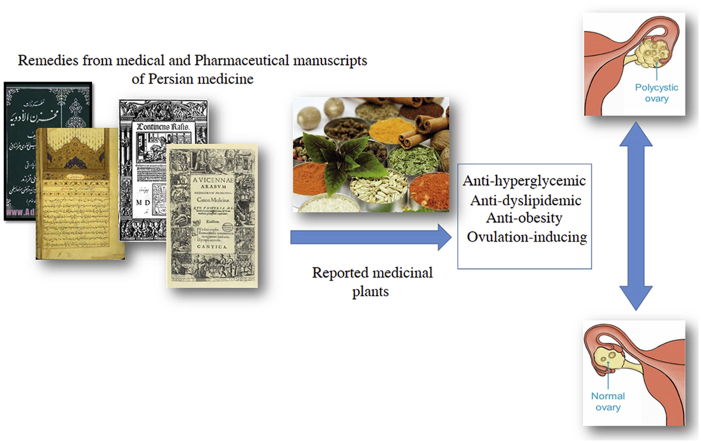
Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age. Its cause is unknown and it remains the most enigmatic of reproductive disorders. The extant written documents of Traditional Persian Medicine (TPM) – with holistic approaches towards human health – contain remedies used for centuries. Before further experimental research on any of these treatments, it is appropriate to study current related scientific evidence on their possible pharmacological actions. This work aims to study PCOS and its treatments in TPM. To collect data from medieval medicinal texts, six of the most famous manuscripts of Persian medicine were studied. Medicinal treatments for a problem similar to PCOS were searched for in these books. The plants were listed and their authentications were confirmed in accordance with botanical books. PubMed and ScienceDirect databases were searched for related mechanisms of action or pharmacological activities of the medicinal plants reported. From numerous articles, the current work tried to cite the latest publications with regard to each reported plant and PCOS-related mechanisms of action. We studied herbal treatments recommended by ancient Persians to treat a condition called Habs-e-tams, which had the same symptoms of PCOS. It could be concluded that ancient physicians not only wanted to treat the irregular menstrual cycle—which is the most obvious symptom of PCOS—but also their treatment options were aimed at ameliorating the related underlying metabolic dysfunctions. The recommended herbs, which have the most scientific proof for their related actions, can be studied further in experimental analyses.
Keywords: Traditional Persian Medicine, Polycystic ovary syndrome, Metabolic dysfunction, Medicinal plant, Pharmacopoeia, Persia
Graphical abstract
1. Introduction
There are many medical issues for which common treatments are suboptimal. The majority of medicines today contain single active ingredients that are active against a single biological target. Owing to the complexity of the human body, this approach might seem rather simplistic.1 The scientific viewpoint, in many studies, still reflects reductionist logic. Although it has provided us valuable cellular information, it lacks an overall vision. This approach started to change from the early years of the second millennium. In recent years, a more comprehensive and holistic approach was applied in health-related studies.2 Traditional medicines of the world often adopt holistic approaches towards human health as well.3 Unlike conventional drugs, traditional medicine contains medications that are often multicomponent and, therefore, multi-target.1 In medical practice, one of the areas in which physicians find difficulties in curing patients are syndromes that have a set of signs and symptoms correlated with each other and with a specific disease. One of these syndromes is the polycystic ovary syndrome (PCOS), which affects up to 17.8% of women of reproductive age. The medical management of this problem requires a multidisciplinary approach. At present, conventional therapies are not effective, and some have unwanted side effects. Women with PCOS prefer alternative therapies.4 The extant written documents of traditional medicinal systems contain a list of drugs that have been used for centuries. These can be good sources for finding possible new drugs for medical conditions which do not have a satisfying treatment. One of the traditional systems of medicine still practised today is Traditional Persian Medicine. This system of medicine was replaced in Iran by Western medicine in the late 19th century. Although physicians were not allowed to apply the treatments of this system of medicine, valuable information was preserved in the books of scholars. Iranian people still seek help for their health-related problems from local herbal shops, which have kept the practice of TPM alive. In the past few years, Iranian universities have changed their policies towards TPM. Today, specialists are studying this system of medicine from different aspects. Recently, traditional Persian therapies have been evaluated by modern methods.5 Iranian pharmaceutical companies are interested in formulations based on TPM remedies. In this work, we aim to study the polycystic ovary syndrome and its treatments in TPM. The recommended herbs, which have had the most scientific proof for their related pharmacological actions in the treatment of PCOS, have been preferred for the experimental research.
1.1. Modern description of PCOS and its treatments
This syndrome presents itself in menstrual irregularity, androgen excess and polycystic ovaries. It is the most common endocrine disorder in such women.6 It is associated with insulin resistance, hyperinsulinism7 and diabetes.8 Women with this syndrome often suffer from dyslipidemia and obesity as well.9, 10 Today, the treatment of PCOS includes restoring normal menstruation cycle and ovulation, reducing hirsutism and acne, and also reducing cardiovascular risk for the patients.6 Oral contraceptives and metformin are commonly prescribed forms of medication for these patients. Lifestyle modification is recommended to overweight/obese patients with PCOS.11 One of the main characteristics of the PCOS is obesity. It affects fertility in women suffering from PCOS through different mechanisms. Excess androgen levels, insulin resistance and increased luteinizing hormone (LH) play the main role.12 Research has suggested that weight loss in these women can restore ovulatory cycles, which allows spontaneous pregnancy.13
1.2. Description of PCOS in Traditional Persian Medicine
Traditional Persian Medicine deploys a holistic approach. It is a protracted method of practice from ancient Persia. Though terminology that is the same as PCOS cannot be found in TPM textbooks, that does not mean that evidence is absent. Regarding signs and symptoms of PCOS in current literature, there is a reasonable description of this issue in Persian resources. Symptoms of PCOS have been indicated and described under the topic of ‘uterus and ovary’-related disorders, such as ‘female infertility’, ‘uterine inflammation’, and ‘amenorrhea’.14 Signs and symptoms of the latest disorder in TPM textbooks, titled Ehtebās-e-Tams (lack of menstruation), come very close to PCOS.15 The main sign that can be attributed to PCOS is prolonged intervals of menstrual bleeding (more than two months). According to TPM books, it could occur as a result of two groups of factors: intrinsic and extrinsic. Intrinsic factors are related to the genitourinary (GU) system itself and other factors focus on the entire human body and interconnected body systems. In the first category, there are: severe cold temperament of GU system, hyper-dense humor (caused by cold temperament), and plethora of phlegmatic humor. There are also general classifications of extrinsic factors. These are cold and dry dystemperament, cold dystemperament with excess of phlegm, or black bile and overt obesity.16 Some of the aforementioned terms—such as overweight—have been approved in recent literature as causes of PCOS. The TPM therapeutic approach for these ailments emphasizes the removal of the cause instead of the symptom. As regards the abovementioned pathologic categorization, a TPM practitioner should follow the curative plan in a holistic manner. Obeying a specific diet regimen and lifestyle modifications are the first essential steps for a patient's cure. If nutritional and lifestyle instructions are not appropriately responded to, the treatment strategy would be converted to medicinal options.17 Most of the medicinal choices are herbal medicine, which have been defined in detail in ancient Persian pharmacopoeias. Some of these medicaments are single herbs and some are combined preparations. The present work considers these herbs. Their respective efficacies have been reviewed in multiple in vitro or in vivo surveys.
2. Current methods
To collect data from medieval medicinal texts, six main manuscripts of Persian medicine were studied. These texts are currently known as the main university reference books for research into TPM in departments of traditional medicine and pharmacy in Iranian universities. Since the end of the 19th century, when TPM was replaced in Iran by Western medicine, no written document on the practice of this system has been available. The definition and causes of Ehtebās-e-Tams was studied in Exir-e-Azam (Azam Khan, 19th century) and Moalejat (Aghili, 18th century). Medicinal treatments for this problem were searched in Kitab al-hawi fı al-tibb (Rhazes, 9th–10th centuries), Canon of Medicine (Avicenna, 10th–11th centuries), Tuhfat al-muminin (Daylami Tunakabuni, 17th century), and Makhzan al-adviyah (Aghili, 18th century).16, 18, 19, 20, 21 The plants used in the treatment of Ehtebās-e-Tams, according to Persian manuscripts, are listed in Table 1. Authentications of the plants were also confirmed by botanical books such as Dictionary of Medicinal Plants, Matching the Old Medicinal Plant Names with Scientific Terminology, Indian Medicinal Plants, and Dictionary of Iranian Plant Names.22, 23, 24, 25 PubMed and ScienceDirect databases were searched for related mechanisms of action or pharmacological activities of the medicinal plants that were reported. The scientific name of each herb was searched along with these keywords: ‘anti-hyperglycemic’, ‘anti-dyslipidemia’, ‘anti-obesity’ and ‘ovulation-inducing’. From numerous articles, the current work tried to cite the latest publications with regard to each reported plant and PCOS-related mechanisms of action.
Table 1.
Herbal Remedies for “Ehtebās-e-Tams” from reports of Traditional Persian Medicine.
| Scientific name | Related Pharmacological activities/method, model or assay |
|||
|---|---|---|---|---|
| Anti-hyperglycemic | Anti-dyslipidemic | Anti-obesity | Ovulation-inducing | |
| Adiantum capillus-veneris L. | + (Aqueous, Methanol extract)/Streptozotocin-induced diabetic rats26 | – | – | – |
| Allium cepa L. | + (Seed ethanol extract) Improvement in FBS and HOMA-IR levels/Diabetic-prone rat27 | + Reduction in FFA and TG levels/obesity-prone diabetic fatty rat27 | + May be effective in formation of oil drop in preadipocyte cells/indict27 | + Antioxidants level compensation to modulate apoptosis/PCO -induced rat28 |
| Allium sativum L. | + (Ethanol extract), Serum glucose level reduction and Increasing serum insulin level/streptozotocin-induced diabetic rat29 | + (Ethanol extract), Decreasing the ALT, AST, cholesterol, urea, uric acid and creatinine29 | + Reduction in body weight, visceral fat, MDA and cholesterol levels/High-fat diet rat30 | – |
| Anethum graveolens L. | + (Leaf hydroalcoholic extract), Regulation of diabetes mellitus/corticosteroid-induced type II in rats31 | + Dill tablets versus Gemfibrozil/(Human study) Reduction of cholesterol (18%) and TG (7%)32 | – | + (Ethanol extract), Increase in estrous cycle duration and diestrus phase and progesterone concentration/Rat33 |
| Apium graveolens L. | – | + (Ethanol extract)/Decrease in TG, LDL-Cholesterol, increase in HLD (rat)34 | + (Aqueous extract)/Based on body and organs weights as well as biochemical parameters (rat)35 | – |
| Artemisia absinthium L. | + Improvement in glucose tolerance by increasing in tyrosyl phosphorylation of insulin receptor/Shikonin treated mice36 | + Improvement in lipid profile/Shikonin treated mice36 | + Decrease in weight, resistance to high fat diet/Shikonin treated mice36 | – |
| Asparagus officinalis L. | + (Methanol extract), improving in insulin secretion and β-cell function/streptozotocin-induced diabetic rats37 | + (Ethanol, aqueous extracts), decrease in cholesterol and LDL/Hyperlipidemic mice38 | + (Ethanol, aqueous extracts), decrease in body weight gain/Hyperlipidemic mice38 | + (Aqueous extract), increase in GnRH, FSH, LH, estrogen, progestin hormones levels and number of ovarian follicles/rats39 |
| Cappari sspinosa L. | + (Aqueous extract), decrease in blood glucose level/Streptozotocin-induced diabetic rats40 | + (Aqueous extract), decrease in TG and cholesterol/normal and Streptozotocin-induced diabetic rats41 | – | – |
| Carum carvi L. | + (Aqueous extracts), decrease in blood glucose/Streptozotocin-induced diabetic rats40 | + (Aqueous extract), hypotriglyceridemic, hypocholesterolemic effects/Normal and Streptozotocin-induced diabetic rats42 | + Reduction in weight, body mass index, body fat percent, and waist-to-hip ratio/Clinical trial43 | – |
| Cicer arietinum L. | + improvement in insulin resistance, preventive effects on postprandial hyperglycemia and hyperinsulinemia/Rat44 | + Reduction in LDL-cholesterol and LDL/HDL levels/Rat44 | + Increase in lipoprotein lipase activity/Rat44 | – |
| Cinnamomum verum J. Presl | + (Aqueous extract), reduction in fasting blood glucose level (no hypoglycemic activity)/Diabetic rats45 | + (Aqueous extract), decrease in levels of total cholesterol, HDL, LDL and TG/Diabetic rats45 | – | – |
| Citrulluscolocynthis (L.) Schrad. | + (Fruit capsules), decrease in HbA1c and fasting blood glucose/Clinical trial46 | – | – | – |
| Citrus × aurantium L. | + (Isolated neohesperidin), increase in oral glucose tolerance and insulin sensitivity, decrease in insulin resistance/KK-Ay diabetic mice47 | + (Neohesperidin), decrease in TG, total cholesterol, leptin level, and liver index/KK-Ay diabetic mice47 | + (Extract), increase glycogenolysis, glycolysis, oxygen uptake, perfusion pressure/Rat48 | – |
| Citrus medica L. | – | – | – | + (Petroleum ether extract), estrogenic effects/Immature ovariectomized rat49 |
| Commiphora mukul (Hook. ex Stocks) Engl. | + (Ethanol extract), preventive effects against alteration in hexokinase, phosphofructokinase, pyruvate kinase, and glucose-6-phosphatase/Streptozotocin-induced diabetic rats50 | + (Ethanol extract), preventive effects against alteration in fatty acid synthase, malic enzyme and lipoprotein lipase/Streptozotocin-induced diabetic rats50 | – | – |
| Commiphora myrrha (Nees) Engl. | + (Aqueous extract), decrease in blood glucose level/Diabetic rats51 | + (Guggulipid), decrease in cholesterol level, LDL, TG and cholesterol/HDL levels/Fruit- and vegetable-enriched prudent diet in hypercholesterolemic patients (Clinical trial)52 | – | – |
| Ficus carica L. | + (Aqueous extract), Insulin-like peripheral effect/Diabetic rats53 | + (Aqueous ethanol extract), increasing the HDL, decreasing the LDL and cholesterol/High-fat diet-induced hyperlipidemic rats54 | – | – |
| Foeniculum vulgare Mill. | + (Essential oil), Correcting the hyperglycemia and activity of serum glutathione peroxidase/Diabetic rat55 | + Ameliorates serum glucose, AST, ALT, GGT, LDH, protein, albumin, liver total lipids/Hyperlipidemic rat56 | – | + (Fennel extract), Increases the serum level of estrogen, progesterone, and prolactin/female mice57 |
| Glycyrrhiza glabra L. | – | + (Root powder), reduction in total lipids, cholesterol, TG, LDL and VLDL, increases in HDL/Hypercholesterolemic rat58 | + (Ethanol extract), reduced weight gain and adipose tissue mass/Rat model of high-fat diet induced hyperlipidemia and obesity59 | – |
| Helianthus annuus L. | + (Ethanol extract), decreased blood glucose level, restored lipid profile/streptozotocin induced diabetic rats60 | – | – | – |
| Hypericum perforatum L. | + (Ethyl acetate extract), reduction in plasma glucose level and fasting blood sugar/Streptozotocin-induced diabetic rats61 | + (Ethyl acetate extract), Reduction in total cholesterol and TG/Streptozotocin-induced diabetic rats61 | + Lowering the total cholesterol and LDL, Inhibiting weight gain, Normalizing the dyslipidemia and improving insulin sensitivity/High-fat-diet induced obese rats62 | – |
| Lepidium sativum L. | + (Seed powder), Decreasing in fasting blood sugar, HbA1C, total cholesterol, TG, lipoprotein fractions, Increase in HDL/Alloxan induced diabetic rats63 | + Reduction in total cholesterol and ALT (6 g/kg diet)/Rats fed with high cholesterol diet64 | – | – |
| Linum usitatissimum L. | + (Ethanol extract), Reduction in serum glucose level in acute and subacute study/Alloxan induced diabetic rat65 | + Reduction in total cholesterol and increasing in HDL/Sprague Dawley rats66 | – | + (Aqueous methanol extract), Increasing in serum estradiol, progesterone, total proteins and cholesterol, ALT and AST activity, Decreasing ovarian cholesterol levels/Immature female rats67 |
| Lupinus albus L. | + (Aqueous suspension), Restore the elevated levels of glucose, urea, creatinine and bilirubin/Alloxan-induced diabetic rats68 | + (Isolated proteins, whole seed), Reduction in total cholesterol and related parameters/Hamsters69 | – | – |
| Matricaria chamomilla L. | + (Ethanol extract), Reducing postprandial hyperglycemia/Streptozotocin-induced diabetic rats70 | – | – | + (Ethanol extract), Decreasing the signs of PCOS in ovarian tissue, helping LH secretion/Polycystic ovary-induced rats71 |
| Melissa officinalis L. | + (Essential oil), Reducing blood glucose and TAG concentrations, improving glucose tolerance and serum insulin levels/Mice72 | + (Ethanol extract), Reducing serum total cholesterol, lipid, ALT, AST and ALP levels, and LPO level in liver tissue/Hyperlipidemic rats73 | + (In a combination), Decreasing the adipose tissue mass and body weight/High-fat diet mice74 | – |
| Nigella sativa L. | + (Oil), Reducing blood glucose and hepatic gluconeogenesis/Streptozotocin-induced diabetic hamsters75 | + (Dietary black seed), Lowering the total cholesterol, LDL and MDA, TG/Rabbits with hypercholesterolemic diet76 | – | + (Hydroalcoholic extract), reduction in the serum level of LH, FSH and estrogen/Female rats71 |
| Origanum majorana L. | + (Extraction, aqueous suspension), Comparable to Glibenclamide/Streptozotocin-diabetic mice77 | – | – | + (Infusion, tea), Reduction in DHEA-S, insulin sensitivity improvement/Hormonal profile, PCO (Clinical trial)78 |
| Petroselinum crispum (Mill.) Fuss | + Reduction in blood glucose and serum alkaline phosphatase activity/Streptozotocin-induced diabetic rats79 | + (Aqueous extract), attenuating the hyperlipidemia/Diabetic rats80 | + (Hydroalcoholic extract), ketohexokinase inhibitory activity, blocking the fructose-induced ATP depletion/Animal81 | – |
| Phaseolus vulgaris L. | + (Aqueous extract), Decline in blood glucose, serum TG, fatty acids, phospholipids, total cholesterol, LDL, and VLDL/Streptozotocin-induced diabetic rat82 | + (Aqueous extract), Decline in lipids and fatty acids, palmitic, stearic, oleic acids, increase in linolenic and arachidonic acids/Streptozotocin-induced diabetic rat83 | + (Dry bean), Weight loss and improve in plasma lipid profile/Diet-induced obesity mice model (74) | – |
| Pimpinella anisum L. | + (Methanol extract, mostly ethyl acetate fraction), α- glucosidase and α-amylase inhibition/in vitro85 | – | – | – |
| Piper longum L. | – | + (Piperine derivative), Decline in TG, increase in HDL levels, and upregulation of HMG-CoA reductase level/High-fat diet-fed rats86 | – | – |
| Prangos ferulacea (L.) lindel. | + (Hydroalcoholic extract), Glucose and lipid profile reduction/Alloxan-induced diabetic rat87 | – | – | – |
| Prunus domestica L. | – | – | + (carbohydrate-free peach and plum), Potentiality to modify the fecal microbial ecology in obese model/Obese Zucker rats88 | – |
| Ruta graveolens L. | + (Infusion), Amelioration of hyperglycemia, hyperlipidemia, insulin and C-peptide concentrations/streptozotocin-nicotinamide-induced diabetic rat89 | + (Hydroalcoholic extract), Decrease in cholesterol, LDL, VLDL and TG/Diabetic rats90 | – | – |
| Thymus vulgaris L. | + (Aqueous extract), Decrease in FBS, LDL, VLDL, TG and cholesterol/Alloxan-induced diabetic rats91 | + (Aqueous extract), Decrease in FBS, LDL, VLDL, TG and cholesterol/Alloxan-induced diabetic rats91 | – | – |
| Trachyspermum ammi (L.) Sprague | – | + (Seed powder), Reduction in lipids, cholesterol, LDL, TG and HMG-COA reductase, Increase in HDL/Hyperlipidemia-induced rabbits92 | – | – |
| Trigonella foenum-graecum L. | + (Soluble dietary fiber fraction), Lowering the serum fructosamine/Type II model of diabetic rats93 | + (Seed powder), Reduction in total cholesterol, LDL, and the atherogenic index, Increase in HDL/Hyperlipidemia-induced rabbits94 | + (Seed extract), Reduction in fat energy intake-total energy expenditure ratio, Decrease in insulin-glucose ratio/Overweight male participants (Clinical trial)95 | + (Seed powder), Increase in circulating plasma progesterone concentrations at 10 and 20 days of gestation/Female white New Zealand rabbits96 |
| Urtica dioica L. | + (Aqueous extract), Strong glucose lowering effect (Pretreatment)/Alloxan-induced diabetic rats97 | + (Aqueous extract), Decrease in the body weight, TG, Cholesterol, and LDL/Type II diabetic model rats98 | – | + (Dry), Decrease in testosterone and DHEA level/Woman with hyperandrogenism (Clinical trial)98 |
| Zataria multiflora Boiss. | + (Extract), insulin, adiponectin, glucose and TG levels improved, PPARγ protein level increased/High fructose diet for rats99 | + (Extract), insulin, adiponectin, glucose and TG levels improved, PPARγ protein level increased/High fructose diet for rats99 | – | – |
3. Current results
Forty herbs—either as single or as a component of a compound medication to treat Ehtebās-e-Tams—were found in TPM books. The majority of these herbs exhibited anti-hyperglycemic (90%) and anti-dyslipidemic (77.5%) effects. Some of these herbs showed significant anti-obesity properties (37.5%). The effect of some of these were studied on ovulation induction and 27.5% had shown positive effects. Table 1 represented herbal remedies for Ehtebās-e-Tams from Reports of Traditional Persian Medicine. In this table, related pharmacological activities and citations of the current proof are also reported.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99
4. Conclusions and further suggestions
Polycystic ovary syndrome is the most common endocrine disorder in women of reproductive age.7 While the cause is unknown, this disorder remains the most enigmatic reproductive disorder. Therefore, there is no known cure for this problem.100 Most common treatments for PCOS are oral contraceptives to suppress the secretion of gonadotropin and decrease free androgen blood levels.101 This can lead to regular menstruation cycles. It is remarkable that the use of oral contraceptives may have unfavorable effects on hyperglycemia and insulin resistance. Metformin is also one of the medications for the treatment of PCOS. Today, the association between PCOS, hyperglycemia, dyslipidemia and obesity is known.9, 10, 102 The metabolic consequences need to be ameliorated in these patients as well. But the pathogenesis of PCOS is not fully understood and there is no single effective treatment for this disorder.9, 10, 102 Traditional systems of medicine often contain information on treatments which have been used for centuries. These can be sources of new drug discoveries.103 One of the difficulties in this area is that the paradigm of medicine was different and so the terminology used in ancient manuscripts is different from what we understand today. Ancient practitioners often had a holistic approach towards the human body as regards health and sickness. But if the medicines used by ancient healers were effective, it should be explicable by a rational mechanism of action as well. The absence of the menstruation cycle was explained in TPM books under the titles Ehtebās-e-Tams or Habs-e-tams or Ehtebās-e-heiz.104 Medicinal treatment options were also explained in detail in these books. Going through ancient Persian books, we can find that they mentioned obesity as one of the extrinsic causes of Habs-e-Tams. In a research, the emmenagogue activity of the herbal treatments used for amenorrhea by the ancient Persians was studied. In that study, of 71 reported plants, only Foeniculum vulgar had an approved emmenagogue activity.104 This could mean that no direct emmenagogue effect was expected from these drugs. In the present work, we reviewed the possible anti-hyperglycemic, anti-dyslipidemic, anti-obesity and ovulation-inducing effects of these remedies. In TPM books, Habs-e-Tams was defined as a condition of prolonged intervals of menstrual bleeding (more than two months).
The majority of drugs used by ancient Iranians to treat this problem showed to have proven effects on lowering blood glucose and lipids (Table 1). Also, many of these herbs had shown anti-obesity effects. But the main point of this data was that one-third of those remedies showed positive effects on ovulation induction through different underlying mechanisms. It is accepted that one of the main problems in patients with PCOS is infertility, which is related to the lack of ovulation. The same as the points and symptoms denoted in current medicine, this condition is also highly remarked upon by Persian scholars. Effects on ovulation induction, however, were studied mainly in animal models and there is a gap in human-related trials (Table 1). According to the table, only Origanum majorana and Urtica dioica had clinical trials on ovulation induction. In this respect, it may be important to evaluate the aforementioned activity on other reported plants. On the other hands, of those medicinal plants, only Allium cepa, Asparagus officinalis and Trigonella foenum-graecum possessed all the related pharmacological activities. These remedies might show effective results in related clinical trials on patients with PCOS or ovary-related amenorrhea.
Going through the medieval and traditional treatments for Habs-e-Tams, as recommended by ancient Persians, one can conclude that they not only wanted to treat the irregular menstrual cycle—which is the most obvious symptom of PCOS—but their treatment options were also aimed to ameliorate the related underlying metabolic dysfunctions. In future work, traditional herbal combinations could be studied and the theoretical role of each ingredient of the formulation could be defined. Furthermore, the effectiveness of these treatments could be investigated in clinical trials after confirmation of their safety.
Conflict of interest
The authors have no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Kim H.U., Ryu J.Y., Lee J.O., Lee S.Y. A systems approach to traditional oriental medicine. Nat Biotech. 2015;33:264–268. doi: 10.1038/nbt.3167. [DOI] [PubMed] [Google Scholar]
- 2.Costantini S., Colonna G., Castello G. A holistic approach to study the effects of natural antioxidants on inflammation and liver cancer. Cancer Treat Res. 2014;159:311–323. doi: 10.1007/978-3-642-38007-5_18. [DOI] [PubMed] [Google Scholar]
- 3.Leonti M. Traditional medicines and globalization: current and future perspectives in ethnopharmacology. Front Pharmacol. 2013;4:92. doi: 10.3389/fphar.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arentz S., Abbott J.A., Smith C.A., Bensoussan A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med. 2014;14:511. doi: 10.1186/1472-6882-14-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorji A. Pharmacological treatment of headache using Traditional Persian Medicine. Trends Pharm Sci. 2003;24:331–334. doi: 10.1016/S0165-6147(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 6.Fauser B.C., Tarlatzis B.C., Rebar R.W. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Sozen I., Arici A. Hyperinsulinism and its interaction with hyperandrogenism in polycystic ovary syndrome. Obstet Gynecol Surv. 2000;55:321–328. doi: 10.1097/00006254-200005000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Legro R.S., Kunselman A.R., Dodson W.C., Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metabol. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 9.Celik O., Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. J Endocrinol Investig. 2012;35:905–910. doi: 10.3275/8371. [DOI] [PubMed] [Google Scholar]
- 10.Gambineri A., Pelusi C., Vicennati V., Pagotto U., Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 11.Legro R.S., Arslanian S.A., Ehrmann D.A. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messinis I.E., Messini C.I., Anifandis G., Dafopoulos K. Polycystic ovaries and obesity. Best Pract Res Clin Obstet Gynaecol. 2015;29:479–488. doi: 10.1016/j.bpobgyn.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Crosignani P.G., Colombo M., Vegetti W., Somigliana E., Gessati A., Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18:1928–1932. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- 14.Tansaz M., Bahmani M. Principles of nutrition in patients with polycystic ovary syndrome in Iranian traditional medicine and comparison with modern medicine. Iran J Med Sci. 2016;41:S49. [PMC free article] [PubMed] [Google Scholar]
- 15.Mokaberinejad R., Zafarghandi N., Bioos S. Mentha longifolia syrup in secondary amenorrhea: a double-blind, placebo-controlled, randomized trials. DARU J Pharm Sci. 2012;20:1. doi: 10.1186/2008-2231-20-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chashti A. Research Institute for Islamic and Complementary Medicine; Tehran: 2004. Exir-e Azam (19th century) [Google Scholar]
- 17.Khorasani A. Research Institute for Islamic and Complementary Medicine; Tehran: 2008. Moalejat (18th century) [Google Scholar]
- 18.Rhazes . Academy of Medical Sciences; Tehran: 2005. Kitāb al-hāwī fī al-Tibb (The Comprehensive Book on Medicine or Liber Continens) [Google Scholar]
- 19.Ibn Sina (Avicenna) Senior Press Superintendent, Jamia Hamdard Printing Press; New Delhi: 1998. Kitāb al-Qānūn fī al-Tibb (Canon of medicine) [Google Scholar]
- 20.Tunakabuni D. Research Center of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Nashre Shahr Press; Tehran: 2007. Tuhfat al-mu'minin (The Present for the Faithful) [Google Scholar]
- 21.Shirazi A. Tehran University of Medical Sciences; Tehran: 2009. Makhzan al-adviyah (The Storehouse of Medicaments) [Google Scholar]
- 22.Ghahraman A., Okhovvat A. Tehran University Press; Tehran: 2004. Matching the Old Medicinal Plant Names with Scientific Terminology. [Google Scholar]
- 23.Khare C.P. Springer; New York: 2007. Indian Medicinal Plants. [Google Scholar]
- 24.Mozaffarian V. Farhang Moaser Press; Tehran: 2006. Dictionary of Iranian Plant Names. [Google Scholar]
- 25.Soltani A. Arjmand Press; Tehran: 2004. Dictionary of Medicinal Plants. [Google Scholar]
- 26.Ranjan V., Vats M., Gupta N., Sardana S. Antidiabetic potential of the whole plant of Adiantum capillus veneris Linn. in streptozotocin-induced diabetic rats. Int J Pharm Chem Res. 2014;6:341–347. [Google Scholar]
- 27.Yoshinari O., Shiojima Y., Igarashi K. Anti-obesity effects of onion extract in Zucker diabetic fatty rats. Nutrients. 2012;4:1518–1526. doi: 10.3390/nu4101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghasemzadeh A., Farzadi L., Khaki A., Khan Ahmadi S. Effect of Allium cepa seeds ethanolic extract on experimental polycystic ovary syndrome (PCOS) apoptosis induced by estradiol-valerate. Life Sci J. 2013;10:170–175. [Google Scholar]
- 29.Eidi A., Eidi M., Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Pintana H., Sripetchwandee J., Supakul L., Apaijai N., Chattipakorn N., Chattipakorn S. Garlic extract attenuates brain mitochondrial dysfunction and cognitive deficit in obese-insulin resistant rats. Appl Physiol Nutr Metabol. 2014;39:1373–1379. doi: 10.1139/apnm-2014-0255. [DOI] [PubMed] [Google Scholar]
- 31.Panda S. The effect of Anethum graveolens L. (dill) on corticosteroid induced diabetes mellitus: involvement of thyroid hormones. Phytother Res. 2008;22:1695–1697. doi: 10.1002/ptr.2553. [DOI] [PubMed] [Google Scholar]
- 32.Mirhosseini M., Baradaran A., Rafeian-Kopaei M. Anethum graveolens and hyperlipidemia: a randomized clinical trial. J Res Med Sci. 2014;19:758–761. [PMC free article] [PubMed] [Google Scholar]
- 33.Monsefi M., Ghasemi M., Bahaoddini A. The effects of Anethum graveolens L. on female reproductive system of rats. DARU J Pharm Sci. 2006;14:131–135. doi: 10.1002/ptr.1959. [DOI] [PubMed] [Google Scholar]
- 34.Mansi K., Abushoffa A.M., Disi A., Aburjai T. Hypolipidemic effects of seed extract of celery (Apium graveolens) in rats. Pharmacogn Mag. 2009;5:301. [Google Scholar]
- 35.Vasanthkumar R., Jeevitha M. Evaluation of antiobesity activity of Apium graveolens stems in rats. Int J Chem Pharm Sci. 2014;5:159–163. [Google Scholar]
- 36.Bettaieb A., Hosein E., Chahed S. Decreased adiposity and enhanced glucose tolerance in shikonin treated mice. Obesity. 2015;23:2269–2277. doi: 10.1002/oby.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hafizur R.M., Kabir N., Chishti S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function in streptozotocin-induced type 2 diabetic rats. Br J Nutr. 2012;108:1586–1595. doi: 10.1017/S0007114511007148. [DOI] [PubMed] [Google Scholar]
- 38.Zhu X., Zhang W., Zhao J., Wang J., Qu W. Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. by-products in mice fed a high-fat diet. J Sci Food Agric. 2010;90:1129–1135. doi: 10.1002/jsfa.3923. [DOI] [PubMed] [Google Scholar]
- 39.Jashni H.K., Jahromi H.K., Ranjbary A.G., Jahromi Z.K., Kherameh Z.K. Effects of aqueous extract from Asparagus officinalis L. roots on hypothalamic-pituitary-gonadal axis hormone levels and the number of ovarian follicles in adult rats. Int J Reprod Biomed. 2016;14:75. [PMC free article] [PubMed] [Google Scholar]
- 40.Eddouks M., Lemhadri A., Michel J.B. Caraway and caper: potential anti-hyperglycaemic plants in diabetic rats. J Ethnopharmacol. 2004;94:143–148. doi: 10.1016/j.jep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Eddouks M., Lemhadri A., Michel J.-B. Hypolipidemic activity of aqueous extract of Capparis spinosa L. in normal and diabetic rats. J Ethnopharmacol. 2005;98:345–350. doi: 10.1016/j.jep.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 42.Lemhadri A., Hajji L., Michel J.-B., Eddouks M. Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2006;106:321–326. doi: 10.1016/j.jep.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Kazemipoor M., Radzi C.W., Hajifaraji M., Haerian B.S., Mosaddegh M.H., Cordell G.A. Antiobesity effect of caraway extract on overweight and obese women: a randomized, triple-blind, placebo-controlled clinical trial. Evid Based Complement Altern Med. 2013;2013:928582. doi: 10.1155/2013/928582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y., Zhou L., Gu Y. Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. Br J Nutr. 2007;98:720–726. doi: 10.1017/S0007114507750870. [DOI] [PubMed] [Google Scholar]
- 45.El-Desoky G.E., Aboul-Soud M.A., Al-Numair K.S. Antidiabetic and hypolipidemic effects of Ceylon cinnamon (Cinnamomum verum) in alloxan-diabetic rats. J Med Plant Res. 2012;6:1685–1691. [Google Scholar]
- 46.Huseini H.F., Darvishzadeh F., Heshmat R., Jafariazar Z., Raza M., Larijani B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of Type II diabetic patients: a randomized, double blind, placebo-controlled clinical trial. Phytother Res. 2009;23:1186–1189. doi: 10.1002/ptr.2754. [DOI] [PubMed] [Google Scholar]
- 47.Jia S., Hu Y., Zhang W. Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-A(y) mice. Food Funct. 2015;6:878–886. doi: 10.1039/c4fo00993b. [DOI] [PubMed] [Google Scholar]
- 48.Peixoto J.S., Comar J.F., Moreira C.T. Effects of Citrus aurantium (bitter orange) fruit extracts and p-synephrine on metabolic fluxes in the rat liver. Molecules (Basel, Switzerland) 2012;17(5):5854–5869. doi: 10.3390/molecules17055854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patil S.J., Patil S.B. Estrogenic activity of petroleum ether extract of seeds of Citrus medica on immature albino rats. Int J Green Pharm. 2008;2:91–94. [Google Scholar]
- 50.Ramesh B., Karuna R., Reddy S.S., Sudhakara G., Saralakumari D. Ethanolic extract of Commiphora mukul gum resin attenuates streptozotocin-induced alterations in carbohydrate and lipid metabolism in rats. EXCLI J. 2013;12:556. [PMC free article] [PubMed] [Google Scholar]
- 51.Helal E.G., Mahmoud A., El-Badawy E.E., Kahwash A.A. Effect of Commiphora myrrha extract on some physiological parameters and histological changes in diabetic albino rats. Egypt J Hosp Med. 2005:148–162. [Google Scholar]
- 52.Singh R.B., Niaz M.A., Ghosh S. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc Drugs Ther. 1994;8:659–664. doi: 10.1007/BF00877420. [DOI] [PubMed] [Google Scholar]
- 53.Perez C., Dominguez E., Canal J., Campillo J., Torres M. Hypoglycaemic activity of an aqueous extract from Ficus carica (fig tree) leaves in streptozotocin diabetic rats. Pharm Biol. 2000;38:181–186. doi: 10.1076/1388-0209(200007)3831-SFT181. [DOI] [PubMed] [Google Scholar]
- 54.Belguith-Hadriche O., Ammar S., del Mar Contreras M. Antihyperlipidemic and antioxidant activities of edible Tunisian Ficus carica L. Fruits in high fat diet-induced hyperlipidemic rats. Plant Foods Hum Nutr. 2016;71:183–189. doi: 10.1007/s11130-016-0541-x. [DOI] [PubMed] [Google Scholar]
- 55.El-Soud N., El-Laithy N., El-Saeed G. Antidiabetic activities of Foeniculum vulgare mill. Essential oil in streptozotocin-induced diabetic rats. Maced J Med Sci. 2011;4:139–146. [Google Scholar]
- 56.Helal E.G., Eid F.A., Wahsh A.M., Ahmed E. Effect of fennel (Foeniculum vulgare) on hyperlipidemic rats. Egypt J Hosp Med. 2011;43:212–226. [Google Scholar]
- 57.Sadeghpour N., Khaki A.A., Najafpour A., Dolatkhah H., Montaseri A. Study of Foeniculum vulgare (Fennel) seed extract effects on serum level of estrogen, progesterone and prolactin in mouse. A general policy. Crescent J Med Biol Sci. 2015;2:59–63. [Google Scholar]
- 58.Visavadiya N.P., Narasimhacharya A.V. Hypocholesterolaemic and antioxidant effects of Glycyrrhiza glabra (Linn) in rats. Mol Nutr Food Res. 2006;50:1080–1086. doi: 10.1002/mnfr.200600063. [DOI] [PubMed] [Google Scholar]
- 59.Malik Z.A., Sharma P.L. An ethanolic extract from licorice (glycyrrhiza glabra) exhibits anti-obesity effects by decreasing dietary fat absorption in a high fat diet-induced obesity rat model. Int J Pharm Sci Res. 2011;2:3010. [Google Scholar]
- 60.Saini S., Sharma S. Antidiabetic effect of Helianthus annuus L., seeds ethanolic extract in streptozotocinnicotinamide induced type 2 diabetes mellitus. Int J Pharm Pharm Sci. 2013;5:382–387. [Google Scholar]
- 61.Arokiyaraj S., Balamurugan R., Augustian P. Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1:386–390. doi: 10.1016/S2221-1691(11)60085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Husain G.M., Chatterjee S.S., Singh P.N., Kumar V. Hypolipidemic and antiobesity-like activity of standardised extract of Hypericum perforatum L. in rats. ISRN Pharmacol. 2011;2011:505247. doi: 10.5402/2011/505247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chauhan K., Sharma S., Agarwal N., Chauhan S., Chauhan B. A study on potential hypoglycemic and hypolipidemic effects of Lepidium Sativum (Garden Cress) in alloxan induced diabetic rats. Am J PharmTech Res. 2012;2:522–535. [Google Scholar]
- 64.Althnaian T. Influence of dietary supplementation of Garden cress (Lepidium sativum L.) on liver histopathology and serum biochemistry in rats fed high cholesterol diet. J Adv Vet Animal Res. 2014;1:216–223. [Google Scholar]
- 65.Ghule A.E., Jadhav S.S., Bodhankar S.L. Effect of ethanolic extract of seeds of Linum usitatissimum (Linn.) in hyperglycaemia associated ROS production in PBMNCs and pancreatic tissue of alloxan induced diabetic rats. Asian Pac J Trop Dis. 2012;2:405–410. [Google Scholar]
- 66.Khalesi S., Jamaluddin R., Ismail A. Effect of raw and heated flaxseed (Linum usitatissimum L.) on blood lipid profiles in rats. Int J Appl Sci Tech. 2011;1:84–89. [Google Scholar]
- 67.Ahmad N., Rahman Z., Akhtar N., Ali S. Effects of aqueous methanolic extract of flax seeds (Linum usitatissimum) on serum estradiol, progesterone, kidney and liver functions and some serum biochemical metabolites in immature female rats. Pak Vet J. 2012;32:211–215. [Google Scholar]
- 68.Mansour H.A., Newairy A.-S.A., Yousef M.I., Sheweita S.A. Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology. 2002;170:221–228. doi: 10.1016/s0300-483x(01)00555-8. [DOI] [PubMed] [Google Scholar]
- 69.Fontanari G.G., Batistuti J.P., Cruz R.J.D., Saldiva P.H.N., Arêas J.A.G. Cholesterol-lowering effect of whole lupin (Lupinus albus) seed and its protein isolate. Food Chem. 2012;132:1521–1526. doi: 10.1016/j.foodchem.2011.11.145. [DOI] [PubMed] [Google Scholar]
- 70.Cemek M., Kağa S., Şimşek N., Büyükokuroğlu M.E., Konuk M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J Nat Med. 2008;62:284–293. doi: 10.1007/s11418-008-0228-1. [DOI] [PubMed] [Google Scholar]
- 71.Farideh Z.Z., Bagher M., Ashraf A., Akram A., Kazem M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J Reprod Infertil. 2010;11:169–174. [PMC free article] [PubMed] [Google Scholar]
- 72.Chung M.J., Cho S.-Y., Bhuiyan M.J.H., Kim K.H., Lee S.-J. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose-and lipid-regulating enzymes in type 2 diabetic mice. Br J Nutr. 2010;104:180–188. doi: 10.1017/S0007114510001765. [DOI] [PubMed] [Google Scholar]
- 73.Bolkent S., Yanardag R., Karabulut-Bulan O., Yesilyaprak B. Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: a morphological and biochemical study. J Ethnopharmacol. 2005;99:391–398. doi: 10.1016/j.jep.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 74.Lee J., Chae K., Ha J. Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis, and Artemisia capillaris in high-fat diet-induced obese mice. J Ethnopharmacol. 2008;115:263–270. doi: 10.1016/j.jep.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 75.Fararh K.M., Atoji Y., Shimizu Y., Shiina T., Nikami H., Takewaki T. Mechanisms of the hypoglycaemic and immunopotentiating effects of Nigella sativa L. oil in streptozotocin-induced diabetic hamsters. Res Vet Sci. 2004;77:123–129. doi: 10.1016/j.rvsc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Pourghassem-Gargari B., Ebrahimzadeh-Attary V., Rafraf M., Gorbani A. Effect of dietary supplementation with Nigella sativa L. on serum lipid profile, lipid peroxidation and antioxidant defense system in hyperlipidemic rabbits. J Med Plant Res. 2009;3:815–821. [Google Scholar]
- 77.Perez Gutierrez R.M. Inhibition of advanced glycation end-product formation by Origanum majorana L. in vitro and in streptozotocin-induced diabetic rats. Evid Based Complement Altern Med. 2012:2012. doi: 10.1155/2012/598638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haj-Husein I., Tukan S., Alkazaleh F. The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J Hum Nutr Diet. 2016;29:105–111. doi: 10.1111/jhn.12290. [DOI] [PubMed] [Google Scholar]
- 79.Ozsoy-Sacan O., Yanardag R., Orak H., Ozgey Y., Yarat A., Tunali T. Effects of parsley (Petroselinum crispum) extract versus glibornuride on the liver of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2006;104:175–181. doi: 10.1016/j.jep.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 80.Soliman H.A., Eltablawy N.A., Hamed M.S. The ameliorative effect of Petroselinum crispum (parsley) on some diabetes complications. J Med Plants. 2015;3:92–100. [Google Scholar]
- 81.Le M.T., Lanaspa M.A., Cicerchi C.M. Bioactivity-guided identification of botanical inhibitors of ketohexokinase. PLoS One. 2016;11:e0157458. doi: 10.1371/journal.pone.0157458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venkateswaran S., Pari L., Saravanan G. Effect of Phaseolus vulgaris on circulatory antioxidants and lipids in rats with streptozotocin-induced diabetes. J Med Food. 2002;5:97–103. doi: 10.1089/109662002760178186. [DOI] [PubMed] [Google Scholar]
- 83.Pari L., Venkateswaran S. Protective role of Phaseolus vulgaris on changes in the fatty acid composition in experimental diabetes. J Med Food. 2004;7:204–209. doi: 10.1089/1096620041224120. [DOI] [PubMed] [Google Scholar]
- 84.Zhu Z., Jiang W., Thompson H.J. Edible dry bean consumption (Phaseolus vulgaris L.) modulates cardiovascular risk factors and diet-induced obesity in rats and mice. Br J Nutr. 2012;108:S66–S73. doi: 10.1017/S0007114512000839. [DOI] [PubMed] [Google Scholar]
- 85.Shobha R., Rajeshwari C., Andallu B. Anti-peroxidative and anti-diabetic activities of aniseeds (Pimpinella anisum L.) and identification of bioactive compounds. Am J Phytomed Clin Ther. 2013;1:516–527. [Google Scholar]
- 86.Bao L., Bai S., Borijihan G. Hypolipidemic effects of a new piperine derivative GB-N from Piper longum in high-fat diet-fed rats. Pharm Biol. 2012;50:962–967. doi: 10.3109/13880209.2012.654395. [DOI] [PubMed] [Google Scholar]
- 87.Farkhad N.K., Farokhi F., Tukmacki A. Hydro-alcoholic extract of the root of Prangos ferulacea (L.) Lindl can improve serum glucose and lipids in alloxan-induced diabetic rats. Avicenna J Phytomed. 2012;2:179. [PMC free article] [PubMed] [Google Scholar]
- 88.Noratto G.D., Garcia-Mazcorro J.F., Markel M. Carbohydrate-free peach (Prunus persica) and plum (Prunus domestica) juice affects fecal microbial ecology in an obese animal model. PLoS One. 2014;9:e101723. doi: 10.1371/journal.pone.0101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed O.M., Moneim A.A., Yazid I.A., Mahmoud A.M. Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozotocin-induced diabetic rats. Diabetol Croat. 2010;39:15–35. [Google Scholar]
- 90.Toserkani A., Jalali M.R., Najafzaheh H. Changes of lipid profiles, glucose, and hemogram after administration of Ruta graveolens extract in diabetic rats. Comp Clin Path. 2012;21:1587–1592. [Google Scholar]
- 91.Ekoh S.N., Akubugwo E.I., Ude V.C., Edwin N. Anti-hyperglycemic and anti-hyperlipidemic effect of spices (Thymus vulgaris, Murraya koenigii, Ocimum gratissimum and Piper guineense) in alloxan-induced diabetic rats. Int J Biosci. 2014;4:179–187. [Google Scholar]
- 92.Javed I., Zia-Ur-Rahman N., Khan M.Z. Antihyperlipidaemic efficacy of Trachyspermum ammi in albino rabbits. Acta Vet Brno. 2009;78:229–236. [Google Scholar]
- 93.Hannan J.M.A., Rokeya B., Faruque O. Effect of soluble dietary fibre fraction of Trigonella foenum graecum on glycemic, insulinemic, lipidemic and platelet aggregation status of Type 2 diabetic model rats. J Ethnopharmacol. 2003;88:73–77. doi: 10.1016/s0378-8741(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 94.Sharma M.S., Choudhary P.R. Effect of fenugreek seeds powder (Trigonella foenum-graecum L.) on experimental induced hyperlipidemia in rabbits. J Diet Suppl. 2016;12:1–8. doi: 10.3109/19390211.2016.1168905. [DOI] [PubMed] [Google Scholar]
- 95.Chevassus H., Gaillard J.B., Farret A. A fenugreek seed extract selectively reduces spontaneous fat intake in overweight subjects. Eur J Clin Pharmacol. 2010;66:449–455. doi: 10.1007/s00228-009-0770-0. [DOI] [PubMed] [Google Scholar]
- 96.Kassem A., Al-Aghbari A., Molham A.-H., Al-Mamary M. Evaluation of the potential antifertility effect of fenugreek seeds in male and female rabbits. Contraception. 2006;73:301–306. doi: 10.1016/j.contraception.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 97.Bnouham M., Merhfour F.-Z., Ziyyat A., Mekhfi H., Aziz M., Legssyer A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia. 2003;74:677–681. doi: 10.1016/s0367-326x(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 98.Das M., Sarma B., Rokeya B. Antihyperglycemic and antihyperlipidemic activity of Urtica dioica on type 2 diabetic model rats. J Diabetol. 2011;2:1–6. [Google Scholar]
- 99.Mohammadi A., Gholamhoseinian A., Fallah H. Zataria multiflora increases insulin sensitivity and PPARgamma gene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci. 2014;17:263–270. [PMC free article] [PubMed] [Google Scholar]
- 100.Vrbikova J. Polycystic ovary syndrome: why there is no cure. Expert Rev Endocrinol Metab. 2012;7:475–477. doi: 10.1586/eem.12.41. [DOI] [PubMed] [Google Scholar]
- 101.Vrbikova J., Cibula D. Combined oral contraceptives in the treatment of polycystic ovary syndrome. Hum Reprod Update. 2005;11:277–291. doi: 10.1093/humupd/dmi005. [DOI] [PubMed] [Google Scholar]
- 102.Shepherd J., Hull Royal Infirmary Hull U. Insulin resistance and oxidative stress in obese PCOS, nonobese PCOS and controls. Insulin. 2016;1:357. [Google Scholar]
- 103.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;1:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elahi A., Fereidooni A., Shahabinezhad F., Tafti M.A., Zarshenas M.-M. An overview of amenorrhea and respective remedies in Traditional Persian Medicine. Trends Pharma Sci. 2016;2(1):3–10. [Google Scholar]