). The mobile phase consisted of n-butanol (50 mL) acetic acid (12 mL) and water (60 mL). The paper chromatogram was developed with ascending technique. The paper was dried in hot air and then spread with 0.5% ninhydrin solution in 95% ethanol. The paper was dried for 1 h at 90 °C. Blue color spot developed on paper was cut and heated with 2 mL of ninhydrin solution on water bath for 5 min. Water (5.0 mL) was added to solution and kept for 1 h. Supernatant (2.0 mL) was decanted and absorbance was measured at 570 nm by using spectrophotometry.16 GABA standard was used to extrapolate absorbances of the samples.
2.9.2. Estimation of lipid peroxidation
Lipid peroxidation was detected by the determination of malondialdehyde (MDA) production determined by the method of Begue and Aust.17
2.10. Statistical evaluation
The statistical analyses were carried out using SPSS version 20.0 and Excel 2010. The measured parameters were expressed as means ± standard error of mean, percentages for antioxidant activity. Linear regression was used to obtain IC50 and TEAC. The anticonvulsant effect was analyzed using One-way ANOVA followed by Dunnett's test. The results were presented as means ± SD (n = 6). Values with P < 0.05 were considered statistically significant.
3. Results
3.1. Phytochemical screening
The results of phytochemical screening are shown in Table 1.
Table 1.
Phytochemical constituents of the ethanolic extract of C. articulatus leaves.
| Constituents | Test | Result |
|---|---|---|
| Alkaloids | Mayer | + |
| Dragendorff | + | |
| Wagner | + | |
| Flavonoid | Shinoda | + |
| Quinone | Bornträger | + |
| Phenols compounds | Ferric chloride | + |
| Saponins | frothing | + |
| Terpenes and steroids | Liebermann–Burchard | + |
(+) positive, (−) negative.
3.2. DPPH free radical scavenging
The antioxidant activity on DPPH free radical of four different concentrations (26.0, 13.0, 6.0 and 3.0 μg/mL) with their respective percentages were determined and placed in Table 2. The half inhibitory concentration (IC50) was 16.50 ± 0.46 μg/mL. Additionally, the extract scavenged DPPH in a dose-dependent. The extract reduced DPPH at 75.50 % for 26 μg/mL.
Table 2.
Antioxidant activity of ethanolic extract of C. articulatus leaves.
| Concentration (μg/mL) | DPPH Scavenging activity (%) |
|
|---|---|---|
| C. articulatus | Trolox | |
| 26 | 75.50 ± 2.1 | 98.50 ± 1.5 |
| 13 | 43.76 ± 1.0 | 97.30 ± 1.0 |
| 6 | 19.13 ± 1.1 | 95.20 ± 2.0 |
| 3 | 9.81 ± 0.6 | 56.61 ± 1.0 |
Results presented here are the mean value of n = 3; ±S.D.
3.3. ABTS+ radical assay
Trolox equivalent antioxidant capacity (TEAC) was 2.20 ± 0.10 mmol trolox/g, calculated by using the equation of linear regression, y = 0.0176x + 0.5911 (r = 0.9903).
3.4. Anticonvulsant effect
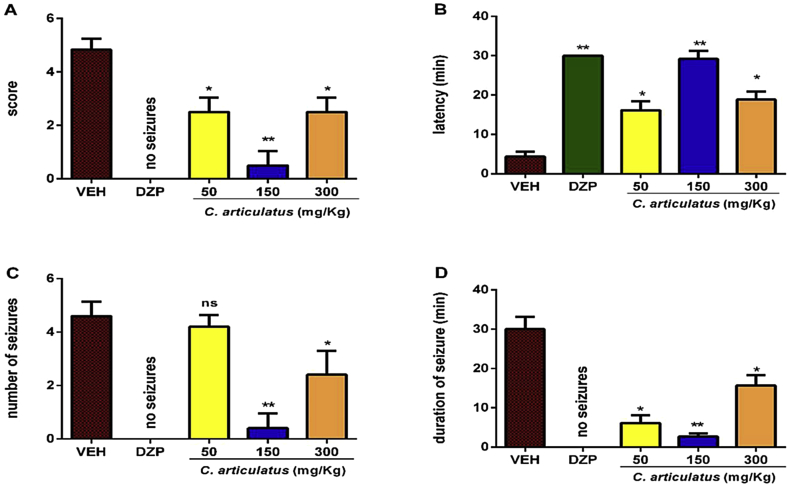
In the anticonvulsant effect, these parameters showed a significant decrease such as onset of seizures, duration of seizures, score and frequency of seizure (P < 0.01) similar to diazepam group (Fig. 1). Treatment with C. articulatus (50, 150 mg/kg, p.o.) and Diazepam (1 mg/kg, i.p.) evidenced significant protection against PTZ induced seizures (Table 3).
Fig. 1.
Analysis of anticonvulsant effect using PTZ (80 mg/kg; i.p.) as chemical inductor of seizures. The bars show the mean ± SEM of all parameters analyzed (n = 6). (A) The first graph shows the score during a seizure according to a modified Racine scale, *P < 0.05, **P < 0.01, compared with the vehicle treated group. (B) Effect of extract on duration of seizure; *P < 0.05, **P < 0.01 compared with vehicle treated group (ANOVA is followed by Dunnett's test). (C) Effect of extract on number of seizure; n.s = non-significant, *P < 0.05, **P < 0.01 compared with vehicle treated group (ANOVA is followed by Dunnett's test). (D) Effect of extract on duration of seizure in pentylenetetrazol induced seizure test; *P < 0.05, **P < 0.01 compared to vehicle treated group (ANOVA is followed by Dunnett's test).
Table 3.
Protection of ethanolic extract of C. articulatus against PTZ.
| Variable | Groups (n = 6) |
||||
|---|---|---|---|---|---|
| VEH | DZP | 50 | 150 | 300 | |
| Alive mice | 0/6 | 6/6 | 5/6 | 6/6 | 4/6 |
| Protection (%) | 0 | 100 | 83.3 | 100 | 66.7 |
DZP: diazepam; PTZ: pentylenetetrazol; VEH: vehicle.
3.5. Biochemical evaluation in brain tissue
In the biochemical evaluation determined by spectrophotometry on GABA level, C. articulatus showed an effectiveness for the dose of 150 mg/kg similar with diazepam (P < 0.01; Dunnett's Test) and lower at dose of 300 mg/kg Table 4.
Table 4.
Estimation of brain GABA and MDA on pentylenetetrazol induced seizure in mice.
| Variable | Groups (n = 6) |
||||
|---|---|---|---|---|---|
| VEH | DZP | PTZ-CA 50 | PTZ-CA 150 | PTZ-CA 300 | |
| GABA (ng/g of brain tissue) | 22 ± 1.5 | 87 ± 1.8∗∗ | 45 ± 2.5∗ | 82 ± 1.2∗∗ | 73 ± 1.1∗∗ |
| TBARS (MDA 10−6 mol/g of brain tissue) | 8.3 ± 0.2 | 4.4 ± 0.2∗ | 3.0 ± 0.2∗∗ | 1.0 ± 2.2∗∗ | 5.1 ± 0.3∗ |
MDA: malondialdehyde; DZP: diazepam; PTZ: pentylenetetrazol; VEH: vehicle; ∗P < 0.05; ∗∗P < 0.01.
MDA levels were decreased in all group treated with C. articulatus and showed an effectiveness for the dose of 150 mg/kg and 300 mg/kg similar with diazepam (P < 0.01; Dunnett's Test) and control group (P < 0.01; Dunnett's Test) Table 4.
4. Discussion
The scientific literature has shown several phytochemical compounds of this plant specially sesquiterpenes as corymbolone and mustakone isolated from the chloroform extract of C. articulatus rhizomes.8 In the current study, the phytochemical screening evidenced the presence of flavonoids, alkaloids, terpenes, steroids, saponins, phenolic compounds and tannins, these metabolites are similar with previous reports of C. articulatus rhizomes extract, with exception of alkaloids.
With regard to oxidative stress, increased formation of free radicals especially reactive oxygen species (ROS) produce an elevated intracellular Ca2+ concentration, which is involved in necrosis or apoptosis mechanisms from the neuronal system. The relationship between free radicals and seizures could have multiples mechanism, for instance, free radicals can induce seizures by a direct inactivation of glutamine synthase, stablishing an abnormal release of excitatory neurotransmitter like glutamic acid.18 Also, the onset of oxygen-induced convulsions in animals is correlated with decreased levels of GABA on the brain cortex due to the inhibition of the enzyme glutamate decarboxylase by oxygen free radicals.19, 20
The antioxidant activity showed in this study against DPPH, ABTS radicals in vitro (TEAC) and MDA in vivo might be linked to the anticonvulsant effect. Seghatoleslam et al21 studied the administration of ascorbic acid in animal studies, demonstrating the reduction of neuronal damage, triggered by free radicals, which are particularly elevated in inflammation processes and neurodegenerative disorders. Pretreated animals with ascorbic acid, prior to pilocarpine induced seizure, have shown a significant 60% reduction in the frequency of hippocampal brain damage induced by seizures, the latency to first seizures, suppression of behavioral seizure episodes, and decrease in lipid peroxidation (MDA), nitrite content and brain damage. Rutin, quercetin, and isoquercitrin have been shown on experimental epilepsy models modulating the GABAA-Cl-channel complex.22
Rakotonirina et al23 established at 300 mg/kg (i.p.) the effective doses for rhizomes extract administered in mice, in our study, the leaves extract of C. articulatus showed effectiveness for test group that received a single dose of 150 mg/kg per oral. These differences could be linked to alkaloids that were reported in this study otherwise rhizomes extract did not show this metabolite. Furthermore, different aspects might be involved such as, solvent, solubility, origin of the studied species.
In previous studies, the mechanism of the anticonvulsant effect of rhizome extract was related to selective inhibition of NMDA receptors according to Ngo et al.24 In this study was demonstrated that leaves extract could have a direct modification on GABA production and protection against lipid peroxidation (MDA) generated on the brain. It is known that lipid peroxidation disturbs biological membranes and is particularly destructive to their structure and function. The leaves extract exerted the inhibition of MDA on brain mice, protecting against neuronal damage produced by PTZ injection, several reports have concluded that epileptic animals reflect elevated MDA levels, and significantly decreased serum total antioxidant capacity like superoxide dismutase (SOD), catalase (CAT), Glutathione (GSH).25
Although the anticonvulsant mechanism of the extract is unknown, the leaves ethanolic extract could be useful to treat the epileptic seizures in the future. Hence the isolation of active substances from leaves could be the next stage to continue with the research. In conclusion the ethanolic extract from C. articulatus leaves showed anticonvulsant effect on pentylenetetrazol induced seizure and antioxidant activity in vitro and in vivo.
Conflict of interest statement
The authors have no actual or potential conflicts of interest.
Acknowledgments
The authors would like to thank The National Institute of Health (Lima, Peru), for providing the samples of Cyperus articulatus to carry out the current research.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Xu C., Fan Y.N., Kan H.D. The novel relationship between urban air pollution and epilepsy: a time series study. PLoS One. 2016;11(8):e0161992. doi: 10.1371/journal.pone.0161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta G., Dua K., Kazmi I., Anwar F. Anticonvulsant activity of Morusin isolated from Morus alba: modulation of GABA receptor. Biomed Aging Pathol. 2014;4(1):29–32. [Google Scholar]
- 3.Coimbra T., Cabral J., Gomes S.R. The role of flavonoids on oxidative stress in epilepsy. Oxid Med Cell Longev. 2015:9. doi: 10.1155/2015/171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govindu S., Adikay S. Evaluation of antiepileptic activity of chloroform extract of Acalypha fruticose in mice. Pharmacogn Res. 2014;6(2):108–112. doi: 10.4103/0974-8490.128970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher R.S., Acevedo C., Arzimanoglou A. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 6.Gschwind M., Seeck M. Modern management of seizures and epilepsy. Swiss Med Wkly. 2016;146:w14310. doi: 10.4414/smw.2016.14310. [DOI] [PubMed] [Google Scholar]
- 7.Gordon-Gray K.D., Ward C.J., Edwards T.J. Studies in Cyperaceae in southern Africa 39: Cyperus articulatus L. and Cyperus corymbosus Rottb. South Afr J Bot. 2006;72:147–149. [Google Scholar]
- 8.Rukunga G.M., Muregi F.W., Omar S.A. Anti-plasmodial activity of the extracts and two sesquiterpenes from Cyperus articulatus. Fitoterapia. 2008;79:188–190. doi: 10.1016/j.fitote.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Ngo E., Rakotonirina A., Rakotonirina S.V., Herrling P. Effects of Cyperus articulatus compared to effects of anticonvulsant compounds on the cortical wedge. J Ethnopharmacol. 2003;87:27–34. doi: 10.1016/s0378-8741(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 10.Abubakar M.S., Abdurahman E.M., Haruna A.K. The repellant and antifeedant properties of Cyperus articulatus against Tribolium casteneum Hbst. Phytotherapy Res. 2000;14(4):281–283. doi: 10.1002/1099-1573(200006)14:4<281::aid-ptr568>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Trease E., Evans W.C. 15th ed. Harcourt Publishers Limited; San Diego, CA: 2002. Pharmacognosy; pp. 343–545. [Google Scholar]
- 12.Brand-Williams W., Cuvelier M., Berset C. Use of free radical method to evaluate antioxidant activity. Leb Wiss Technol. 1995;28:25–30. [Google Scholar]
- 13.Arnao M., Cano A., Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2003;73:239–244. [Google Scholar]
- 14.Ingale S.P., Gandhi F.P. Effect of aqueous extract of Moringa oleifera leaves on pharmacological models of epilepsy and anxiety in mice. Int J Epilepsy. 2016;3:12–19. [Google Scholar]
- 15.Racine R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 16.Tamboli A.M., Rub R.A., Ghosh P., Bodhankar S.L. Antiepileptic activity of lobeline isolated from the leaf of Lobelia nicotianaefolia and its effect on brain GABA level in mice. Asian Pac J Trop Biomed. 2012:537–542. doi: 10.1016/S2221-1691(12)60092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buege J., Aust S. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 18.Seghatoleslam M., Alipour F., Shafieian R. The effects of Nigella sativa on neural damage after pentylenetetrazole induced seizures in rats. J Tradit Complement Med. 2016;6:262e268. doi: 10.1016/j.jtcme.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naziroglu M., Akay M.B., Celik O., Yildirim M.I., Balci E., Yurekli V.A. Capparis ovata modulates brain oxidative toxicity and epileptic seizures in pentylentetrazol-induced epileptic rats. Neurochem Res. 2013;38(4):780–788. doi: 10.1007/s11064-013-0978-3. [DOI] [PubMed] [Google Scholar]
- 20.Nozadze M., Mikautadze E., Lepsveridze E. Anticonvulsant activities of myo-inositol and scyllo-inositol on pentylenetetrazol induced seizures. Seizure. 2011;20:173–176. doi: 10.1016/j.seizure.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Tomaciello F., Leclercq K., Kaminski R.M. Resveratrol lacks protective activity against acute seizures in mouse models. Neurosci Lett. 2016;632:199–203. doi: 10.1016/j.neulet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Cárdenas-Rodríguez N., González-Trujano M.E., Aguirre-Hernández E. Anticonvulsant and antioxidant effects of Tilia americana var. mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxid Med Cell Longev. 2014;2014:10. doi: 10.1155/2014/329172. Article ID 329172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakotonirina V.S., Ngo E., Rakotonirinac A., Bopelet M. Sedative properties of the decoction of the rhizome of Cyperus articulatus. Fitoterapia. 2001;72:22–29. doi: 10.1016/s0367-326x(00)00243-4. [DOI] [PubMed] [Google Scholar]
- 24.Ngo E., Schmutz M., Meyer C., Rakotonirina A., Bopelet M., Portet C. Anticonvulsant properties of the methanolic extract of Cyperus articulatus (Cyperaceae) J Ethnopharmacol. 2001;76:145–150. doi: 10.1016/s0378-8741(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 25.Menon B., Ramalingam K., Kumar R.V. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure. 2012;21(10):780–784. doi: 10.1016/j.seizure.2012.09.003. [DOI] [PubMed] [Google Scholar]