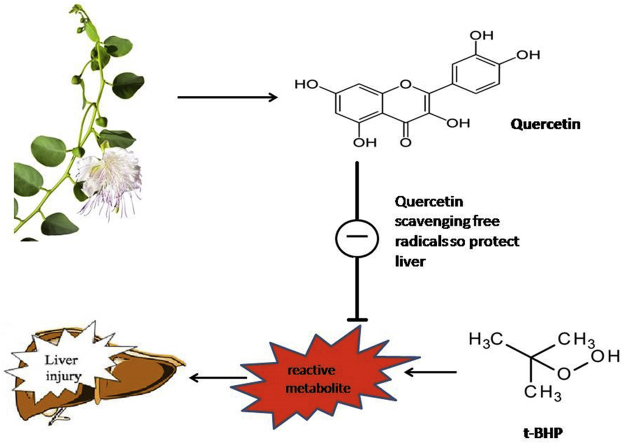
Abstract
The present study investigates the antioxidant and hepatoprotective effects of Capparis spinosa L. and Quercetin in tert-butyl hydroperoxide (t-BHP) induced acute liver damage. Different fractions of C. spinosa were examined for total phenolic content and antioxidant property. Among these fractions, hydroalcoholic extract was used to assess the hepatoprotective effect in tert-butyl hydroperoxide (t-BHP) induced hepatotoxicity model by determining serum biochemical markers, sleeping time and antioxidant assay such as reduced glutathione (GSH) as well as histopathological examination of liver tissues. The total phenolic and Quercetin contents of hydroalcoholic fraction were significantly higher than other fractions. It also showed high antioxidant activity. Pretreatment with hydroalcoholic fraction at the dose of 400 mg/kg and Quercetin at the dose of 20 mg/kg showed liver protection against t-BHP induced hepatic injury, as it was evident by a significant decrease in serum enzymes marker, sleeping time and MDA and an increase in the GSH, SOD and CAT activities confirmed by pathology tests. The final results ascertained the hepatoprotective and antioxidant effects of C. spinosa and Quercetin in a dose-dependent manner. Moreover, this study suggests that possible mechanism of this protection may be associated with its property of scavenging free radicals which may be due to the presence of phenolic compounds.
Keywords: Capparis spinosa, t-butyl hydroperoxide, Quercetin, Antioxidant, Hepatoprotective
Graphical abstract
1. Introduction
Liver is the largest internal organ in the human body that plays a major role in metabolism and detoxification of various chemicals, drugs and other toxic compounds.1 Oxidative stress is a biochemical condition that occurs in the body producing several types of reactive species such as reactive oxygen species (ROS) and reactive nitrogen species (RNS).2 Oxidative stress is regarded as one of the pathological mechanisms that causes initiation and progression of liver damage through inducing irreversible alteration of lipid membranes, proteins and DNA and, more importantly, through modulating pathways that control biological function.3 A number of pro-oxidants are implicated in the oxidative stress and cell injury that result from their intracellular metabolism to free radical intermediates.4 tert-butyl hydroperoxide (t-BHP) is an organic lipid hydroperoxide analogue which is often used as a model compound to induce oxidative stress during in vitro and in vivo studies. t-BHP can be metabolized into free radical intermediates by cytochrome P-450 which subsequently initiates lipid peroxidation and depletes cellular reduced glutathione (GSH) content. t-BHP caused leakage of lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and formation of malondialdehyde (MDA) in hepatocyte. It also mediated DNA base damage in mammalian cells.5, 6, 7
The genus Capparis belonging to the Capparaceae family includes nearly 250 species; many of which are distributed in the mediterranean regions.8 Capparis spinosa L. has been used for pharmaceutical, cosmetic, and nutritional purposes. In many countries, the flower buds, fruits, roots, and seeds of the C. spinosa have been used in folk medicine as an anti-rheumatic, tonic, expectorant, anti-spasmodic, diuretic and analgesic agents.9 They are very good sources of glucosinolates (glucocapparin, glucoiberin, sinigrin and glucobrassicin), flavonoids, phenolic acids and alkaloids; all of which are known to provide health-improving benefits due to their various biological activities such as antioxidant, anticancerogenic, antimicrobial and antimutagenic.10 Furthermore, leaves of C. spinosa is used to prepare Liv-52 which is employed to treat alcoholic liver disease, viral hepatitis, and liver cirrhosis.11
Quercetin is a flavonoid found in many fruits and vegetables including green apple, onion, green tea, lemon as well as in medicinal botanic plants. In addition, C. spinosa is one of the rich sources of Quercetin. It is one of the most active antioxidants owing to its high ability to scavenge free radicals.12
The present study was conducted to investigate the antioxidant activity and phenolic content of C. spinosa fractions. We have also examined the mechanism of C. spinosa and Quercetin as hepatoprotective agents by studying their effects on serum liver function, oxidative stress biomarkers and hepatic histopathology of mice subjected to t-BHP-induced hepatotoxicity.
2. Materials and methods
2.1. Chemicals
Quercetin, chloroform, ethyl acetate, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), reduced glutathione (GSH), trichloro acetic acid (TCA), thiobarbituric acid (TBA), bovine serum albumin (BSA) and bradford reagent were purchased from Sigma–Aldrich chemical company (St. Louis, MO), USA. All chemicals and reagents used were analytical grade. Tert-butyl hydroperoxide (t-BHP) was also purchased from Roche chemical company (Germany).
2.2. Plant extraction
Plant was collected from the Iranian province of Khuzestan during spring 2015. Plant materials were taxonomically identified by central herbarium of Ahvaz Jundishapur University of Medical Sciences, Iran. The leaves of C. spinosa were shade-dried, ground and soaked in 80% aqueous-ethanol, ethyl acetate and chloroform in separate containers for three days with occasional shaking.
Each solvent was filtered through a filter paper (Whatman No. 2) and then was removed under vacuum in a rotary evaporator until dryness. The percentage yields of extracts in different solvents were 15.75% (w/w) for dried hydroalcoholic, 4.51% for ethyl acetate and 3.9% for chloroformic fraction, respectively.
2.3. HPLC analysis
A high performance liquid chromatography system (HPLC) (Shimadzu-02-0600, Japan) was used for quantitative and qualitative analysis of Quercetin. Analyses were conducted using a C18 column (4.6 mm × 150 mm, 5 μm diameter particle sizes, Waters, Symmetry, Milford, USA). The mobile phase used was methanol: acetonitrile: water (10:10:80) with a flow rate of 1 ml/min and injection volume was 20 μl. An amount of 0.5 g of each extracts were dissolved in 10 ml of solution (methanol–acetic acid–water (1:2:1)) for 1 h on a shaker at laboratory temperature. 2 ml of the solutions were centrifuged for 10 min at 2000 × g. Then, the solutions were filtered through a micro filter with regenerated cellulose membranes of the pore size 0.22 μm. The filtrate was applied for HPLC. Standard solutions of Quercetin were also prepared by dissolving different amount in methanol. Suitable concentrations were injected to find the RT (retention time) values and check the linearity between concentration and peak areas. The spectrum was detected at 370 nm using a UV detector.
2.4. Total phenolic content (TPC)
Total phenolic contents of crude extracts were determined by a spectrophotometer using the Folin-Ciocalteu's reagent.13 Different dilutions of each dry extract (3.75, 6.25, 12.5 and 25 mg/ml) were prepared in 10 ml of their own solvent. Moreover, different concentrations of tannic acid (0.02, 04, 06 and 08 mg/ml) were prepared as standards. Then, 0.5 ml of extracts or standards was transferred into a 5 ml volumetric flask and was swirled with 2.5 ml of Folin-Ciocalteu's reagent (diluted 1:10, v/v). After 5 min, 2 ml of Na2CO3 (7.5%, v/v) solution was added and mixed. The solution was thoroughly mixed and placed at ambient temperature for 2 h until the characteristic blue color was developed. The absorbance was measured at 765 nm after 30 min. Quantification of TPC was based on a standard curve generated with tannic acid (TAC) at 765 nm.
All tests were conducted in triplicate and averaged. Results were expressed as tannic acid equivalent (mg tannic acid/g dried extract). Additional dilution was done in case the absorbance value measured was over the linear range of the standard curve.
2.5. In-vitro free radical scavenging activity
2.5.1. 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
Radical scavenging activity was determined by a spectrophotometric method based on the reduction of the methanol solution of DPPH.14, 15 Tests were carried out in triplicate. A methanol solution (0.1 ml) of the test sample at various concentrations was added to 3.9 ml of a DPPH solution (25 mg/lit). The decrease in absorbance at 515 nm was ceaselessly determined every 1 min with a UV–Vis spectrophotometer for 30 min. The percentage inhibition (I %) of DPPH radical was calculated in the following way:
A blank is the absorbance of the control reaction (containing all reagents except the test compound) while A sample is the absorbance of the test compound. The percentage of inhibited DPPH radical was calculated by the above equation; then, it was plotted against the sample/standard concentration to obtain the amount of antioxidant necessary to decrease the initial concentration of DPPH by 50% (IC50). Based on the parameter IC50, the result was expressed in terms of mg dry matter of sample/standard equivalent g−1 DPPH in the reaction medium.
2.5.2. Ferric reducing antioxidant potential (FRAP) assay
The FRAP assay was performed according to Benzie and Strain method.16 The principle of this method is based on the reduction of a ferric-tripyridyltriazine complex to its ferrous colored form in the presence of antioxidants. Briefly, the FRAP reagent contained 2.5 ml of a 10 mmol/L TPTZ (2,4,6-tripyridy-s-triazine, sigma) solution in 40 mmol/L HCl plus 2.5 ml of 20 mmol/L FeCl3·6H2O and 25 ml of 0.3 mol/L acetate buffer, pH 3.6 and was prepared freshly and warmed at 37 °C. First, absorption of fresh FRAP reagent were measured at the wavelength of 593 nm. Then, 100 μl sample or standards were mixed with 300 μl distilled water and 3 ml FRAP reagent and the absorbance of reaction mixture at 593 nm was measured spectrophotometrically. Finally, the absorbance was measured again after 30 min incubation at 37 °C. The FRAP values were achieved by standard calibration curve obtained by using different concentrations of FeSO4·7H2O. The final result was expressed as the concentration of antioxidants having a ferric reducing ability equivalent to that of 1 mmol/L FeSO4 (equivalent concentration 1 = EC1).
We then selected the most potent antioxidant extract for in vivo examination.
2.6. Animals
56 male swiss albino mice (6–8 weeks old, 25–30 g) were obtained from animal house of Ahvaz Jundishapur University of Medical Science, Iran. Mice were kept in polycarbonate cages under standard condition (temperature 25 ± 2 °C) with 12 h light/dark cycle. They were provided with standard pellet diet and free access to drinking water ad libitum. Animals were acclimated to the environment for a minimum of seven days prior to the experiment. The investigation was performed according to the Animal Ethics Committee Guidelines of Ahvaz Jundishapur University of Medical Sciences for the use of experimental animals.
2.7. Study design
Plant extracts were dissolved in normal saline prior to be applied to the mice. t-BHP was diluted in normal saline, and then; it was administered intraperitoneally in a single dose of 1.5 mM/kg. Animals were distributed into seven groups, each containing 8 mice. Group 1, as the negative control group, received normal saline for 5 days while group 2 received oral hydroalcoholic fraction of C. spinosa (400 mg/kg) for five consecutive days. Group 3 as the positive control received t-BHP (1.5 mM/kg/IP) only on the 5th day and groups 4 to 7 received C. spinosa orally in doses of 100, 200 and 400 mg/kg and Quercetin (20 mg/kg/po) respectively for 5 days and t-BHP (1.5 mM/kg/IP) on the 5th day, 1 h after the last dose of extract and Quercetin administration.
2.8. Hexobarbital-induced sleeping time
On the 6th day, hexobarbital was administered at the dose of 40 mg/kg body weight I.P, and the sleeping time was recorded in minutes from the onset of sleep to its natural arousal, i.e. loss of righting reflex to its recovery.17
2.9. Sample collection
On the 6th day, 24 h after t-BHP administration, animals were anaesthetized with diethyl ether and their blood samples were collected by cardiac puncture. They were then sacrificed by decapitation and their livers were isolated and washed with saline quickly. Afterward, the liver was weighed and divided into two parts. One part was used for pathological studies and the other part was homogenized for tissue antioxidant assays.
2.10. Biochemical assays
The blood samples were left to clot for 40 min at room temperature. Serum was separated by centrifugation at 1050 × g at 24 °C for 5 min and was stored at −20 °C until further analyses. Activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum were determined employing the Reitman-Frankel method.18 Alkaline phosphatase (ALP) was estimated according to king's method.19
In order to estimate the tissue biochemical parameters, the liver tissues were chopped into small pieces with scissors. Then, it was homogenized in ice-cold phosphate buffer (pH 8) at a concentration of 10% (weight by volume) using a wisetis HG-150 homogenizer. The unbroken cells and cell debris were removed by centrifugation at 200 × g for 10 min using a (HettichZentrifugen, Germany) refrigerated centrifuge. Protein content in homogenates was measured by the method of Bradford20 using crystalline BSA as standard. The supernatant was used for the evaluation of GSH, malondialdehyde (MDA), superoxide dismutase (SOD) and catalase employing standard methods.
The GSH content in the tissue was determined using a modified version of the method described by Ellman, based on the formation of a yellow colored complex with Ellman's reagent (DTNB).21 Free endogenous GSH was assayed in a 3 ml volume glass by the addition of 40 μl of 0.5 mM DTNB prepared in 0.2 M phosphate buffer (pH = 8) to 2 ml phosphate buffer and 40 μl of the supernatant. The absorbance was read at 412 nm using a UV–visible spectrophotometer.
The lipid peroxidation was expressed by measuring the amounts of malondialdehyde (MDA) based on the thiobarbituric acid (TBA) color reaction and using the method described by Buege and Aust.22 Briefly, 0.5 ml of liver supernatant was mixed with 1.5 ml of TCA (10%, w/v). The samples were centrifuged at 3000 × g for 10 min; and 1.5 ml of each sample supernatant was transferred to a test tube containing 2 ml of TBA solution (0.67%, w/v). The mixture was kept in boiling water for 30 min, forming a pink color solution. The mixture was then immediately cooled and the absorbance was measured at 532 nm by spectrophotometer. The concentration of MDA was calculated based on the absorbance coefficient of the TBA–MDA complex (ε = 1.56 × 105 cm−1 M−1) and it was expressed as nmol/mg protein.
Catalase (CAT) activity was measured by the method of Aebi and Bergmeyer.23 100 μl of liver supernatant was added to a quartz cuvette and the reaction was initiated by the addition of 1.9 ml of freshly prepared H2O2 (30 mM) in phosphate buffer (50 mM, pH 7.0). The decomposition rate of H2O2 was measured spectrophotometrically at 240 nm during 60s. The activity of CAT was expressed as μmol H2O2/mg tissue/min.
Superoxide dismutase SOD activity was measured using a calorimetrically enzymatic assay kit (ZellBio GmbH, Ulm, Germany). In this assay, the SOD activities were expressed as units per microgram of liver protein.
The levels of MDA, SOD and GSH were normalized with protein.
2.11. Histopathological assessments
For the histological examination, the livers were fixed in 10% formalin solution for at least 24 h. Then, the liver tissues were dehydrated with a sequence of ethanol solutions, embedded in paraffin, cut into 5 μm sections and stained with hematoxylin and Eosin dye (H&E stain). These sections were then examined under a photomicroscope for the presence of cell necrosis, fatty change, ballooning degeneration, edema and mononuclear/polymorphonuclear cell infiltration.
2.12. Statistical analysis
Data analysis was carried out using GraphPad Prism software version 5. Results were expressed as Mean ± SEM and all statistical comparisons were made by means of one-way ANOVA test followed by Tukey's post hoc analysis. p-value less than 0.05 was considered to be significant.
3. Results
The physical properties and percentage (%) yield of different extracts are demonstrated in Table 1.
Table 1.
Physical properties, total phenolics and Quercetin content of different extract of C. spinosa.
| Fraction | Color | Consistency | % Yield (w/w) | Quercetin content (mg/g) | Total phenolics (mg/g) |
|---|---|---|---|---|---|
| Hydro-alcoholic | Greenish brown | Semi-solid | 15.75 | 7.415 ± 0.405 | 26.58978 ± 1.191 |
| Ethyl acetate | Dark green | Semi-solid | 4.51 | 2.897 ± 0.312a | 6.53532 ± 0.425a |
| Chloroform | Dark green | Semi-solid | 3.9 | 4.986 ± 0.309a | 13.65529 ± 1.361a |
Values are presented as mean ± S.E.M for 3 replications.
Significantly different compared to hydro-alcoholic fraction (p < 0.05).
3.1. HPLC analysis
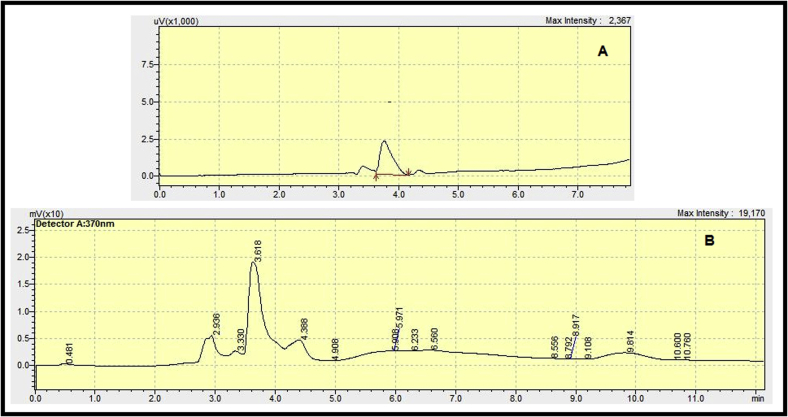
Evaluation of Quercetin in different fractions of C. spinosa by HPLC revealed that hydroalcoholic fraction had the highest Quercetin content (7.415 ± 0.405 mg/g) followed by chloroformic (4.986 ± 0.309 mg/g) and ethyl acetate fraction (2.897 ± 0.312 mg/g). The results are shown in Table 1. Moreover, the chromatograms of Quercetin standard solution and hydroalcoholic fraction of C. spinosa are shown in Fig. 3.
Fig. 3.
. (A): HPLC chromatogram of 100 ng/ml Quercetin standard solution. (B): HPLC Chromatogram of hydroalcoholic extract of C. spinosa containing Quercetin.
3.2. Total phenolic content (TPC)
The amount of total phenolics is varied in different extracts (Table 1). The highest total phenolic levels were detected in hydroalcoholic extract (26.58978 ± 1.191 mg/g extract) followed by chloroformic (13.65529 ± 1.361 mg/g) and ethyl acetate extract (6.53532 ± 0.425 mg/g).
3.3. In-vitro free radical scavenging activity
3.3.1. DPPH radical scavenging activity
The evaluation of antioxidant activities of extracts and Quercetin by DPPH assay showed that Quercetin has better scavenging activity and has the lower IC50 followed by hydroalcoholic extract, chloroformic and ethyl acetate extract, respectively. The antioxidant activities of Quercetin, hydroalcoholic and chloroformic extracts were similar (p > 0.05) while ethyl acetate extract exhibited a weak antioxidant activity (Table 2).
Table 2.
In vitro antioxidant activity of C. spinosa extracts and Quercetin as determined by the DPPH and FRAP assays.
| Extract | DPPH (IC 50 value; mg/ml) | FRAP (EC1 value; mg/ml) |
|---|---|---|
| Hydro-alcoholic | 0.034891 ± 0.0017 | 1.655187 ± 0.1219 |
| Ethyl acetate | 0.277039 ± 0.0122a,b | 15.90301 ± 1.082a,b |
| Chloroformic | 0.0392 ± 0.0011 | 3.908373 ± 0.3171a |
| Quercetin | 0.01158 ± 0.00107 | 0.4903 ± 0.05911 |
Values are presented as mean ± S.E.M for 3 replications.
Significantly different compared to Quercetin (p < 0.05).
Significantly different compared to hydro-alcoholic fraction (p < 0.05).
3.3.2. FRAP assay
The FRAP assay measures the antioxidant activity of any substance in the reaction medium by its reducing ability. Antioxidant potential of different fractions of C. spinosa and Querctin was estimated according to their ability to reduce the TPTZ-Fe (III) complex to a TPTZ-Fe (II) complex and was expressed as EC1. The hydroalcoholic fraction of C. spinosa and Quercetin showed highest FRAP antioxidant activity as recorded in DPPH methods. The order of FRAP activity of respective samples is as follows: Quercetin > hydroalcoholic > chloroformic, followed by ethyl acetate extract (Table 2). As in the case of DPPH, the ferric reducing antioxidant capacity of ethyl acetate extract was found to be significantly lower than other samples (p < .005).
In addition, a significant correlation was observed between TPC and the FRAP assays (R2 = 0.699; data not shown). Moreover, a significant correlation of R2 = 0.586 was calculated between TPC and the IC 50 of DPPH radical-scavenging activity.
3.4. Biochemical changes
The hepatic enzymes of serum ALT, AST and ALP are well known as biomarkers for early acute hepatic damage. The effects of pretreatment with C. spinosa extracts and Quercetin on the t-BHP-induced elevation of ALT, AST and ALP are shown in Table 3. Data analyses demonstrated that t-BHP (2 mmol/kg/ip) intoxication develop severe hepatic damage as it was evident from a significant increase in the serum activities of ALT, AST and ALP (p < 0.05) as compared to the normal control group (Table 3). Pretreatment with C. spinosa (100, 200 and 400 mg/kg) and Quercetin (20 mg/kg/po) significantly decreased the elevation of AST and ALT (p < 0.05). In addition, C. spinosa decreased ALP in all doses by a dose-dependent manner; however, high dose of C. spinosa extract (400 mg/kg) and Quercetin (20 mg/kg/po) significantly reduced the elevation of ALP. Furthermore, no significant difference was observed between the control and second group which received high doses of C. spinosa (400 mg/kg).
Table 3.
Effects of the pretreatment with hydroalcoholic fraction of C. spinosa and Quercetin on the serum activities of AST, ALT, ALP and sleeping time in t-BHP-induced hepatotoxicity.
| Groups | ALT (U/l) | AST (U/l) | ALP (U/l) | Sleeping time (min) |
|---|---|---|---|---|
| 1 – Normal saline (control) | 77.35 ± 5.073 | 119.1 ± 7.898 | 303.9 ± 7.426 | 15.44 ± 1.613 |
| 2 – Extract (400 mg/kg) | 90.94 ± 3.54b | 113.3 ± 6.617b | 301.4 ± 11.17b | 18.29 ± 1.604b |
| 3 – t-BHP (50 mg/kg) | 357.33 ± 25.94a | 491.7 ± 26.53a | 430.9 ± 15.25a | 43.53 ± 2.977a |
| 4 – Extract (100 mg/kg) + t-BHP | 241.41 ± 17.19a,b | 308.5 ± 22.23a,b | 405.2 ± 6.501a | 38.85 ± 2.667a |
| 5 – Extract (200 mg/kg) + t-BHP | 160.21 ± 17.72a,b | 311.4 ± 16.43a,b | 378.8 ± 13.67a | 30.97 ± 1.771a,b |
| 6 – Extract (200 mg/kg) + t-BHP | 129.80 ± 15.93b | 236.8 ± 16.49a,b | 340.7 ± 21.67b | 24.60 ± 2.011b |
| 7 – Quercetin (200 mg/kg) + t-BHP | 192.42 ± 14.90a,b | 219.4 ± 12.70a,b | 326.7 ± 21.44b | 26.80 ± 2.092a,b |
Animals in group 1 received normal saline solution, while group 2 received C. spinosa extract (400 mg/kg, po) for 5 days, group 3 received t-BHP (0.18 mM/kg, ip) on the fifth day. The mice in groups 4, 5 and 6 were pretreated with C. spinosa extract (100, 200 and 400 mg/kg, p.o, respectively) once daily for five consecutive days. Group 7 were pretreated with Quercetin (1.5 mM/kg, p.o), once daily for five consecutive days. One hour after the final treatment, the mice were treated with t-BHP (0.18 mM/kg, ip). Hepatotoxicity was determined 24 h later by quantifying the sleeping time and serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) as well as alkaline phosphatase (ALP).
Each value represents the mean ± SEM for eight mice.
Significantly different from the control (p < 0.05).
Significantly different from t-BHP group (p < 0.05).
3.5. Effect on oxidative stress
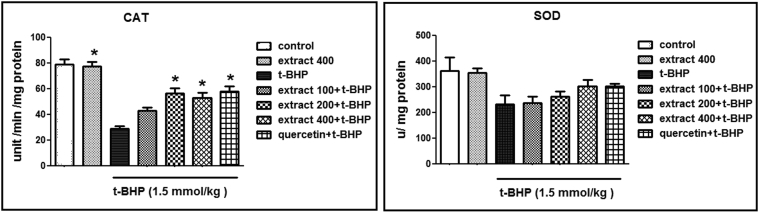
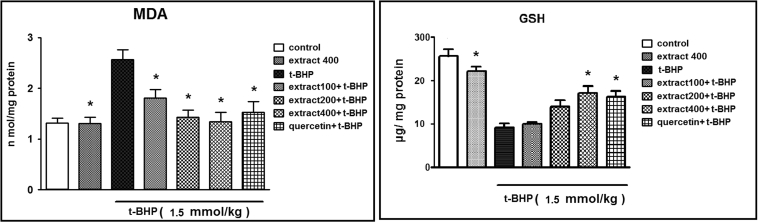
In order to further evaluate the possible mechanism involved in the protective effect of C. spinosa and Quercetin on hepatotoxicity, MDA formation, GSH content and antioxidant enzymes (SOD and CAT) were measured. As seen in Fig. 1, Fig. 2, t-BHP treatment increased MDA formation and depleted liver GSH level. Compared to the control group, t-BHP also significantly decreased SOD and CAT activities. Regarding the inhibition of lipid peroxidation, we observed that the groups which were pretreated with C. spinosa and Quercetin significantly reduced the amount of MDA in liver homogenate, as compared with the t-BHP-intoxicated group (p < 0.05). Moreover, the group pretreated with 400 mg/kg of C. spinosa had even lower MDA levels than the other treatment group. GSH forms the main intracellular antioxidant to scavenge the free radicals. Administration of t-BHP significantly reduced the levels of GSH. Pretreatment with C. spinosa and Quercetin significantly recovered the levels of GSH depletion produced by t-BHP. Likewise, 400 mg/kg of C. spinosa and Quercetin showed the best result.
Fig. 1.
Pretreatment effects of C. spinosa and Quercetin on the hepatic SOD and CAT activity. Each value represents means ± S.E.M. of 8 mice per group. *: Significantly different (p < 0.05) from the positive control group.
Fig. 2.
Pretreatment effects of C. spinosa and Quercetin on the hepatic GSH and MDA content. Each value represents means ± S.E.M. of 8 mice per group. *: Significantly different (p < 0.05) from the positive control group.
Antioxidant enzymes form the first line of defense against free radical-induced toxicity. In the present study, SOD and CAT activities were significantly decreased (p < 0.05) in the t-BHP group when compared to the control group, indicating that t-BHP damaged Antioxidant defence. Quercetin and C. spinosa in all doses raised SOD and CAT activities as compared to the mice treated with t-BHP. The increase was also significant in CAT activity.
3.6. Histopathology
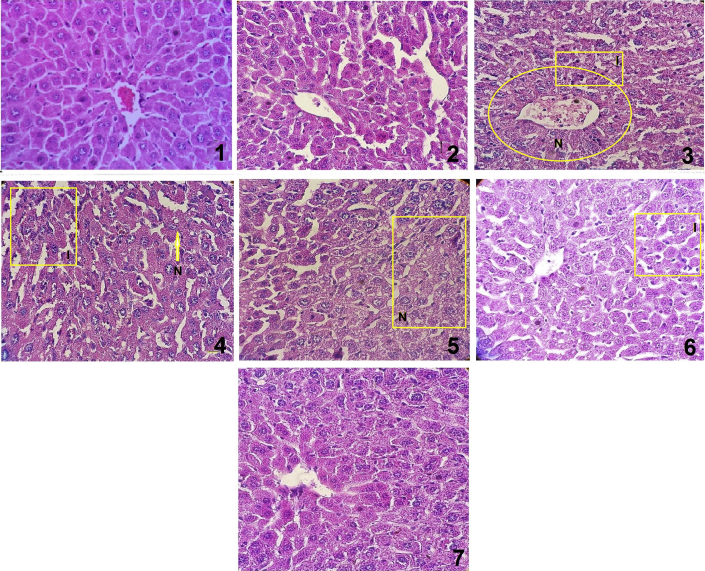
The histopathological pictures of liver are shown in Fig. 4. The pattern of liver in the negative control group and the second group with only crude extract showed normal hepatic architecture. The hepatocytes are within normal limits and are separated by narrow blood sinusoids. Structure of liver lobules was preserved and no cell necrosis, fatty change and inflammation were seen (Fig. 4.1 and 4.2). t-BHP intoxicated group exhibited severe histopathological changes such as centrilobular hepatic necrosis, fatty changes, vacoulization, and infiltrating lymphocytes (Fig. 4.3).
Fig. 4.
Histopathological observations of liver sections stained with hematoxylin and eosin(×100). (1 and 2) Liver sections of mice treated with saline and hydroalcoholic extract of the C. spinosa (400 mg/kg, p.o.), respectively, showing normal architecture and central vein and normal hepatic cells; (3) Liver section of mice treated with t-BHP (1.5 mmol/kg, i.p.) showing centrilobular necrosis, congestion and severe inflammation; (4) Liver section of C. spinosa (100 mg/kg, p.o.) + t BHP (1.5 mmol/kg, i.p.)-treated mice, showing small areas of necrosis and partially severe inflammation.; and (5) Liver section of mice treated with C. spinosa (200 mg/kg, p.o.) + t BHP (1.5 mmol/kg, i.p.) showing partially preserved hepatocytes as well as the architecture with small areas of necrosis and inflammatory cell infiltration. (6 and 7) Liver section of mice treated with C. spinosa (400 mg/kg, p.o.) and Quercetin (20 mg/kg, p.o.), respectively + tBHP (1.5 mmol/kg, i.p.) showing well preserved hepatocytes and architecture with a few to milder degree of inflammation. N: necrosis I: inflammatory cell infiltration.
Pretreatment with 100 mg/kg of C. spinosa extract showed mild improvement and congestion, diffuse necrosis, accumulation of lymphocytes in the portal. Vacuolization and severe edema were also observed in hepatocytes (Fig. 4.4). Administration of 200 mg/kg of C. spinosa extract showed mild to moderate congestion and necrosis, moderate lymphocytic aggregation in portal, sporadic and brief fatty changes in hepatocytes and partial edema (Fig. 4.5). In the group treated with 400 mg/kg of C. spinosa extract, slight congestion, mild lymphocytic accumulation and edema were seen (Fig. 4.6). The pattern of hepatic tissue in the Quercetin group was similar to the group received 400 mg/kg of C. spinosa (Fig. 4.7).
4. Discussion
Increasing evidence has indicated that cellular damage mediated by oxidative stress contribute to the initiation and progression of many liver diseases.24 Many efforts have been made to develop the complementary and alternative medicines for reducing oxidative stress and improving liver functions.25, 26 Therapeutic agents from natural sources, such as Quercetin and silymarin, are particularly attractive for treatments as they have the potential to reduce the risk of drug toxicity.27 Total phenolic content of extract often determines its pharmacological effects such as antioxidant activity. The in vitro study demonstrated that hydroalcoholic extract with higher total phenolic content exhibit higher antioxidant activity followed by chloroformic and ethyl acetate extract. Therefore, components like phenolic compounds may play an important role in its antioxidant activity. Several studies have reported a direct relationship between phenolic content and antioxidant activity.28
HPLC analysis of C. spinosa extracts showed different amounts of Quercetin in these extracts, and the Quercetin content of hydroalcoholic fraction of C. spinosa was higher than chloroformic and ethyl acetate extracts. This finding indicated that solvent can affect phytochemical composition of each extract.
T-BHP is a dangerous chemical that is highly reactive, flammable and toxic.29 The mechanism by which t-BHP causes damage involves the biotransformation of t-BHP by the cytochrome P-450 system into a free radical intermediates, peroxyl (tBuOO) and alkoxyl (tBuO) radical; all of which cause lipid peroxidation eventuating in cells damage.30 Aminotransferases (AST and ALT) are the first enzymes to be used in diagnosis of liver damage. Since these are normally located in the cytosol, toxicity affecting the liver with subsequent breakdown in membrane architecture of the cells leads to their spillage into plasma while their concentration rises in the bloodstream.31, 32, 33 Administering t-BHP to mice markedly increases serum transaminase (AST and ALT) and ALP levels. This increase commonly reflects the severity of liver injury.34 Pretreatment with C. spinosa and Quercetin attenuated these increased enzyme activity and was followed by recovery towards normal levels. These findings strongly prove the ability of these substances in protecting hepatocyte against membrane fragility and decreasing the leakage of enzymes into the circulation.
A number of studies have demonstrated that the neutralization of free radical by antioxidant compounds may reduce the amounts of t-BHP induced oxidative damage; and thus, protect the liver.35, 36 Metabolism of t-BHP leads to the formation of highly unstable free radicals to initiate peroxidation and other oxidative damage in the cells.30 Hence, the antioxidant capacities to scavenge of free radicals can inhibit t-BHP – induced lipid peroxidation.37
Both enzymatic and non-enzymatic antioxidant system are essential for cellular response in order to deal with oxidative stress under physiological condition. Therefore, an increase in a variety of oxidative stress biomarkers like MDA and a decrease in other antioxidants like GSH and antioxidant enzyme including CAT and SOD strongly support the presence of oxidative stress in liver damage.38, 39, 40 Antioxidant enzymes (SOD and CAT) form the first line of defense against oxidative tissue-damage.41 SOD plays an important role in scavenging toxic intermediates and converting O2 into H2O2. Moreover, CAT metabolizes H2O2 to non-toxic products.27 GSH, an important intracellular antioxidant, acts both as a co-factor for glutathione peroxidase and as a direct scavenger to remove reactive species such as hydroxyl radical, peroxynitrite, and singlet oxygen. Enhanced levels of GSH also protect liver against a potential oxidative damage.42 MDA is one of the lipid peroxidation products which has been used as a biomarker of lipid peroxidation for several decades. It has been used as a facile means for assessing lipid peroxidation in biological materials.43 Our findings showed that MDA levels were increased in response to t-BHP treatment while GSH, SOD and CAT levels were decreased. These preliminary findings indicate an oxidative damage associated with t-BHP. In this work, Quercetin and C. spinosa extract (400 mg/kg) brought the MDA and antioxidant enzymes to the normal levels, implying that these substances prevent the oxidative damage of t-BHP. In addition, these pretreated groups have shown an increase in hepatic GSH content that indicate their ability to enhance the antioxidant capacity of liver. Moreover, histopathological studies under light microscope confirms the protective effects of C. spinosa and Quercetin against the t-BHP-induced liver damage as it was evident by the reversal of centrilobular necrosis, vacuolization and scattered lymphocytes infiltrate in hepatic parenchyma.
5. Conclusion
This study demonstrated that C. spinosa and Quercetin are effective for the prevention of t-BHP-induced hepatic damage in mice; and therefore, it could be used as a hepatoprotective agent. The protective effects against acute liver injury may be due to the higher antioxidant capacity, free radical scavenging effect, inhibition of lipid peroxidation and increased antioxidant activity. According to the results obtained, abundant flavonoid compounds such as Quercetin are considered as the main hepatoprotective factor in the hydroalcoholic extract of C. spinosa.
Conflict of interest statement
The authors don't have any conflict of interest.
Acknowledgement
This work was supported by the grant number u-95074 provided by Deputy of Research of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Forouzandeh H., Azemi M.E., Rashidi I., Goudarzi M., Kalantari H. Study of the protective effect of Teucrium polium L. extract on acetaminophen-induced hepatotoxicity in mice. Iran J Pharm Res. 2013;12(1):123. IJPR. [PMC free article] [PubMed] [Google Scholar]
- 2.Ermak G., Davies K.J. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38(10):713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y., Wang N., Ye X. Hepatoprotective effect and its possible mechanism of coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in rats. J Ethnopharmacol. 2011;138(3):683–690. doi: 10.1016/j.jep.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Williams G.M., Jeffrey A.M. Oxidative DNA damage: endogenous and chemically induced. Regul Toxicol Pharmacol. 2000;32(3):283–292. doi: 10.1006/rtph.2000.1433. [DOI] [PubMed] [Google Scholar]
- 5.Duh P.-D., Wang B.-S., Liou S.-J., Lin C.-J. Cytoprotective effects of pu-erh tea on hepatotoxicity in vitro and in vivo induced by tert-butyl-hydroperoxide. Food Chem. 2010;119(2):580–585. [Google Scholar]
- 6.Yau M.-H., Che C.-T., Liang S.-M., Kong Y.-C., Fong W.-P. An aqueous extract of Rubus chingii fruits protects primary rat hepatocytes against tert-butyl hydroperoxide induced oxidative stress. Life Sci. 2002;72(3):329–338. doi: 10.1016/s0024-3205(02)02239-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang C.-J., Wang J.-M., Lin W.-L., Chu C.-Y., Chou F.-P., Tseng T.-H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38(5):411–416. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 8.Argentieri M., Macchia F., Papadia P., Fanizzi F., Avato P. Bioactive compounds from Capparis spinosa subsp. rupestris. Ind Crops Prod. 2012;36(1):65–69. [Google Scholar]
- 9.Kalantar M., Goudarzi M., Foruozandeh H., Siahpoosh A., Javad M. The topical effect of Cappariss spinosa L. Extract on burn wound healing. Jundishapur J Nat Pharm Prod. 2016 doi: 10.17795/jjnpp-20670. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulisic-Bilusi T., Schmller I., Schnbele K., Siracusa L., Ruberto G. The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa) Food Chem. 2012;132:261–267. doi: 10.1016/j.foodchem.2011.10.074. [DOI] [PubMed] [Google Scholar]
- 11.Tlilia N., Ferianib A., Saadouid E., Nasria N., Khaldid A. Capparis spinosa leaves extract: source of bioantioxidants with nephroprotective and hepatoprotective effects. Biomed Pharmacother. 2017;87:171–179. doi: 10.1016/j.biopha.2016.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Moghaddasian B., Asli D.E., Alaghemand A. Quantitative analysis of Quercetin in different parts of Capparis spinosa by HPLC. Ann Biol Res. 2012;3(12):5775–5778. [Google Scholar]
- 13.Mahboubi M., Kazempour N., BolandNazar A.R. Total phenolic, total flavonoids, antioxidant and antimicrobial activities of Scrophularia striata Boiss extracts. Jundishapur J Natur Pharmac Prod. 2013;8(1):15–19. [PMC free article] [PubMed] [Google Scholar]
- 14.Burits M., Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Cuendet M., Hostettmann K., Potterat O., Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helvetica Chim Acta. 1997;80(4):1144–1152. [Google Scholar]
- 16.Benzie I.F., Strain J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 17.Girish C., Pradhan S. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J Pharmacol Pharmacother. 2012;3(2):149. doi: 10.4103/0976-500X.95515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitman S, Frankel S. A colorimetric method for determination of serum glutamic oxalacetic glutamic pyruvic transminases 1957; 28(1):56–63. [DOI] [PubMed]
- 19.King J. Van Nostrand Company Limited; London: 1965. The phosphohydrolases and alkaline phosphatases: practical clinical enzymology. [Google Scholar]
- 20.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Ellman G.L. Tissue sulfhydryl groups. Archives Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Cicho Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol WJG. 2014;20(25):8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao L., Du J., Ding W. Hepatoprotective and antioxidant effects of dietary Angelica sinensis extract against carbon tetrachloride induced hepatic injury in Jian Carp (Cyprinus carpio var. Jian) Aquac Res. 2016;47(6):1852–1863. [Google Scholar]
- 26.Jiang W., Guo M.-H., Hai X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J Gastroenterol. 2016;22(46):10180. doi: 10.3748/wjg.v22.i46.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J.-G., Kan Y.-J., Wu Y.-B., Yi J., Chen T.Q., Wu J.-Z. Hepatoprotective effect of ganoderma triterpenoids against oxidative damage induced by tert-butyl hydroperoxide in human hepatic HepG2 cells. Pharm Biol. 2016;54(5):1–10. doi: 10.3109/13880209.2015.1091481. [DOI] [PubMed] [Google Scholar]
- 28.Gonalves S., Gomes D., Costa P., Romano A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Ind Crops Prod. 2013;43:465–471. [Google Scholar]
- 29.Correia S., Vaz C.V., Silva A., Cavaco J.E., Socorro S. Regucalcin counteracts tert butyl hydroperoxide and cadmium induced oxidative stress in rat testis. J Appl Toxicol. 2017;37(2):159–166. doi: 10.1002/jat.3333. [DOI] [PubMed] [Google Scholar]
- 30.Suksen K., Charaslertrangsi T., Noonin C. Protective effect of diarylheptanoids from Curcuma comosa on primary rat hepatocytes against t-butyl hydroperoxide-induced toxicity. Pharm Biol. 2016;54(5):853–862. doi: 10.3109/13880209.2015.1088550. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen N.P.E., Bessems J.G.M., Straat R.V.D. Molecular aspects of paracetamol-induced hepatotoxicity and its mechanism-based prevention. Drug Metab Rev. 1992;24:367–407. doi: 10.3109/03602539208996298. [DOI] [PubMed] [Google Scholar]
- 32.Jagadeesan G., Kavitha A.V. Recovery of phosphatase and transaminase activity of mercury intoxicated Mus musculus (Linn.) liver tissue by Tribulus terrestris (Linn.) (Zygophyllaceae) extract. Trop Biomed. 2006;23(1):45–51. [PubMed] [Google Scholar]
- 33.Lin S.C., Yao C.J., Lin C.C., Lin Y.H. Hepatoprotective activity of Taiwan folk medicine: eclipta prostrate Linn. against various hepatotoxins induced acute hepatotoxicity. Phytother Res. 1996;10:483–490. [Google Scholar]
- 34.Giannini E., Risso D., Botta F. Validity and clinical utility of the aspartate aminotransferase alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus related chronic liver disease. Archives Intern Med. 2003;163(2):218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 35.Noh J.-R., Gang G.-T., Kim Y.-H. Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl 4-and high-fat diet-treated mice. Food Chem Toxicol. 2010;48(11):3177–3183. doi: 10.1016/j.fct.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Chen H., Tappel A. Protection by multiple antioxidants against lipid peroxidation in rat liver homogenate. Lipids. 1996;31(1):47–50. doi: 10.1007/BF02522409. [DOI] [PubMed] [Google Scholar]
- 37.Wang B.-J., Wang B.-J., Liu C.-T., Tseng C.-Y., Wu C.-P., Yu Z.-R. Hepatoprotective and antioxidant effects of Bupleurum kaoi Liu (Chao et Chuang) extract and its fractions fractionated using supercritical CO2 on CCl4-induced liver damage. Food Chem Toxicol. 2004;42(4):609–617. doi: 10.1016/j.fct.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res; 42(8):741–749. [DOI] [PubMed]
- 39.Dey A., Lakshmanan J. The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct. 2013;4(8):1148–1184. doi: 10.1039/c3fo30317a. [DOI] [PubMed] [Google Scholar]
- 40.Karabulut A., Karabulut E., Kiran T., Ocak S., Otlu O. Oxidant and antioxidant activity in rabbit livers treated with zoledronic acid. Transplant Proc. 2010;42(9):3820–3822. doi: 10.1016/j.transproceed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez C., Mayo J.C., Sainz R.M. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 42.Hosseini-Zijoud S.-M., Ebadi S.A., Goodarzi M.T. Lipid peroxidation and antioxidant status in patients with medullary thyroid carcinoma: a case-control study. J Clin Diagn Res. 2016;10(2):4–10. doi: 10.7860/JCDR/2016/17854.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]