Abstract
Cancer and depression are known as two of the most debilitating disease and disorder increasing evidence suggest an urgent need for new therapeutic agents with lower toxicity and high efficacy. Some Thyme species extracts have remarkably been shown to positively affect depression and cancer cells. In the present study, we investigated the effect of Thymus kotschyanus on depression and cancer cells. To this end, in experiment 1, NMRI mice were treated orally with the ethanolic extract of T. kotschyanus (50, 150 and 250 mg/ml) for seven days and then depression-like behavior was measured by Forced Swim Test (FST) and Tail Suspension Test (TST). In experiment 2, the pharmacological effect of the extract on the lung (A549) and cervical (Hela) cancer cell lines was also evaluated by MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) in various concentration_(10, 5, 2.5, 1.25, 0.63, 0.31, 0.15 and 0.08 mg/ml). The results indicated that T. kotschyanus extract treatment (150 and 250 mg/kg) decreased depression-like behavior in the FST and TST tests in adult mice. Moreover, the treatment inhibited cancer cell growth and viability in a dose and time-dependent manner. Collectively these findings suggest that T. kotschyanus have antidepressant and anticancer effects.
Keywords: Thymus kotschyanus, Depression, Lung cancer, Cervical cancer, Mice
Graphical abstract
1. Introduction
In recent years, extensive research have been done on cancer and depression, the two prime causes of mortality and morbidity in people, however there are two main problems: the first is the etiology and the second is the treatment of these diseases.1 A case study conducted by Onitilo et al. indicated that cancer and depression are two independent risk factors for mortality rate in humans; hence, their effects on all-cause of mortality are additive, not synergistic.2, 3
Depression is a debilitating chronic disorder, significantly affecting the quality of life of large population worldwide.4, 5, 6 There are several contributing factors including genetic predisposition, epigenetic alterations, neuroendocrine and immunological dysregulations, monoamine neurotransmitter deficiencies in the brain, affecting depression-related behavior.7, 8, 9
Cervical and lung cancers are the principal causes of cancer-related death.10, 11 Cervical cancer is caused by chronic infection with a range of high-risk Human Papilloma Virus (HPV) leading to an estimated 274,000 death globally every year. Moreover, lung cancer is a highly aggressive, progressive and heterogeneous malignant disease predominantly results from smoking tobacco with few options of treatment.12, 13, 14, 15 For the treatment, chemotherapy with drugs such as Cisplatin and Iressa and psychotherapy with antidepressant drugs like fluoxetine, sertraline, and citalopram are widely used. However, these drugs have several side effects leading to further research for treatment with herbal medicines to reduce adverse side effects.16, 17, 18
There is a wide geographical distribution of the genus Thymus in Asia, North America, Europe and Africa, Thymus has several species including Thymus vulgaris, Thymus daenensis, Thymus schimperi, Thymus zygis and Thymus kotschyanus etc.19, 20, 21, 22 T. kotschyanus is a dicot herbal plant belonging to Lamiaaceae's family. The name of T. kotschyanus is gained from Greek “Thymos” meaning power and courage.23 In traditional medicine thymus species are used as antibacterial, antifungal, antiviral, anti-helminthic, antioxidative, antispasmodic, sedative and diaphoretic drugs.24, 25, 26 Carvacrol and Thymol are known as the major phenolic monoterpenes of thyme oil especially in T. kotschyanus oil showing anticancer and anti-depressant effects.27
Insufficient attention has so far been devoted to T. kotschyanus. Therefore, the goal of this study was to investigate the potential effects of T. kotschyanus on human lung and cervical cancer cell growth in in vitro cell culture and depression-like behavior in male mice.
2. Material and method
2.1. Experimental design
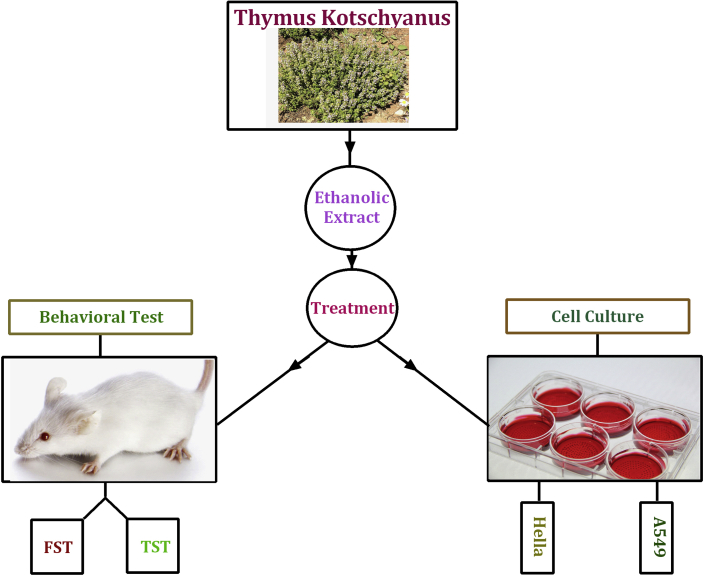
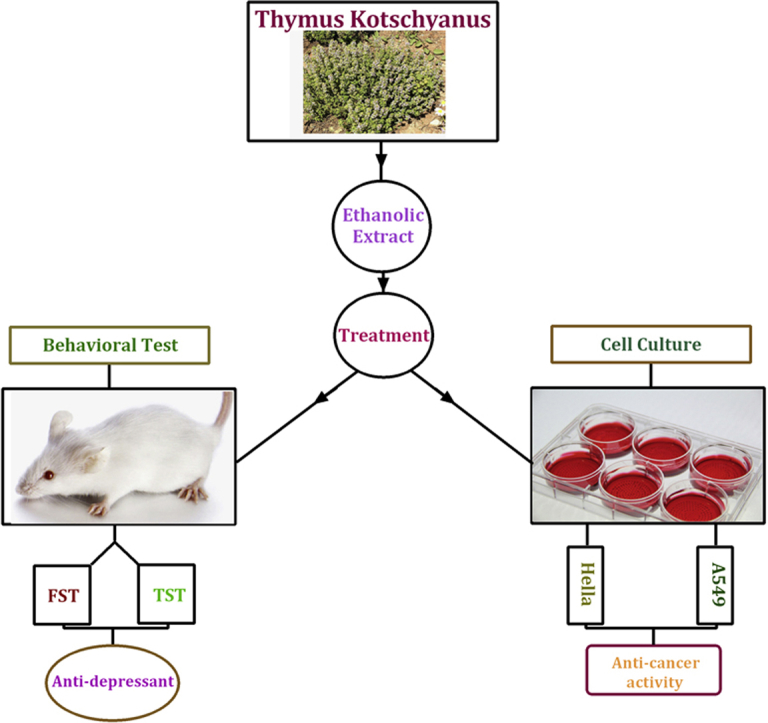
A summary of experimental design is shown in Fig. 1. The mice were randomly divided into four groups (six animals per groups), three groups orally received T. kotschyanus extract (50, 150 and 250 mg/kg) once daily for seven days and the one received saline as control group. Each animal was tested only once, with ten-day intervals between behavioral tests. In parallel with the behavioral tests, we investigated the effect of extract (10, 5, 2.5, 1.25, 0.63, 0.31, 0.15 and 0.08 mg/ml) on two cancer cell lines (A549 and Hella) for 24 and 48 h.
Fig. 1.
Experimental design: the effects of T. kotschyanus exposure on depression-like behavior and cancer cell growth inhibition in male mice and cancer cell lines respectively. TST and FST performed on the same mice with ten days interval.
2.2. Plant extract
Leaves of T. kotschyanus (collected from Dizin, North Tehran province of Iran) were dried at room temperature and ground into powder form by a grinder. The ethanolic extract was extracted from the powdered plant with one liter of solvent (70% ethanol), agitated for two days then filtered for twice with Whatman No. 41 filter paper at the room temperature. The extract was evaporated at 30 °C in the rotary evaporator. The evaporation process involved the total removal of ethanol and water was used for the extraction. The yield of the extract was 10 g/200 g powder. The extract was dissolved in DMSO and then diluted with distilled water (DW) to make required concentration for oral administration. The orally administrated dosses are expressed as milligram of dry extract per kilogram of mice body weight. In the cell culture study, before sterilizing with a 0.22 μm pore size syringe filter (Nuc, Denmark), 20 mg of the extract was dissolved in 100 μl DMSO and diluted with RMPI-1640 to make appropriate concentration for cell culture administration.27, 28
2.3. Animals
Male NMRI mice were used throughout the study. The animals were obtained from Pasture Institute of Iran. Mice were housed under the standard condition with 12/12 light-dark cycle (lights on 08:00–20:00 h) and controlled humidity and temperature (23 ± 1 °C) in all experiments. All animals had access to food and water ad libitum. All procedures were approved by the Cellular and Molecular Research Center at the Baqiyatallah University of Medical Sciences.
2.4. Forced Swim Test
Forced Swim Test is widely used for measuring anti-depressant effect in pharmacology. Briefly, mice were gently dropped into the transparent glass cylinder, 25 cm height and 10 cm diameter in order to avoid mice touch the bottom of the cylinder. The cylinder filled with water at 25 ± 1 °C to a depth of 15 cm. Moreover water was replaced by fresh one between each test. The total duration of immobility was recorded by a blind observer during the last 4 min of 6 min testing period. The immobility was defined as an absence of any struggling exclude movement requisite for a mouse to keep its head above the water. A decline in immobility time is a demonstration of the anti-depressant-like properties.29
2.5. Tail Suspension Test
TST is a valuable behavioral test which is widely used to screen antidepressant-like activity. Briefly, mouse was individually suspended by the tail using a clamp 2 cm from the end. Total immobility was measured during 5 min test period. Moreover, immobility was described as times spent completely immobile posture except those caused by respiration.30
2.6. Cell culture
A549 and HeLa cell lines were acquired from the National Cell Bank of Iran (NCBI, Tehran) and were grown in the RPMI 1640 medium (Gibco) supplemented with 10% (v/v) Fetal Bovine Serum (FBS), penicillin/streptomycin (100 IU/ml, 100 μg/ml respectively) (Sigma, Germany). The cells were incubated and maintained in a humidified atmosphere at 37 °C and 5% CO2. Upon reaching 80% confluence, cells were rinsed with phosphate-buffered saline (PBS) then Cells were detached and harvested from 25 cm2 flasks by using 0.25% Trypsin/EDTA solution (sigma, Germany). Finally, cells were sub-cultured into a 96-well plate according to experiment. The experiment was performed in duplicate.
2.7. MTT assay
The cytotoxicity of the extract was evaluated on HeLa cells as well as A549 cells by using the MTT method. The method is according to the ability of viable cells to produce blue formazan crystals from yellow tetrazolium salt MTT by the mitochondrial dehydrogenase. The cells were plated into 96-well plate (Nunc, Denmark) at a density of 104 cells/well/200 μl. The next day the media were replaced with fresh complete medium containing different extract concentrations (10, 5, 2.5, 1.25, 0.63, 0.31, 0.15, 0.08 mg/ml) and 0.2% DMSO (Sigma, USA) as negative control. Then 10 μl of MTT labeling reagent was added to each well, then the cells were administered with the extract for 24 and 48 h. The plates were incubated at 37 °C and 5% CO2 for 4 h. Afterwards, supernatants were discarded and 100 μl of the Solubilization solution was added to each well and plates were incubated overnight in humidity atmosphere. Finally, cytotoxicity was detected by measuring the absorbance at a wavelength of 570 nm using an ELISA plate reader (Lab System). The percentage of cell cytotoxicity and viability was calculated according to following formula31:
%Cytotoxicity = 1 − Mean absorbance of toxicant/Mean absorbance of negative control × 100
%Viability = 100 − %Cytotoxicity
2.8. Statistical analysis
All data were analyzed according to the analysis of variance (ANOVA) followed by Tukey's test using the statistical program SPSS (IBM, Version 20). All values were presented as the means ± SEM and a P value of less than 0.05 was considered statistically significant.
3. Result
3.1. Tail Suspension Test
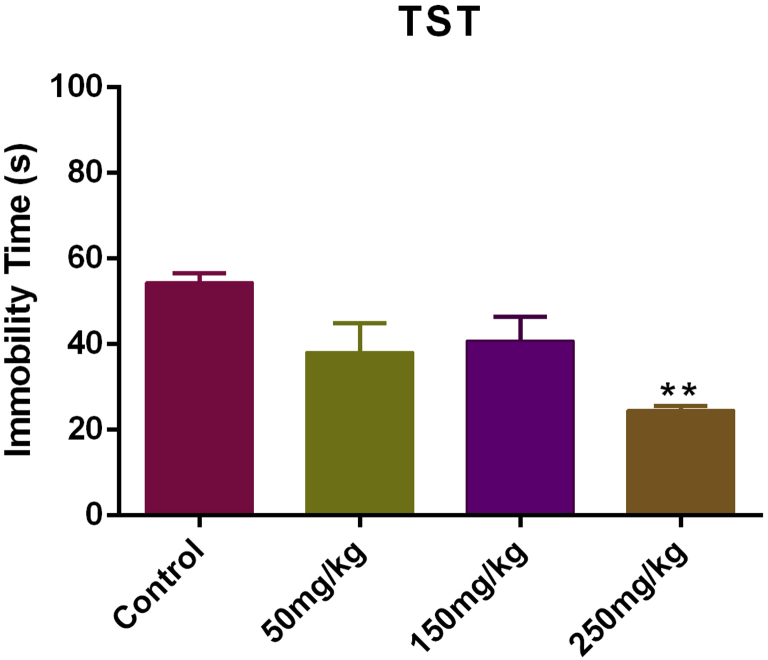
We determined that the effect of T. kotschyanus extract on depression-like behavior in the TST. Fig. 2 illustrates that the extract at dose of 250 mg/kg significantly decreased immobility time [F(3, 20) = 7.387, P < 0.01] in mice in comparison with control group. These data show that T. kotschyanus extract treatment can reduce depression-like behavior in mice.
Fig. 2.
Effects of Thymus kotschyanus extract in male mice in the TST (2) and FST (3). Values are presented as mean ± SEM (N = 6) of total duration of immobility. **P < 0.01 and ***P < 0.001.
3.2. Forced Swim Test
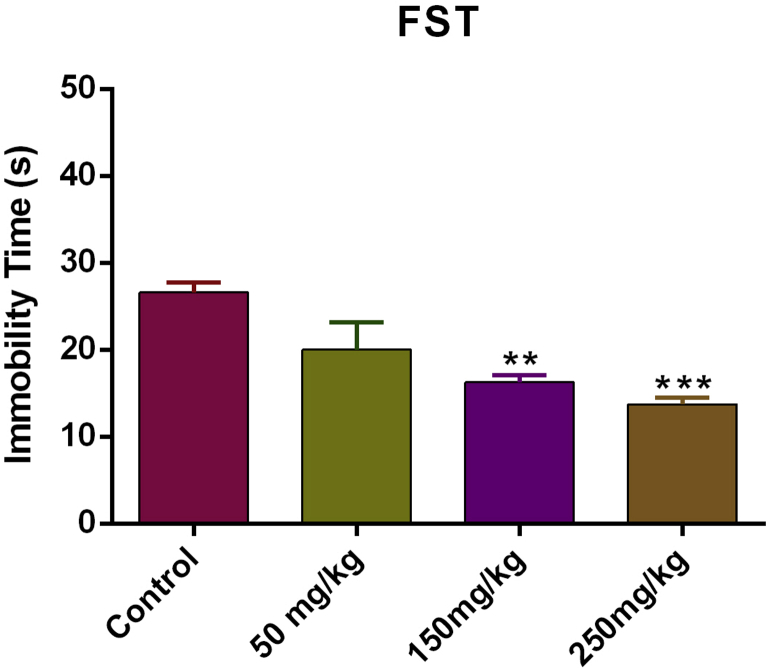
The FST was conducted after the TST and its data is presented in Fig. 3. The analysis indicated that the extract treatment at doses of 150 and 250 mg/kg significantly resulted in decreased the immobility time [F(3, 20) = 10.137, P < 0.001] in mice, as compared with control group. These results confirmed the TST data, showing that T. kotschyanus extract treatment can reduce depression-like behavior in mice.
Fig. 3.
Effects of Thymus kotschyanus extract in male mice in the TST (2) and FST (3). Values are presented as mean ± SEM (N = 6) of total duration of immobility. **P < 0.01 and ***P < 0.001.
3.3. Cell viability
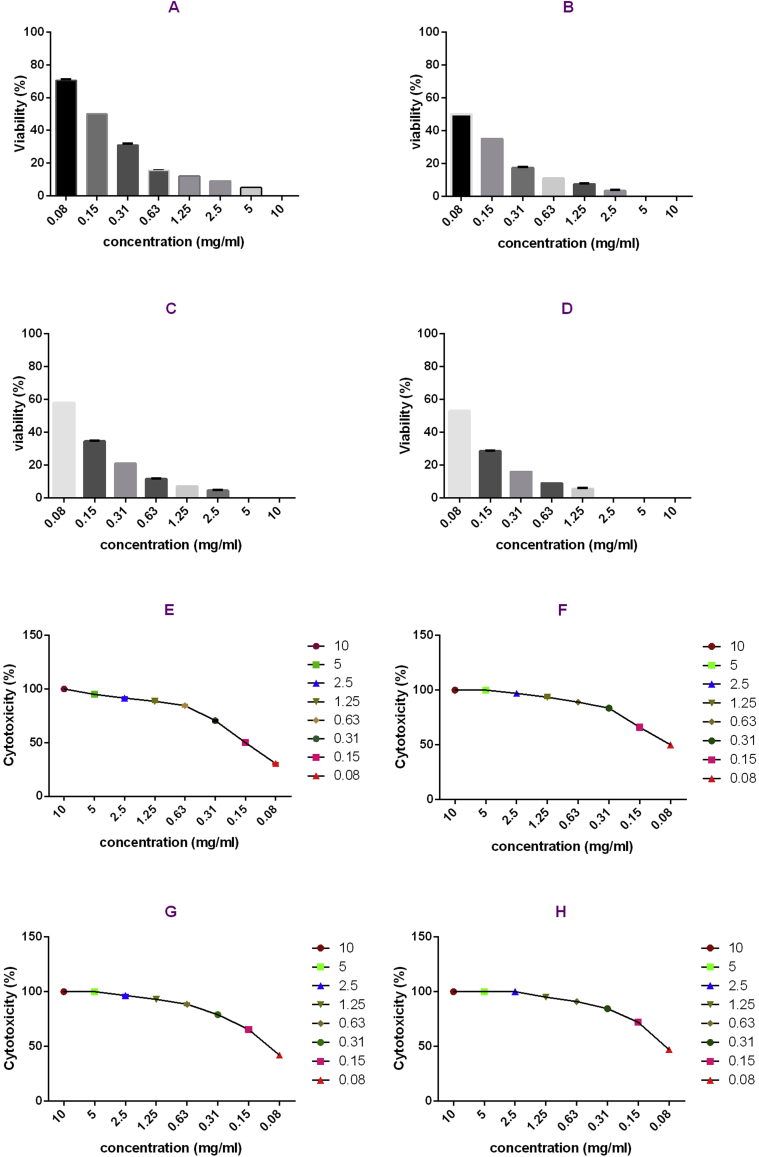
The cytotoxic effect of T. kotschyanus extract was determined by MTT assay which is depicted in Fig. 4, A–H. After 24 h the extract a dose-dependent reduction in cell viability was found. The long-term extract treatment at higher dose resulted in the maximal cytotoxicity on cells. While A549 (Lung Carcinoma-derived cells) and HeLa (Cervical adenocarcinoma-derived cells) cell lines were treated for 24 h with the extract the viability (%) of HeLa cells was less than A549 cells, the viability of HeLa cells at a concentration of 0.08 mg/ml was more than A549 cells in the same concentration in 48 h treatment. However, the grater viability loss was observed in 48 h treatment including 2.5, 5 and 10 mg/ml for HeLa as well as 5 and 10 mg/ml for A549 cells. The extract at the dose of 10 mg/ml produced the most significant cytotoxicity (in both times) than the other doses.
Fig. 4.
A–D. Shows the inhibition of A549 and Hela cells growth that was measured by MTT assay (A. viability after 24 h in A549, B. viability after 48 h in A549, C. viability after 24 h in Hela, D. viability after 48 h in Hela). E–H. Shows the cytotoxic effect of the extract of Thymus kotschyanus in A549 and Hela cells that were measured using MTT assay (E. 24 h _A549, F. 48 h_ A549, G. 24 h_ Hela, H. 48 h_ Hela).
4. Discussion
Herbal plants are predominantly used because of not only the toxicity and undesirable side effects of synthetic drugs, but also increasing resistance to chemical drugs as well as their high cost.19, 32, 33 Among rich variety of medicinal plants containing bioactive substances, Thyme family are well-known as one of the most important spices, and used for culinary purpose34 in spite of their anti-inflammatory, anti-oxidant, anti-bacterial (in vitro antimicrobial activity against Salmonella, E. coli, Listeria, and Staphylococcus) and anxiolytic properties.35, 36 T. kotschyanus has been mainly popularly used over centuries in common cold, inflammation, treat headache, vomit and irritation of urinary organs by Iranian.37
On the contrary, some useful properties of T. kotschyanus have been demonstrated in the recent studies, no studies have so far been specifically evaluated its ethanolic extract effects on both depression and cancer.
4.1. Behavioral effect of T. kotschyanus
We observed a significant decrease in depression-like behavior in mice treated orally with 150 and 250 mg/ml after 7 days compared with control animals. The major components in T. kotschyanus are Carvacrol and Thymol.38, 39 A study conducted by Deng et al. indicated that the Thymol administration to mice enhanced serotonin (5-HT) and norepinephrine (NE) in the hippocampus, while negatively regulated the release of pro-inflammatory cytokines, Interleukin-1β (IL-1β), Interleukin-6 (IL-6) and Tumor Necrosis-alpha (TNF-α) in this region of the brain.40 Several parallel studies indicated that the augmentation of pro-inflammatory cytokines levels declines tryptophan, a crucial neurotransmitter precursor in serotonin biosynthesis41 Moreover, another recent study showed that Carvacrol resulted in reduced immobility time in the TST and FST tests; however, the mechanism appears to be quite different from that by which Thymol leads to decreased depression-like behavior.42 Interestingly, the anti-depressant effect of Carvacrol, unlike Thymol, could associated to the enhancement of dopamine transmission in the synaptic cleft which occurs in the limbic system.42 Therefore, it may be concluded that increased level of serotonin or dopamine besides the decrease in pro-inflammatory cytokines affect depression-like behavior in male mice.
4.2. Effect of Thymus kotschyanus on cancer cells
Ideally, besides having fewer side effects and toxicity for normal host tissues, successful anticancer drugs or promising anticancer herbs should inhibit tumor growth through the reduction of cell proliferation and the induction of cell apoptosis.43 Previous studies have provided conflicting findings about the anticancer activity of T. kotschyanus extract. However, our results led to a significant inhibition in A549 and HeLa cell lines growth which may be attributed to the phenolic compounds, in particular, Thymol and Carvacrol; moreover, based on some evidence these substantial substances are also involved in antioxidant properties of T. kotschyanus.44 In this regard, some studies have demonstrated Thymol cell growth inhibition properties.45, 46 The antiproliferative effect of Carvacrol on lung and cervical cancer cell line, chronic myeloid leukemia cells and human metastatic breast cancer cells (MDA-MB231) have been reported.47, 48, 49 The cytotoxicity of T. kotschyanus ethanolic extract on A549 and HeLa cell lines has indicated that the herb may exert its inhibitory and killing activity through multiple mechanisms leading to cell death. The possible mechanism seems to be associated with Thymol resulting in apoptosis.45 Nevertheless, some researchers have reported that the apoptosis induction by Thymol is lower when compared with Carvacrol cell death potential.47
Therefore, since T. kotschyanus extract was found to decrease the level of depression and inhibit the lung and cervical cancer cell growth, it is possible antidepressant and anticancer effect of T. kotschyanus were mediated by Carvacrol and Thymol, the major components of T. kotschyanus. Further, studies are needed to find which components could be responsible for these effects.
Conflict of interest
None declared.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Mayes R., Armistead B. Chronic disease, prevention policy, and the future of public health and primary care. Med Health Care Philos. 2013;16:691–697. doi: 10.1007/s11019-012-9454-0. [DOI] [PubMed] [Google Scholar]
- 2.Zonderman Alan B., Costa Paul T., McCrae Robert R. Depression as a risk for cancer morbidity and mortality in a nationally representative sample. JAMA. 1998;9:1191–1195. [PubMed] [Google Scholar]
- 3.Onitilo Adedayo A., Nietert Paul J., Egede Leonard E. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. General Hosp Psychiatry. 2006;28:396–402. doi: 10.1016/j.genhosppsych.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Khan D., Fernando P., Cicvaric A. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl Psychiatry. 2014;4:e363. doi: 10.1038/tp.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haque R., Enger S.M., Chen W., Petitti D.B. Breast cancer risk in a large cohort of female antidepressant medication users. Cancer Lett. 2005;221:61–65. doi: 10.1016/j.canlet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Gerhard D.M., Wohleb E.S., Duman R.S. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquini M., Berardelli I., Biondi M. Ethiopathogenesis of depressive disorders. Clin Pract Epidemiol Ment Health. 2014;10:166. doi: 10.2174/1745017901410010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelzangs N., De Jonge P., Smit J., Bahn S., Penninx B. Cytokine production capacity in depression and anxiety. Transl Psychiatry. 2016;6:e825. doi: 10.1038/tp.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnsten A.F., Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Wei L.-H., Kuo M.-L., Chen C.-A. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene. 2001;20:5799–5809. doi: 10.1038/sj.onc.1204733. [DOI] [PubMed] [Google Scholar]
- 11.Kwei K., Kim Y., Girard L. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27:3635–3640. doi: 10.1038/sj.onc.1211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thekkek N., Richards-Kortum R. Optical imaging for cervical cancer detection: solutions for a continuing global problem. Nat Rev Cancer. 2008;8:725–731. doi: 10.1038/nrc2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J., Wu L., Kohlmeier M., Ye F., Cai W. Association between MTHFR C677T, MTHFR A1298C and MS A2756G polymorphisms and risk of cervical intraepithelial neoplasia II/III and cervical cancer: a meta-analysis. Mol Med Rep. 2013;8:919–927. doi: 10.3892/mmr.2013.1589. [DOI] [PubMed] [Google Scholar]
- 14.Team NLSTR Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;2011:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung R.J., McKay J.D., Gaborieau V. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 16.Valentovic M., Murphy R., Lamyaithong A.B., Stafford R., Schnelle A., Ball J.G. Protective effect of resveratrol on mitochondria and oxidative stress induced by cisplatin in HK-2 cells. FASEB J. 2016;30(1 suppl) 711.712–711.712. [Google Scholar]
- 17.Zheng N., Zhao C., He X.-R. Simultaneous determination of gefitinib and its major metabolites in mouse plasma by HPLC–MS/MS and its application to a pharmacokinetics study. J Chromatogr B. 2016;1011:215–222. doi: 10.1016/j.jchromb.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Rothman R.B., Baumann M.H. Serotonin releasing agents: neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav. 2002;71:825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- 19.Hossain M.A., AL-Raqmi K.A.S., AL-Mijizy Z.H., Weli A.M., Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3:705–710. doi: 10.1016/S2221-1691(13)60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal J., Adhikari R., Negi J. Effect of nitrogen, phosphorus and potassium on growth and green herb yield of Thymus serphyllum. Int J Curr Microbiol Appl Sci. 2016;5:406–410. [Google Scholar]
- 21.Soare J.R., Dinis T.C., Cunha A.P., Almeida L. Antioxidant activities of some extracts of Thymus zygis. Free Radic Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 22.Amirghofran Z., Ahmadi H., Karimi M.H. Immunomodulatory activity of the water extract of Thymus vulgaris, Thymus daenensis, and Zataria multiflora on dendritic cells and T cells responses. J Immunoass Immunochem. 2012;33:388–402. doi: 10.1080/15321819.2012.655822. [DOI] [PubMed] [Google Scholar]
- 23.Mousavi S.R., Ardakani M.R., Mirza M., Vazan S., Paknejad F. The effect of NaCl salinity and temperature on the germination of three thyme populations. Int J Biosci. 2014;5:50–59. [Google Scholar]
- 24.Korkmaz M. Dose-dependent medicinal effects of Thymus haussknechtii Velen grown wild in Turkey. Pak J Pharm Sci. January 2016;1:179–183. [PubMed] [Google Scholar]
- 25.Al-Bayati F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J Ethnopharmacol. 2008;116:403–406. doi: 10.1016/j.jep.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Dogu-Baykut E., Gunes G., Decker E.A. Impact of shortwave ultraviolet (UV-C) radiation on the antioxidant activity of thyme (Thymus vulgaris L.) Food Chem. 2014;157:167–173. doi: 10.1016/j.foodchem.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 27.El Astal Z., Ashour A., Kerrit A. Antimicrobial activity of some medicinal plant extracts in Palestine. Pak J Med Sci. 2005;21:187–193. [Google Scholar]
- 28.Chakraborty S., Roy M., Taraphdar A.K., Bhattacharya R. Cytotoxic effect of root extract of Tiliacora racemosa and oil of Semecarpus anacardium nut in human tumour cells. Phytotherapy Res. 2004;18:595–600. doi: 10.1002/ptr.1501. [DOI] [PubMed] [Google Scholar]
- 29.Qi C.-C., Shu Y.-M., Chen F.-H., Ding Y.-Q., Zhou J.-N. Sensitivity during the forced swim test is a key factor in evaluating the antidepressant effects of abscisic acid in mice. Behav Brain Res. 2016;300:106–113. doi: 10.1016/j.bbr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary O.F., Bechtholt A.J., Crowley J.J., Hill T.E., Page M.E., Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology. 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- 31.Moghaddam M.M., Barjini K.A., Ramandi M.F., Amani J. Investigation of the antibacterial activity of a short cationic peptide against multidrug-resistant Klebsiella pneumoniae and Salmonella typhimurium strains and its cytotoxicity on eukaryotic cells. World J Microbiol Biotechnol. 2014;30:1533–1540. doi: 10.1007/s11274-013-1575-y. [DOI] [PubMed] [Google Scholar]
- 32.Singh M., Shakya S., Soni V.K., Dangi A., Kumar N., Bhattacharya S.-M. The n-hexane and chloroform fractions of Piper betle L. trigger different arms of immune responses in BALB/c mice and exhibit antifilarial activity against human lymphatic filarid Brugia malayi. Int Immunopharmacol. 2009;9:716–728. doi: 10.1016/j.intimp.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Adelodun V.O., Elusiyan C., Olorunmola F. Evaluation of antitrypanosomal and anti inflammatory activities of selected Nigerian medicinal plants in mice. Afr J Tradit Complement Altern Med. 2013;10:469–476. doi: 10.4314/ajtcam.v10i6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarimeseli A., Coskun M.A., Yuceer M. Modeling microwave drying kinetics of thyme (Thymus vulgaris L.) leaves using ANN methodology and dried product quality. J Food Process Preserv. 2014;38:558–564. [Google Scholar]
- 35.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Komaki A., Hoseini F., Shahidi S., Baharlouei N. Study of the effect of extract of Thymus vulgaris on anxiety in male rats. J Traditional Complement Med. 2016;6:257–261. doi: 10.1016/j.jtcme.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miraldi E., Ferri S., Mostaghimi V. Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran) J Ethnopharmacol. 2001;75:77–87. doi: 10.1016/s0378-8741(00)00381-0. [DOI] [PubMed] [Google Scholar]
- 38.Rustaiyan A., Masoudi S., Monfared A. Volatile constituents of three Thymus species grown wild in Iran. Planta Medica. 2000;66:197–198. doi: 10.1055/s-0029-1243136. [DOI] [PubMed] [Google Scholar]
- 39.Mazooji A., Salimpur F., Danaei M., Akhoondi Darzikolaei S., Shirmohammadi K. Comparative study of the essential oil chemical composition of Thymus kotschyanus Boiss. & Hohen. Var kotschyanus from Iran. Ann Biol Res. 2012;3:1443–1451. [Google Scholar]
- 40.Deng X.-Y., Li H.-Y., Chen J.-J. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res. 2015;291:12–19. doi: 10.1016/j.bbr.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 41.Sohani Z., Samaan Z. Does depression impact cognitive impairment in patients with heart failure? Cardiol Res Pract. 2012;2012 doi: 10.1155/2012/524325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo F.H.C., Moura B.A., de Sousa D.P. Antidepressant-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: involvement of dopaminergic system. Fundam Clin Pharmacol. 2011;25:362–367. doi: 10.1111/j.1472-8206.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang S.-T., Yang R.-C., Yang L.-J., Lee P.-N., Pang J.-H.S. Phyllanthus urinaria triggers the apoptosis and Bcl-2 down-regulation in Lewis lung carcinoma cells. Life Sci. 2003;72:1705–1716. doi: 10.1016/s0024-3205(03)00016-x. [DOI] [PubMed] [Google Scholar]
- 44.Kumar D., Rawat D.S. Synthesis and antioxidant activity of Thymol and Carvacrol based Schiff bases. Bioorg Med Chem Lett. 2013;23:641–645. doi: 10.1016/j.bmcl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Deb D.D., Parimala G., Devi S.S., Chakraborty T. Effect of Thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chemico-Biol Interact. 2011;193:97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Ait M'Barek L., Ait Mouse H., Jaâfari A. Cytotoxic effect of essential oil of thyme (Thymus broussonettii) on the IGR-OV1 tumor cells resistant to chemotherapy. Braz J Med Biol Res. 2007;40:1537–1544. doi: 10.1590/s0100-879x2007001100014. [DOI] [PubMed] [Google Scholar]
- 47.Koparal A.T., Zeytinoğlu M. Animal Cell Technology: Basic & Applied Aspects. Springer; 2003. Effects of Carvacrol on a human non-small cell lung cancer (NSCLC) cell line, A549; pp. 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arunasree K. Anti-proliferative effects of Carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine. 2010;17:581–588. doi: 10.1016/j.phymed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Mehdi S.J., Ahmad A., Irshad M., Manzoor N., Rizvi M.M.A. Cytotoxic effect of Carvacrol on human cervical cancer cells. Biol Med. 2011;3:307–312. [Google Scholar]